Isolation and characterization of five novel mini-Mconotoxins from the venom of mollusk-hunter snail Conus bandanus
Nguyen Bao, Jean-Pière LE CAER, Phan Thi Khanh Vinh
1Faculty of Food Technology, Nha Trang University, 02 Nguyen Dinh Chieu, Nha Trang, Khanh Hoa, Vietnam
2Institut de Chimie des Substances Naturelles, Centre de Recherche de Gif - FRC3115, - UPR 2301, F-91198 Gif-sur-Yvette, France
ABSTRACT
KEYWORDS: Mini-M conotoxin; Conus bandanus; Cone snail venom
1.Introduction
The predatory gastropods, cone snails of genus Conus, use venom peptides as a weapon for prey capture, competition, and defence from predators.These peptides can target different classes of ion channels with a high degree of specificity[1].The structural and functional diversity of Conus venom peptides have been disclosed after being studied for about 40 years[2].Most bio-active components of these venoms are disulfide-rich peptides, namely conotoxins[3].The variety of specific conotoxin generates through different types of cysteine framework(s), modification of aminoacid residues within the inter-cysteine loop, cysteine connectivity,post-translational modifications[4].
Disulfide-rich peptides belonging to the M-superfamily have the cysteine arrangement: C1C2-C3-C4-C5C6, where the dashes represent inter-cysteine loops one, two, and three, respectively.So far, M-superfamily conotoxins were classified into five branches,labeled M1 through M5.The branch number shows the number of residues that exist in the third cysteine loop between C4and C5[5].M1-M3 branches are the mini-M conotoxins, and M4-M5 branches are the maxi-M conotoxins.The mini-M conotoxins usually have less than 22 amino acids, while the maxi-M conopeptides have more than 22 residues[6].The specific target receptors of the maxi-M conotoxins have been identified, such as voltage-gated sodium channels (µ-conotoxins)[7], voltage-gated potassium channels (κMconotoxins)[8], nicotinic acetylcholine receptors (ψ-conotoxins)[9].In contrast, the target receptors of the mini-M conotoxins have been rarely identified.
As part of a project for searching new small disulfide-rich peptides from the cone snail venom, characterization of conopeptides of Conus bandanus (C.bandanus) has been carried out.These cone snails were collected in the Nha Trang Bay (Vietnam).Here, the peptide sequences of five new conotoxins were reported.The primary structure was achieved using tandem mass spectrometry and Edman degradation.
2.Materials and methods
2.1.Isolation and purification of native conotoxins
The specimens of each C.bandanus were collected at Ke Ga reef of the Nha Trang Bay, Vietnam (with latitude: 12.291132; longitude:109.207772).The venom was extracted from the dissected whole apparatus, with a solution of 0.1% trifluoroacetic acid (TFA) three times.All subfractions were performed on a Shimadzu LC-class 10 system with 220 nm absorbance at room temperature.The lyophilized venom was dissolved in A buffer, and the extract was loaded in batches of ~3 mg on a Vydac analytical C18column (300 Å, 5 µm, 4.6 mm i.d.×250 mm).The gradient program was 0% of B buffer (900 mL CH3CN/100 mL H2O/1 mL TFA) in 10 min, then 0%-67.5% of B buffer in 60 min with the flow rate of 1 mL/min.Further purification steps were carried out using the same column with appropriate gradient programs.
2.2.Complete reduction and alkylation of peptide fractions
Twenty µL of the purified fraction was reduced completely by incubation for 15 min at 65 ℃ in 40 µL of 20 mM tris-2-carboxyethyl-phosphine (TCEP) in 0.5 mol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid.Alkylation was then achieved by the addition of 30 µL of 50 mM iodoacetamide (IAA) and incubated for 30 min at 25 ℃ in darkness.The mixture was finally desalted by solid-phase extraction on a Zip Tip C18column (Millipore, USA).
2.3.Analysis by matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS) and tandem mass spectrometry (MS/MS)
The investigated fractions were analyzed with a 4800 MALDITOF/TOF mass spectrometer (AB Sciex, France).The instrument was externally calibrated using a peptide mixture (700-3 700 Da,AB Sciex).Aliquots of 1 µL of a purified fraction were mixed with 1 µL of a solution of 4 mg/mL of cyano-4-hydroxycinnamic acid.The samples were irradiated with an Nd: YAG laser operating at 355 nm wavelength, producing 3 ns wide pulses.Acquisitions were performed on positive reflection mode.For MS/MS experiments,precursor ions were accelerated at 8 keV, and the MS/MS spectra were acquired using 2 keV collision energy, with collision-induced dissociation (CID) gas at a pressure of 3.5×10-6Torr.MS and MS/MS data were treated using DataExplorer 4.9 (AB Sciex).
2.4.Peptide sequencing by Edman degradation
The amino acid sequences of the native peptide were determined by automated Edman degradation using a Procise protein sequencer(Applied Biosystem model 492, Applied Biosystem, USA).The 2µL of pure peptides (50-500 pmol) was dissolved in 25 µL of 50%(v/v) aqueous TFA for sequencing.
3.Results
3.1.Venom fractionation and purification
The venom ducts from 11 specimens of C.bandanus were dissected,cut into small pieces, and extracted with a solution of 0.1% TFA.The soluble components were fractionated on a Vydac column (300 Å, 5 µm, 4.6 mm i.d.×250 mm) and yielded 25 major peaks (Figure 1A).Each peak was pooled on the same column.Figure 1B, 1C,and 1D showed some peaks of the final purified products from the previous steps.These peaks were purified near to homogeneity on the same reversed-phase analytical column just by optimizing the elution gradient slope.
