Metabolite profiling and antidiabetic attributes of ultrasonicated leaf extracts of Conocarpus lancifolius
Syed Ali Raza, Ayoub Rashid Chaudhary✉, Muhammad Waseem Mumtaz, Ahmad Adnan, Hamid Mukhtar,Muhammad Tayyab Akhtar
1Department of Chemistry, Government College University, Lahore, 54000 Pakistan
2Department of Chemistry, University of Gujrat, Gujrat Pakistan
3Institute of Industrial Biotechnology, Government College University, Lahore, 54000 Pakistan
ABSTRACT
KEYWORDS: Antioxidant; Antidiabetic; Conocarpus lancifolius;Metabolite profiling; Diabetic mice model
1.Introduction
Diabetes mellitus type 2 (T2DM) is recognized as a metabolic disorder with permanent hyperglycemic conditions due to insulin resistance or low insulin secretion[1].The T2DM results in metabolic dysfunctions at the physiological level along with some associated ailments.The current speed of diabetes mellitus expansion over the globe can lead to 592 million diabetic people until 2035[2].According to the WHO report in 2016, Pakistan may be ranked 7th in the world with an increased number of diabetic patients by 2030.The situation is alarming as the socio-economic burden is becoming massive due to the rapid spread of diabetes mellitus in urban as well as in rural populations of Pakistan[3,4].
The T2DM involves alterations at a physiological level guided by pathogenesis under multiple factors.Physiological systems involve the production of reactive oxygen species (ROS) as a function of incomplete oxygen reduction.A high level of ROS production by internal or external stimuli is detrimental and causes oxidative stress.Oxidative stress is produced as a result of an imbalance in ROS and antioxidant defense system of the body, leading to activation of redox-based cell signaling system followed by damages at the cellular level[5].Scientific information is available revealing the role of ROS in the initiation and propagation of diabetes mellitus,aging, cancer, cardiovascular diseases and many other disorders[6,7].Antioxidants are substances that react with ROS to minimize their harmful impacts and reduce the intensity of stress.Although synthetic antioxidants are available, toxicity aspects associated with them emphasize the need for safe and natural functional agents.Many synthetic drugs to control hyperglycemia are available, but their performances are associated with serious health disorders[8].Undesirable side effects with synthetic drugs put the accent on an exploration of safe therapeutic agents, especially from plants.Plants are a rich source of natural phenolics and other functional bioactive ingredients recognized as potent antioxidants.Many synthetic drugs originated from plants and the natural balance in metabolites and synergism factors makes the plant more effective and safe.Secondary metabolites in plants which are biologically active act as strong antioxidants to eradicate ROS and maintain the antioxidant homeostasis for normal metabolic processes[9].
Plants are frequently used as folk medicines in many parts of the world and may control the phenomenon of oxidation at the cellular level, hence helping in the therapy of stress-related diseases like T2DM.Inhibition of the α-glucosidase enzyme delays the process of carbohydrates absorption, hence minimizes the postprandial blood glucose.Plants are cheap and safe sources of phytotherapeutic agents that have prominent inhibitory properties toward α-glucosidases[10].The identification of biologically active ingredients in plants is essential to provide evidence to support the alternative medicinal system.Modern trends in plant metabolomics surround the scope to explore plants for the presence of biologically active and important metabolites in a more comprehensive way.Recently, metabolomics has emerged as an excellent technique to identify metabolites of functional nature for the benefit of mankind mainly with the help of chromatography, nuclear magnetic resonance spectroscopy and mass spectrometry[11].Pakistan is rich in the availability of medicinal flora, but the evidence-based scientific evaluation is not up to the mark to accept these plants as effective alternative medicine systems.The use of modern analytical techniques under the umbrella of metabolomics may provide leads for the development of the evidence-based efficient complementary medicinal system with minimum side effects.
Conocarpus lancifolius (C.lancifolius) is a tree of family Combretaceae and found in many regions including Pakistan.Previously, the anti-urease, phytotoxic and antioxidant activity of C.lancifolius was reported.The impressive bioactivities were probably due to phenolic and flavonoid contents[12].Another work reported the in vitro antioxidant and antibacterial activity of C.lancifolius in which crude methanolic extract obtained through simple maceration exhibited excellent anti-α-glucosidase activity[13].A study also reported the isolation of ellagic acid and kaempferol derivatives responsible for the antidiabetic potential of C.lancifolius[14].This plant is traditionally used to treat diabetes but little scientifically proved information exists about the antidiabetic role of C.lancifolius and it can be explored on a broader spectrum for the identification of nutraceuticals and phytotherapeutic agents responsible for its medicinal properties.Limited information was found on the metabolites present in C.lancifolius, so there is a need to investigate the C.lancifolius leaves in animal models to conclude the authenticity of its folkloristic intake to treat diabetes and to evaluate its oral toxicity.Conjunctive use of freeze-drying and ultrasonication may enhance the biological attributes of C.lancifolius.The available antidiabetic evidences on C.lancifolius leaves served as an impetus to assess freeze-dried and ultrasonicated C.lancifolius leaf extracts at full potential for antioxidant activity, α-glucosidase, and α-amylase inhibition, metabolite's profiling and in vivo antidiabetic activity.