3.2.Three disulfide conotoxins
Each HPLC fraction contained conopeptides (Figure 1B, 1C,1D), which were analyzed by MALDI-TOF MS.The native peak 10*showed monoisotopic ion signals at m/z 1 683.43 (Figure 2A).Otherwise, the native peak 6*yielded two monoisotopic ion signals at m/z 1 680.69 and 1 664.7 (Figure 2C).The difference in mass of 16 Da (one oxygen atom) of mass spectra of two peptides could indicate that m/z 1 680.69 was most probably the hydroxylated form of m/z 1 664.7.The native 16*also yielded two monoisotopic ion signals at m/z 1 755.4 and 1 813.4 (Figure 2E).These two peaks contained two different peptides, which were inseparable with the changes of the elution gradient slope in RP-HPLC purification.Following complete reduction with TCEP (Figure 2B, 2D, 2F),signals of these peaks were shifted by 6 Da presenting three disulfide bonds.Thus, the fractions of 10*, 6*, and 16*showed five different small conotoxins containing six cysteines.
3.3.MS/MS sequencing for conotoxins
The investigated compounds showed five of 25 peaks, as indicated by asterisked numbers in Figure 1A.Five new conotoxins corresponding to peaks 10*, 6*(containing two peptides), and 16*(containing two inseparable peptides) were provisionally designated Bn3b (m/z 1 683.43 Da); Bn3c (m/z 1 680.69 Da), Bn3d (m/z 1 664.7 Da); Bn3e (m/z 1 755.4 Da) and Bn3f (m/z 1 813.4 Da), respectively.
3.3.1.Sequencing of Bn3b peptide
The CID MS/MS fragmentation technique was employed to determine the amino acid sequence of 10*-peak.This technique generated b and y-type product ions predominantly.Figure 3 showed the CID mass spectra of reduced 10*-peak.The MS/MS spectrum of the parent ion at m/z 1 689.97 (Figure 3) showed mostly a complete series of b and y-type ions from positions 1 to 15.From the differences between the intense product ions of b-type series (b9to b14) and y-type series (y7to y14), the initial tag of the sequence was characterized as CDWENCDH(L/I)CSCC.There was a limit in distinguishing leucine/isoleucine residue (mass 113 Da).Besides, the mass of cysteine at the N-terminus has been inferred from m/z 1 586.53 y14ion.Thus, peak-10*linear sequence exhibited a cysteine framework Ⅲ, with the following pattern CC-C-C-CC-.The theoretical calculation offered an amidated glycine (G*) at the C-terminus.The initial sequence assignment of m/z 1 689.97 parent ion was CCDWENCDH(L/I)CSCCG*with the name Bn3b, as shown in Figure 3.
3.3.2.Sequencing of Bn3c, Bn3d peptides
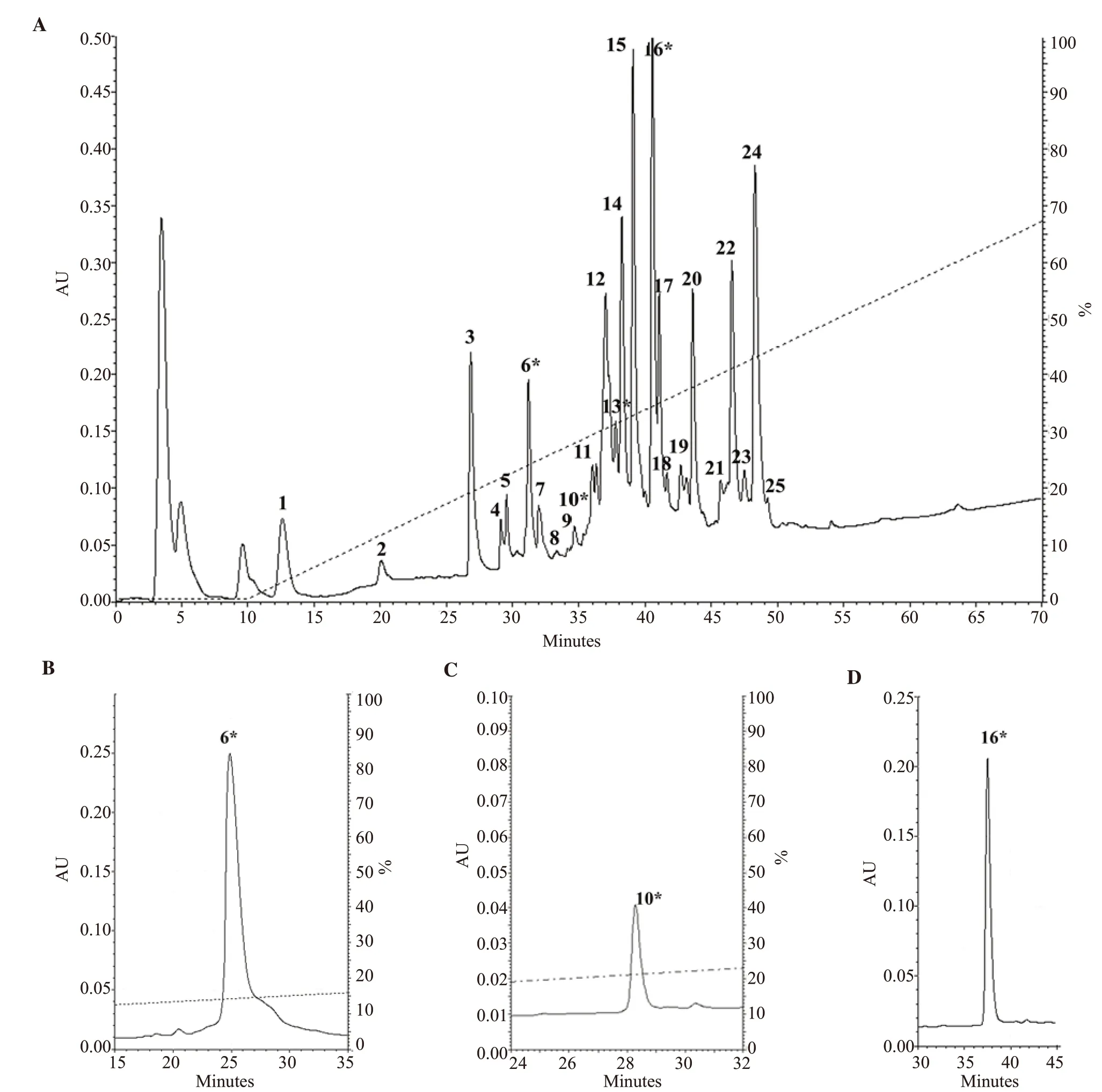
Figure 1.HPLC profiles of dissected Conus bandanus venom, and conopeptide purifications.(A) isolation was carried out with Vydac analytical C18 column.(B,C, D) further purification of the peaks 6*, 10*, 16* was carried out with appropriate gradients, respectively.
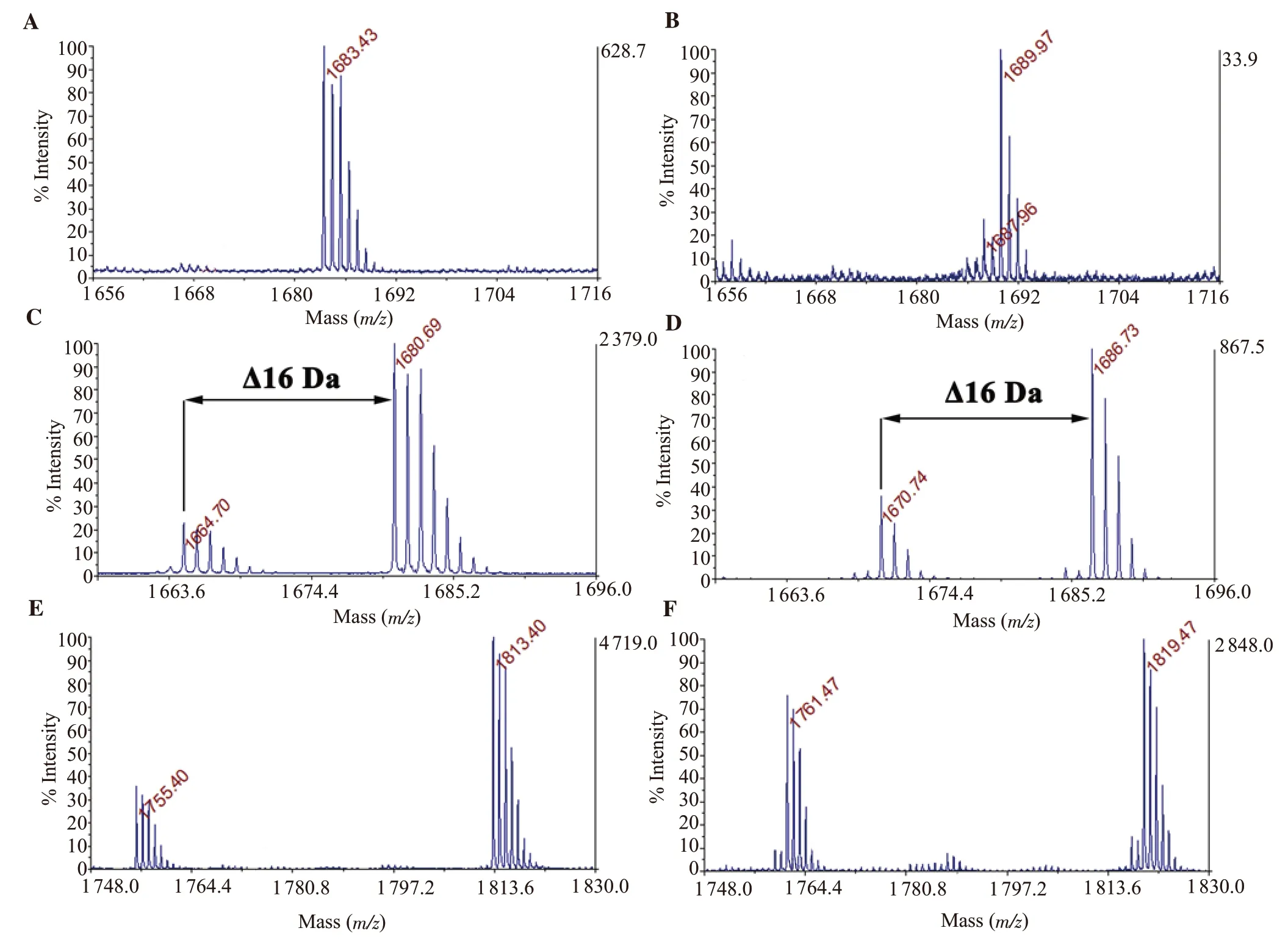
Figure 2.Determination of the cysteine number in the investigated native conopeptides of peak 10*, 6*, 16*, respectively (A, C, E) and on its reduced forms (B,D, F) by matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS).
The native peak 6*was reduced, then alkylated by IAA.There were two IAA-labeled ions m/z 2 028.81, 2 044.8 (data not shown).Figure 4A showed the m/z 2 044.8 parent ion spectrum obtained from the fragmentation of singly charged species.Although fragment ion spectra were obtained with less intense peaks being distributed over the m/z region 256 to 1 863.This m/z region was enlarged in the inset, which showed complete series of b-type ions from positions 1 to 16.The m/z increment of 160 Da between (b1/b2, b6/b7, b10/b11, b13/b14, b14/b15) ions confirmed the presence and position of 5 IAA-labeled cysteines.From the differences between the product ions of b- and y-type series (b1to b15, y1to y14), the initial tag of sequence was characterized as CAPSACR(L/I)GCQOCC with O as hydroxyproline.The mass of an IAA-labeled cysteine at the N-terminus was inferred from m/z 161 b1ion.Thus, the primary sequence may exhibit the same cysteine framework Ⅲ conotoxin with Bn3b.The mass of an arginine (R) at the C-terminus was also inferred from the theoretical calculation and m/z 175 y1ion([R+H2O+H]+).The initial sequence assignment of m/z 2 044.8 parent ion was CCAPSACR(L/I)GCQOCCR with the name Bn3c,as shown in Figure 4A.