2.Materials and methods
2.1.Chemicals and reagents
All the chemicals and reagents used were of high purity analytical grade mainly purchased from Sigma USA and Merck (Germany).Ethanol, methanol, liquid nitrogen, Folin Ciocalteu reagent, Na2CO3,gallic acid, NaNO2, AlCl3, NaOH, 2,2-diphenyl-1-picrylhydrazyl(DPPH), butylated hydroxyanisole (BHA), acarbose, α-glucosidase,α-amylase enzyme, ρ-nitrophenyl glucopyranoside, and alloxan monohydrate were utilized.
2.2.Hydroethanolic extracts preparation
C.lancifolius leaves were collected from canal road Lahore Pakistan and were identified by Prof.Dr.Zaheer-ud-Din Khan of Department of Botany, Government College University Lahore and a voucher specimen (GC.Herb.33.Bot.3378) was submitted to the botanical herbarium of the university.Fresh leaves were immediately quenched with liquid nitrogen and ground to powder by a mechanical electric grinder.Ten grams of powdered leaf material was immersed in 100 mL of ethanol and water-based solvent system in different proportions(Aqueous, 80:20%, 60:40%, 40:60%, 20:80%, and pure ethanol)followed by ultrasonication with a soniprep 150 sonicator (MSE, UK)for 30 min at temperature <10 ℃.After sonication, samples were shaken for 2 h for maximum extraction of bioactive ingredients.The mixtures obtained were then filtered and excess solvent was evaporated under vacuum using a rotary evaporator at 40 ℃.The crude extracts were freeze-dried and stored at -68 ℃[15].Percentage extract yield was calculated to discriminate solvent efficacy.
2.3.Total phenolic content (TPC)
The previously established method was utilized with some modifications.Initially, 0.1 mL of methanolic (1 mg/mL) plant extract was completely dissolved in 1 mL of Folin Ciocalteu reagent.After that, the mixture was mixed with 2 mL of 10% Na2CO3, and incubated for 2 h followed by absorbance measurement at 765 nm by UV-1700 spectrophotometer (Schimadzu Japan).Findings were reported as mg of gallic acid equivalent per gram plant extract (mg GAE/g PE)[16].
2.4.Total flavonoid content (TFC)
The aluminum chloride-based colorimetric method was used to determine the TFC content of the extracts.Briefly, 10-100 µg of crude plant extracts were dissolved in 2 mL of methanol followed by the addition of 4 mL distilled water.Afterward, 0.6 mL of 5%NaNO2solution and 0.6 mL of 10% AlCl3solution were added to the mixture and stayed for 10 min at room temperature.The resultant reaction mixture was enriched with 4 mL of 1 mol/L NaOH solution and the final volume was made up to 20 mL with distilled water.The resultant mixture stayed for 25 min after which the absorbance was taken at 510 nm on the UV-1700 spectrophotometer (Schimadzu Japan).Results were reported as mg rutin equivalent per gram of plant extract (mg RE/g PE)[17].
2.5.DPPH radical scavenging assay
The in vitro antioxidant activity of the plant extracts was determined by DPPH free radical scavenging assay[18].DPPH solution in methanol was made by dissolving 2.5 g of DPPH in 10 mL methanol.Each plant extract (50-200 µg) was dissolved in 1 mL methanol to form concentrations of 50-200 ppm.Two hundred µL of each extract solution was then added to 1.0 mL of DPPH solution.The reaction mixture was then allowed to stay in the dark for 35 min to complete the reaction and absorbance was then measured at 517 nm on UV-1700 spectrophotometer (Schimadzu Japan).Free radical scavenging potential was finally calculated using the following formula,
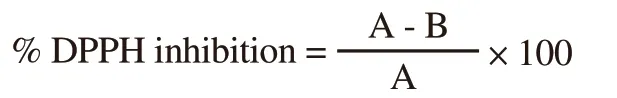
Where A represented the absorbance of blank and B represented the absorbance of the sample.The BHA was used as a standard antioxidant reference and results were represented in IC50value (µg/mL).