The other MS/MS spectrum of the m/z 1 664.7 parent ion (TCEPreduced form, also IAA-alkylated form with m/z 2 028.81) showed partly series of b- and y-type ions from position 3 to 15 (Figure 4B).The differences between these product ions exhibited almost the same residues of Bn3c sequence.Figure 2C showed the isotopic cluster of the singly protonated m/z species of two reduced-form peptides in the peak 6*.A difference in mass of 16 Da corresponded to the mass of an oxygen atom, which was inferred proline in the 13th position of m/z 1 664.7 conotoxin.The difference in mass between the b12/b14; y2/y4ions in the spectrum (ΔM = 200) indicated Pro-Cys residues.Thus, the m/z 1 664.7 peptide, named Bn3d, had the sequence CCAPSACR(L/I)GCQPCCR.The Bn3d was a variant of conotoxin Bn3c.
3.3.3.Sequencing of Bn3e, Bn3f peptides
The same approach for peptide sequencing was used for peak 16*.The sequences were obtained by fragmentation of the reduced and alkylated peptides in the purified peak 16*.Figure 5 showed the parent ion spectra obtained from fragmentation of the singly charged([M + H]+; m/z = 2 103.5 and 2 161.49) species.The b- and y-ions were presented in the insets of the figure.These two peptides shared commonly main fragments such as CCDWDWCDH(L/I)CTCC.This result revealed that two conotoxins belong to the cysteine framework Ⅲ.Thus, the native m/z 1 755.4 peptide was named Bn3e, and the native m/z 1 813.4 peptide was named Bn3f.The mass calculation offered an amidated glycine (G*) at the C-terminus for conotoxin Bn3e.However, there was ambiguous in distinguishing amidated-aspartic acid/asparagine/glycine-glycine residue (mass 114 Da) of Bn3f at the C-terminus.Herein, we proposed the case of glycine-glycine residues at the C-terminus, which was shown in Figure 5B.
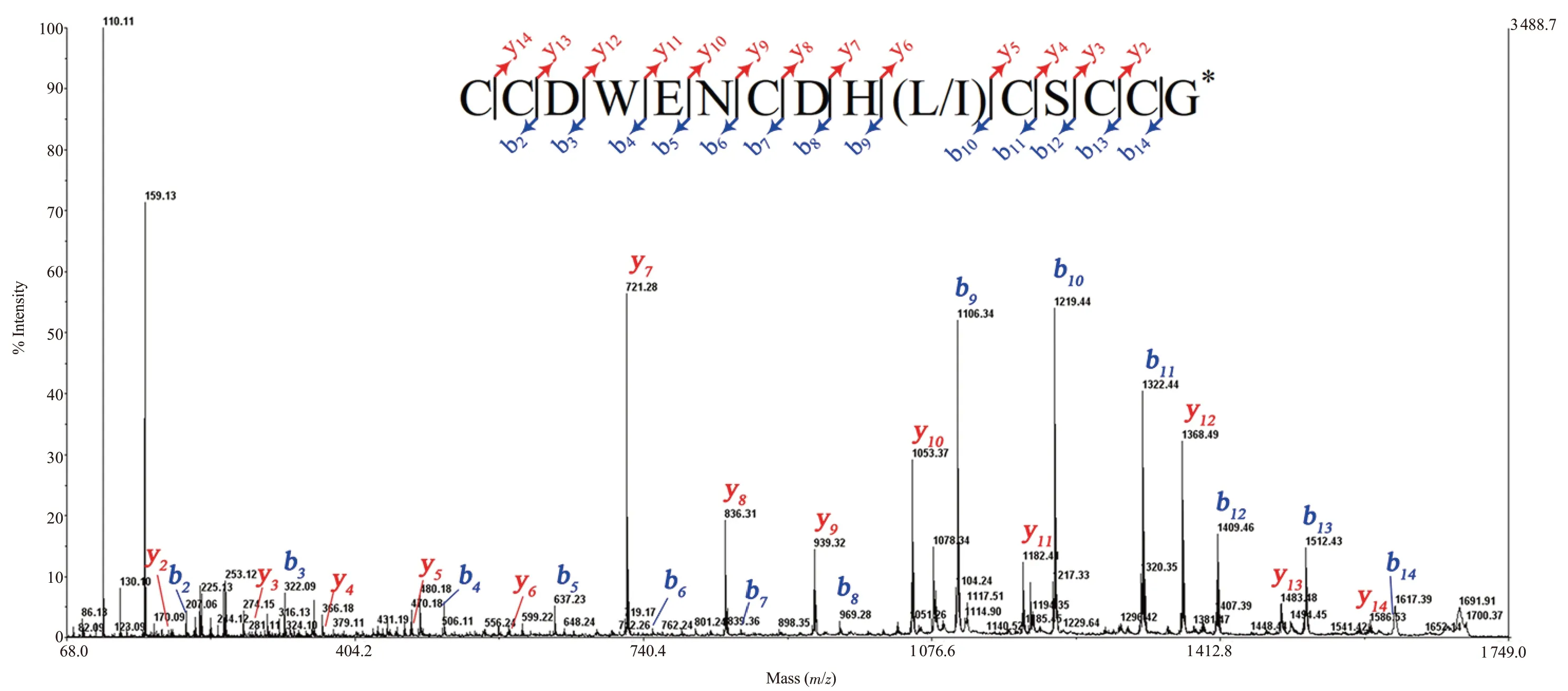
Figure 3.Collision-induced dissociation (CID) tandem mass spectrometry (MS/MS) spectrum of tris-2-carboxyethyl-phosphine (TCEP)-reduced Bn3b, recorded with the 4800 MALDI-TOF MS.Insets show sequences derived from these MS/MS spectra.Note *: C-terminal amidation.
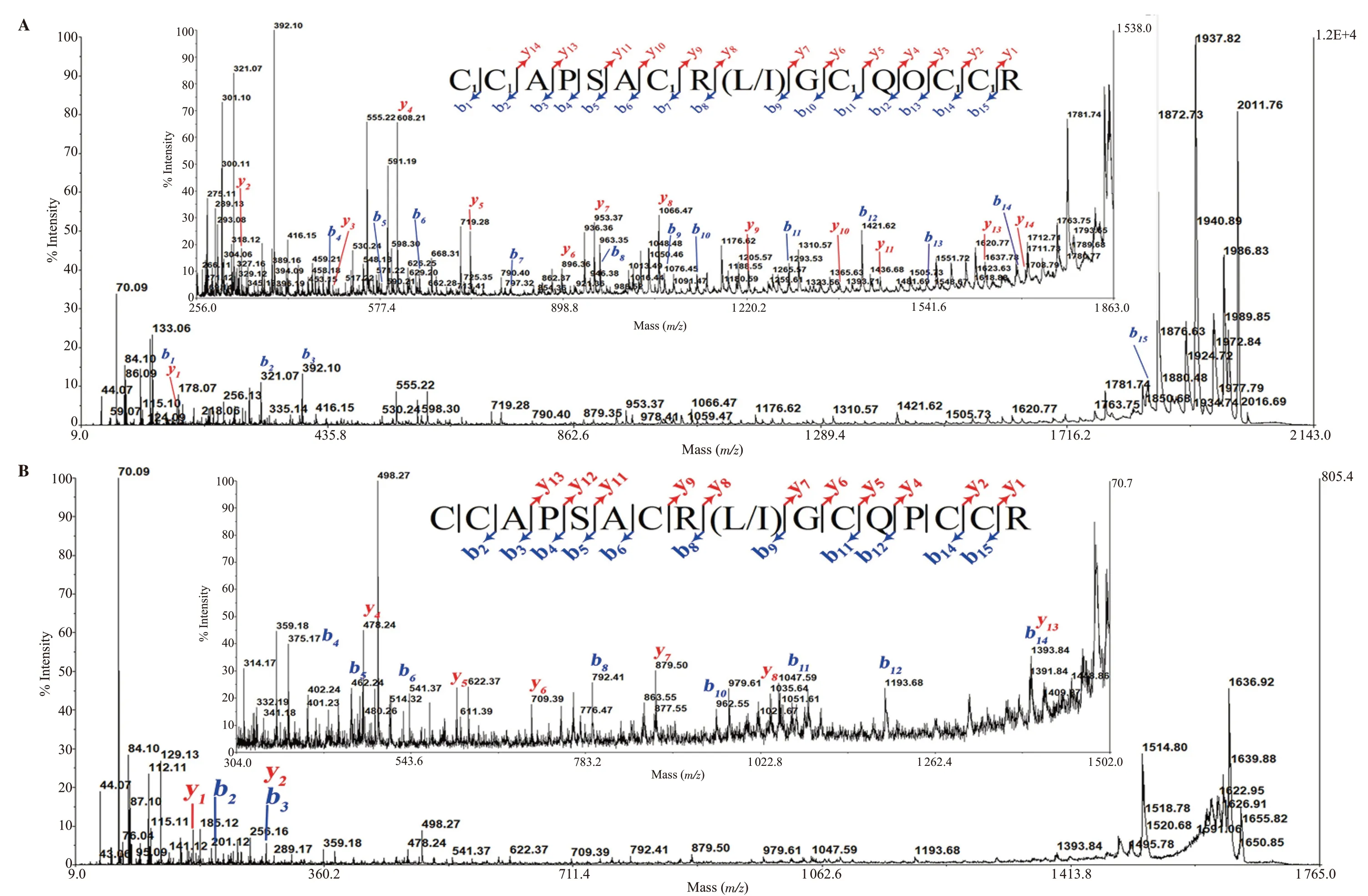
Figure 4.CID MS/MS spectrum of peak 6*, recorded with the 4800 MALDI-TOF/TOF mass spectrometer.MS/MS fragmentation of iodoacetamide (IAA)-labeled Bn3c (A) and TCEP-reduced Bn3d (B).Insets show their zoom of spectra and their sequences.Note: C1: carbamidomethyl-cysteine; O: hydroxyproline.
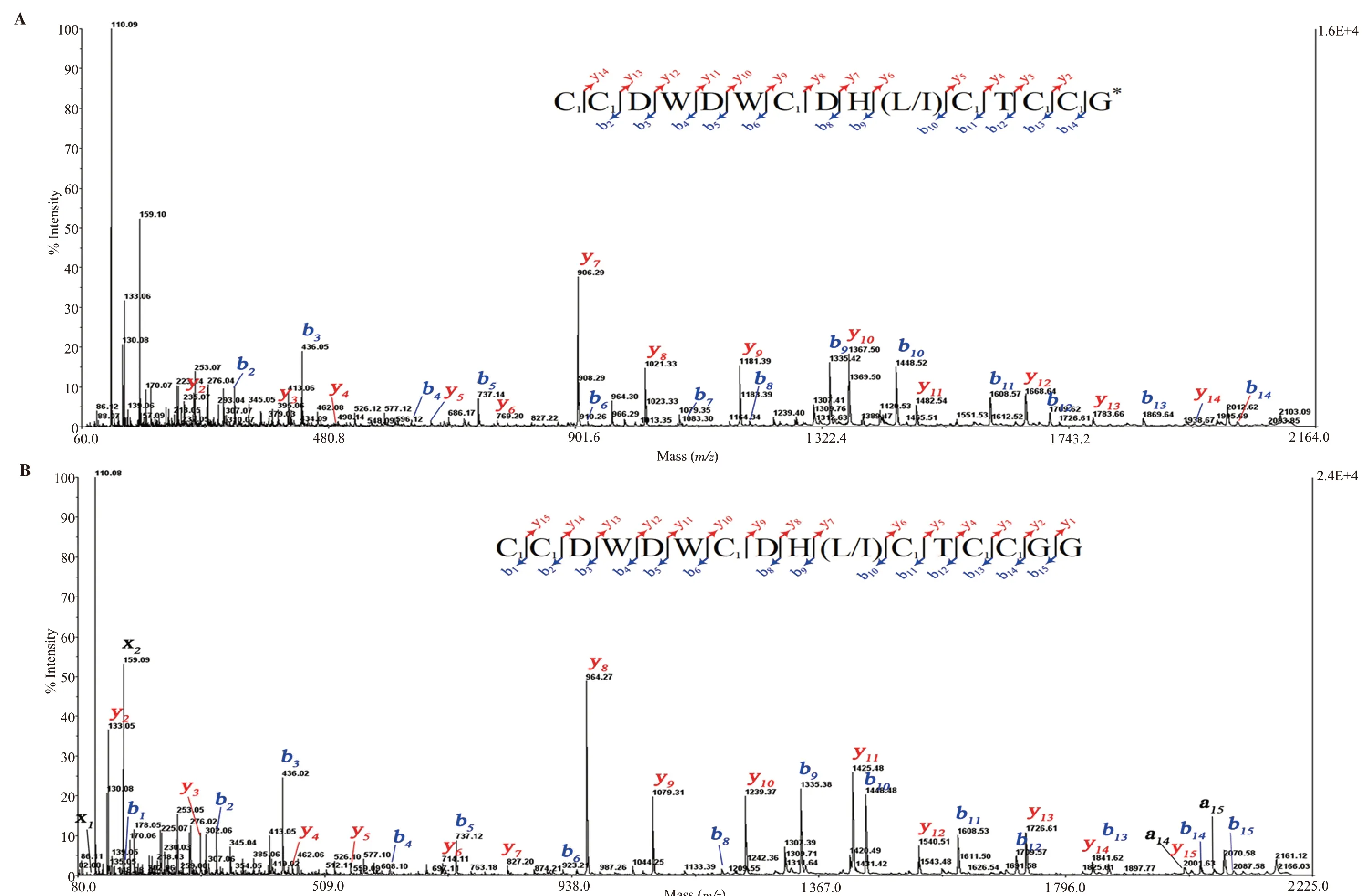
Figure 5.CID MS/MS spectrum of of IAA-labeled peak 16*, recorded with the 4800 MALDI-TOF/TOF mass spectrometer.MS/MS fragmentation of Bn3e (A) and Bn3f (B).Insets show sequences derived from these MS/MS spectra.Note: C1: carbamidomethyl-cysteine; *: C-terminal amidation.
3.4.Leu/Ile differentiation of Bn3b, Bn3e, Bn3f conotoxins
The method of Edman degradation was applied to differentiate Leu/Ile of some new M-superfamily conotoxins.The native Bn3b was submitted to Edman degradation with amount ~ 0.5 nmol.Figure 6A presented the release of Bn3b PTH-amino acid residues for each cycle.The sequence had entirely 15 amino acid residues with 6 cysteines at positions "1, 2, 7, 11, 13, 14" which were identified by CID MS/MS analysis.At the 10th position, it was confirmed that the leucine-residue had an amount of 3.4 pmol.No data were observed at the last 15th cycle.Thus, the complete primary Bn3b sequence is CCDWENCDHLCSCCG*with glycine amidation at the C-terminus.The native 16*-peak was submitted to Edman degradation with a high concentration ~0.5 nmol.Figure 6B presented the release of the PTH-amino acid residues for each cycle.The sequence had totally 15/16 amino acid residues (respectively Bn3e, Bn3f) with 6 cysteines at positions "1, 2, 7, 11, 13, 14" which supported well the CID MS/MS obtained data.At the 10th position, it was confirmed that the leucineresidue had an amount of 120 pmol in place of isoleucine.Thus, the complete primary Bn3e sequence is CCDWDWCDHLCTCCG*with amidated glycine at the C-terminus.For Bn3f sequencing, the MS/MS spectrum data and theoretical calculation offered previously three possibilities in Bn3f peptide at the C-terminus, such as an amidated aspartic acid, asparagine, or two glycines.This Edman sequencing experiment obtained 40.19 and 65.43 pmol of glycine at the two last cycles (Figure 6B).Thus, the complete linear Bn3f sequence is CCDWDWCDHLCTCCGG.
4.Discussion
This report described the isolation and characterization of five novel conotoxins from the venom of mollusk-hunter snail C.bandanus.This species is abundantly distributed along the coast of the Nha Trang Bay in the South-Central Coast of Vietnam.Despite its abundance, the venom components of this species have not been studied.So far, only one peptide sequence of C.bandanus venom has been determined such as BnⅢD at the protein research[10].Otherwise, the analysis of nucleotide sequences from cDNA libraries had yielded the translated peptide sequences of three peptides.These peptides are thought to belong to the A-superfamily of conotoxins[11].Here, five new conopeptides of C.bandanus have been characterized.They had 15 or 16 amino acid residues and owned the cysteine framework -CC-C-C-CC-.
Conus capture their own prey using a cocktail of conopeptides.Based on the number of cysteine bonds, conopeptides are categorized into disulfide-rich and disulfide-poor conopeptides.The “conotoxins”term is often related to cysteine-rich peptides, while cysteine-none/-poor peptides are usually recognized as conopeptides.Among 29 superfamilies, M-superfamily conotoxins belong to one of two complicated groups, including 9 different cysteine frameworks[12].They possess a typical range of 13-35 amino acid residues, which are widely distributed in three feeding types of Conus snails.To the best of our knowledge, most M-superfamily conotoxins possess the characteristic Cys framework Ⅲ: -C1C2-C3-C4-C5C6-.Thus, five new conopeptides could belong to M-superfamily conotoxins.They were named as Bn3b, Bn3c, Bn3d, Bn3be, and Bn3f following the nomenclature suggested by Olivera et al[13].
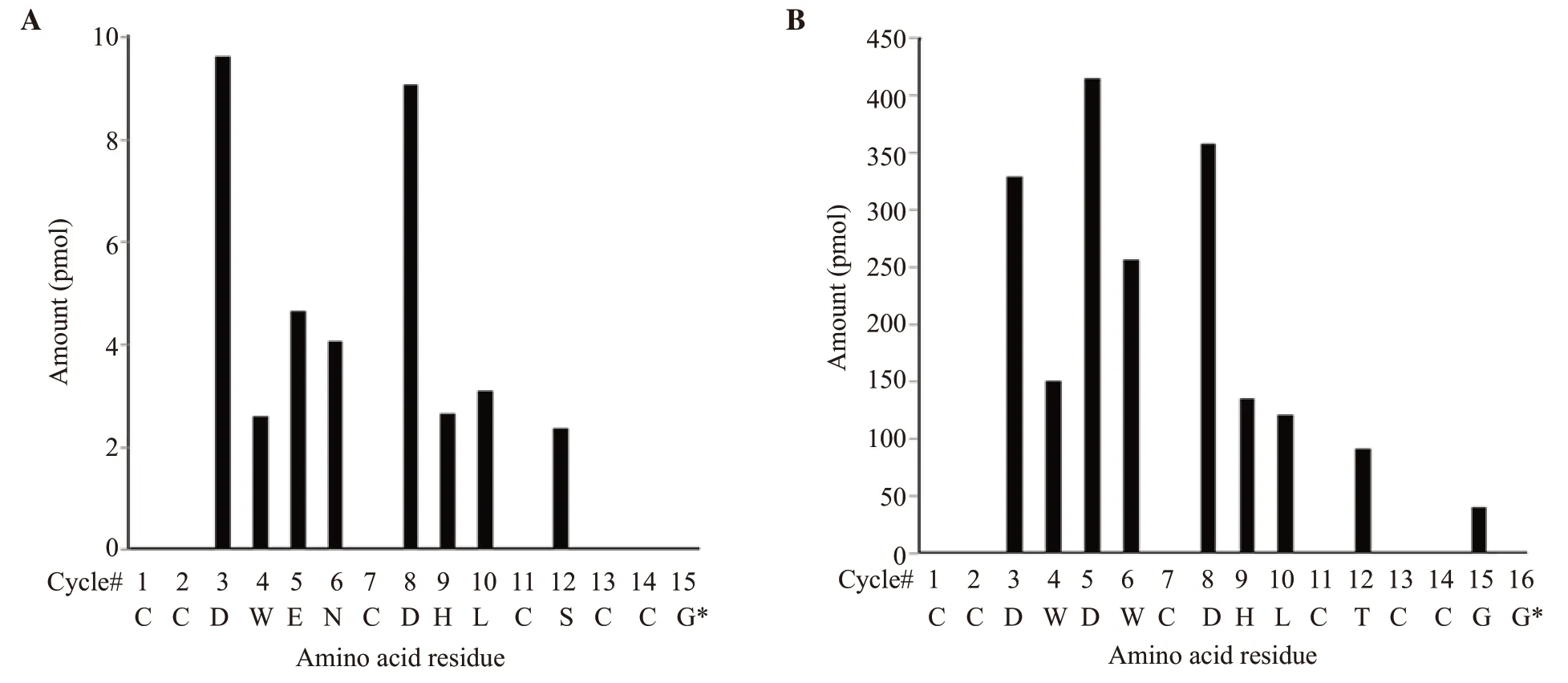
Figure 6.Solid-phase Edman degradation of native Bn3b (A) and Bn3f (B).Note: *: C-terminal amidation.
The M-superfamily conotoxins have been divided into five branches: M1 to M5.The branch number indicates the number of amino acids between C4and C5.The sequences of the identified M-1 and M-2 branch of mini-M conotoxins were compared in Table 1.Remarkably, this pattern exists mostly in mollusk and worm-hunting cone snails.According to the criterion above, Bn3b, Bn3e, Bn3f conotoxins belonged to the M1 branch.It is significant to note that the peptide Bn3b did not possess any posttranslational modifications at the 4th and 5th residues unlike BnⅢD, except residue at C-terminus, which is amidated glycine.Besides, Bn3e and Bn3f shared high homology 93%, only one different amidated residue at C-terminus.The divergence of C-terminal amidation in conotoxins from invertebrate cone snails suggested a more general role for amidation in biology.Moreover, Bn3e and Bn3f were also similar to the M1 branch conotoxins from Conus marmoreus (C.marmoreus)(Mr3.8, Mr3.18) in that these peptides had the sequence WCDHLC as part of loop-2.
Bn3c, Bn3d conotoxins could fall into the M2 branch.The M2-branch conotoxins from C.bandanus can be distinguished from their molluscivorous counterparts in having an uncharged polar residue adjacent to the consensus O/P in loop 2.Two post-translational modifications were widespread in M-superfamily conotoxins:proline hydroxylation and C-terminal amidation[14].However, the M-superfamily conotoxins characterized from mollusk-hunting had most Pro residues, but in Bn3c and MrⅢC, prolines were found to be hydroxylated.Furthermore, the C-termini (-CysArg) of Bn3c and Bn3d was determined C-terminal free acid.As shown in Table 1,the Bn3c-, Bn3d-sequences were compared to other M2 conotoxins of different Conus species that we collected on the ConoServer[12].The Bn3c and Bn3d conotoxin shared 62.5% homology with three conotoxins of the mollusk-hunter Conus textile.In addition, these two peptides also exhibit high homology 93.75% with a C.marmoreus conotoxin sequences (e.g., MrⅢC), only one different residue at the 12th position.Besides, there is Leu residue at the 9th position of MrⅢC.It is important to note that C.marmoreus and C.bandanus are close relative interspecies.Their venoms shared a 24.4% similarity of the conotoxin composition in the previous study[15].Thus, there is a high probability that the 9th residue in Bn3c and Bn3d might be a leucine.
The conotoxin framework-Ⅲ, according to ConoServer data, can be represented by µ-, ɩ-, ψ-, and κ-conotoxins.The new mini-M conotoxins and LtⅢA (derived from Conus litteratus) had the same cysteine framework as -CCx1x2x3x4Cx1x2x3Cx1(x2)CC- with MrⅢE of C.marmoreus (Table 2).Significantly, the conotoxin MrⅢE had been shown to have C1-C5/C2-C4/C3-C6disulfide connectivities[17],while KⅢA (derived from Conus kinoshitai) were proved their disulfide connectivities as C1-C4/C2-C5/C3-C6[21].We suggested that the new mini-M conotoxins could share the same disulfide connectivities as the MrⅢE conotoxins, at least Bn3b, Bn3e,Bn3f.Further work might be necessary to attribute the disulfide connectivity of these mini-M conotoxins definitively.Unfortunately,the pharmacological targets of MrⅢE have not been identified yet.The mini-M conotoxins have been poorly explored for their specific pharmacological targets.For instance, conotoxin LtⅢA was reported as an agonist of tetrodotoxin-sensitive voltage-gated sodium channel without delaying the inactivation (ɩ-conotoxin).Meanwhile, another mini-M conotoxin KⅢA, having different cysteine pattern, blocked tetrodotoxin-resistant sodium channels (µ-conotoxins).
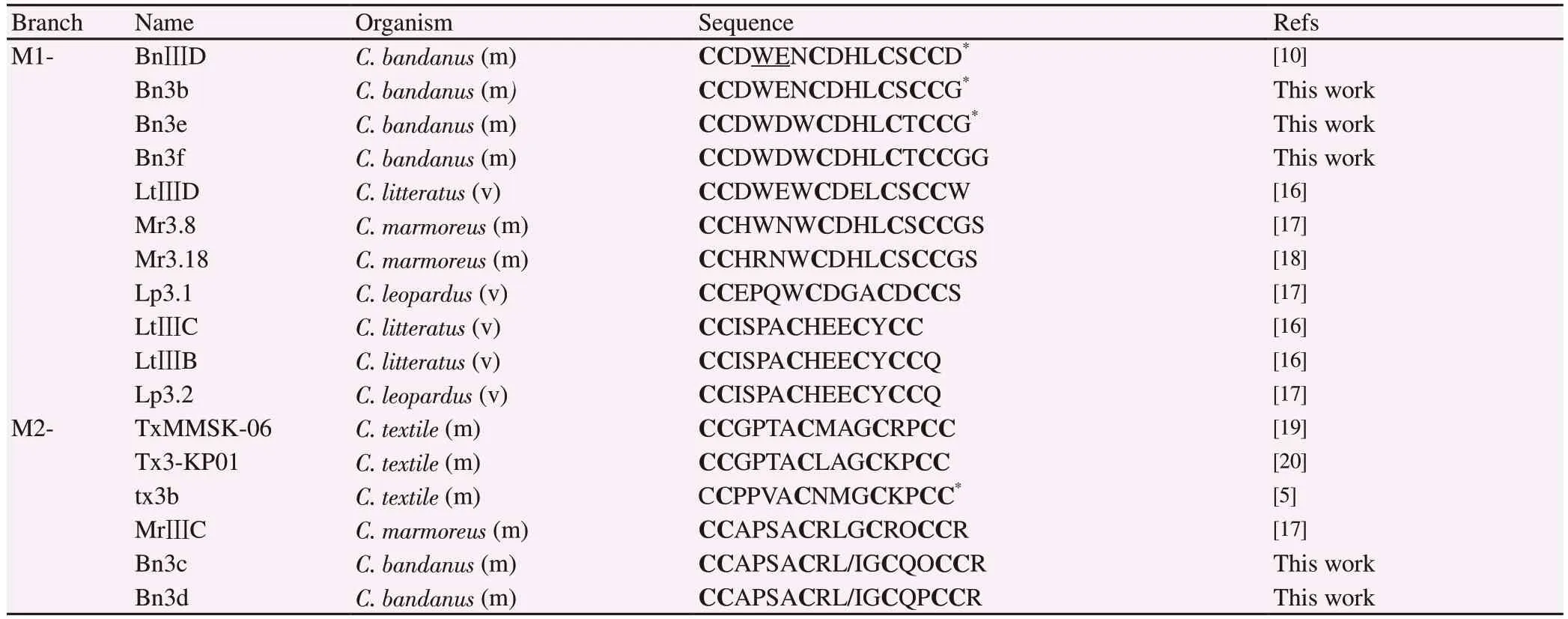
Table 1.Conus bandanus sequence similarity with other conotoxins from different Conus species belonging to the M1-, M2- superfamily branches.
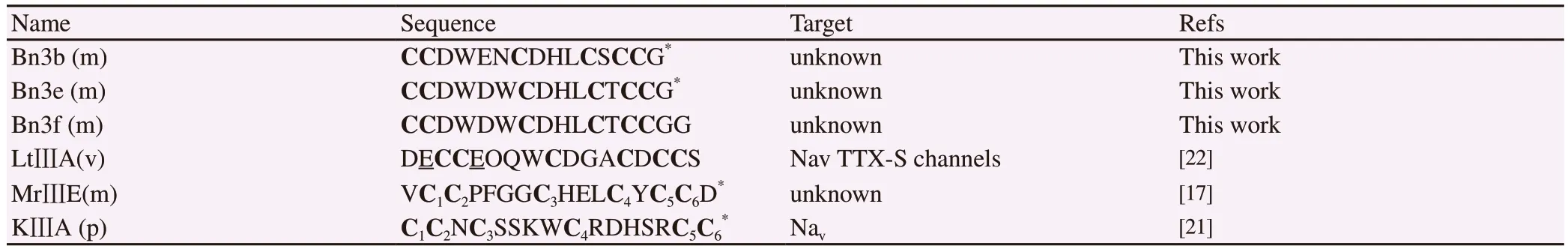
Table 2.Inter-cysteine variation, cysteine connectivity, pharmacological target of mini-M conotoxins.
Conflict of interest statement
We declare that there is no conflict of interest.
Funding
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 106-NN.02-2015.14.
Authors' contributions
NB supervised the entire project, participated in analyzing the results.JPLC did spectrometry analysis and participated in data interpretation.PTKV participated in fractionation and peptide purification.All authors wrote and finalized the manuscript.
 Asian Pacific Journal of Tropical Biomedicine2020年8期
Asian Pacific Journal of Tropical Biomedicine2020年8期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Metabolite profiling and antidiabetic attributes of ultrasonicated leaf extracts of Conocarpus lancifolius
- Immunosuppressive and antibacterial activities of dihydromorin and norartocarpetin isolated from Artocarpus heterophyllus heartwoods
- Peanut testa extracts enhance anticancer effect of cisplatin against human cholangiocarcinoma cells via modulation of histone deacetylase inhibitory activity
- Gymnema montanum improves endothelial function via inhibition of endoplasmic reticulum stress by activating Nrf2 signaling