2.6.The α-glucosidase and α-amylase inhibitory assay
Antidiabetic potential of plant extracts was investigated by measuring their inhibitory action against α-glucosidase.A reaction mixture(100 µL) consisted of 70 µL of (50 mM) phosphate buffer (pH 6.8),10 µg of each ethanolic extract and α-glucosidase (1 unit/mL) from yeast was prepared.The mixture was incubated at 37 ℃ for 10 min.The reaction was initiated by adding 10 µL of (5 mM) ρ-nitrophenyl glucopyranoside.After 30 min incubation, the absorbance was measured at 405 nm for released ρ-nitrophenol.All the measurements were made in triplicate.The following formula was used to calculate percentage inhibition[19].
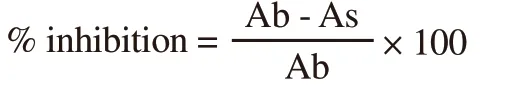
Where Ab was the absorbance of blank and As was the absorbance of the sample.The acarbose was used as a standard reference and results were represented as IC50(µg/mL) values for each extract.
The anti-α-amylase potential of extracts was determined by the previously reported procedures with little modification[20].Briefly,250 µL of each extracts ranging from 1.0-10 mg/mL were added 0.02 M sodium phosphate buffer (pH 6.9) having 0.5 mg/mL of porcine α-amylase.The resultant mixture was incubated for 10 min at 25 ℃.The dinitrosalicylic acid was added to stop the reaction.The samples were further incubated for 5 min and diluted with 5 mL distilled water to take absorbance at 540 nm.A control was also run(without extract) and acarbose was used as a standard compound for comparison.
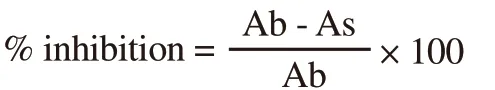
Where Ab was the absorbance of blank and As was the absorbance of the sample.The results were represented as IC50(µg/mL) values for each extract.
2.7.UHPLC-Q-TOF-MS/MS analysis
The UHPLC-Q-TOF-MS/MS technique is highly efficient for plant extract analysis to identify the secondary metabolites.This technique is highly reliable due to rapid data acquisition and stable resolution over m/z range along with high mass accuracy.The clear MS/MS is obtained due to the TOF section.Briefly, the most potent 60% ethanolic extract was extracted with appropriate solvent and filtered with polytetraflouroethylene filter having 0.45 µm pore size.The prepared extract sample was subjected to UHPLCQ-TOFMS/MS (AB Sciex 5600-1 equipped with Eksigent UHPLC).The analysis conditions were characterized by a scan range of 50-1 200 m/z for MS/MS with negative ionization mode, column Thermo Hypersil Gold (100 mm × 2.1 mm × 3 µm).A gradient mobile phase comprised of water and acetonitrile (each having 0.1% formic acid and 5 mM ammonium formate).The gradient programme started from 10% acetonitrile to 90% acetonitrile with a 0.25 mL/min mobile phase flow rate.An injection volume of 20 µL was used and the instrument was also equipped with duo spray ion source with curtain gas (N2), ion source gas (GS1) and turbo gas (GS2) at 25, 40 and 40 psi, respectively.The desolvation temperature (TEM) was 500 ℃ and ion spray voltage was -4 500 V.The Sciexpeak views 2.1 software, ACD labs MS fragmenter software and Chemispider database were utilized for data interpretation.Resolved peaks were also identified with the help of literature.
2.8.Mice model for in vivo investigation
Mice model was adopted to evaluate the potential of plant extracts on blood glucose level and haematological parameters.Sixty albino mice (5-6 weeks old) with an average bodyweight of (28.50 ± 2.30)g were kept in polypropylene containers lined with fine husk under 12 h light and dark cycle.Mice were acclimatized for 10 d to adapt to a new environment at (28.0 ± 2.0) ℃ with a relative humidity of(68.75 ± 4.50)%.Mice were fed on high fat diet (HFD) composed of 30% protein, 50% corn starch, 10% sucrose, 5% corn oil, 2.5%cholesterol, 4% vanaspati ghee, 1% coconut oil, 10% milk butter,5% minerals and 1% vitamins.Whereas normal diet contained 30%protein, 50% corn starch, 10% sucrose, 5% corn oil, 5% minerals and 1% vitamins.The changes in body weights were measured on a weekly basis and the feeding of HFD continued for eight weeks.For oral glucose tolerance, mice fasted for 6 h with an ad libitum supply of water.The aqueous glucose solution at a dose of 2 g/kg body weight (b.w) was orally administrated in a quantity of 1 mL/kg body weight.Blood glucose level was checked at 30, 60 and 120 min after administration of glucose solution[21].The acute toxicity test was determined in female mice of 8-12 weeks age having body weight between 25-30 g.One of the mice was fed with 2 000 mg/kg b.w extract dose and monitored for the first four h after the interval of 30 min.The periodic monitoring for 24 h was carried out.After that, four other healthy female mice of mentioned age and weight were administrated with extract at a dose of 2 000 mg/kg b.w and were observed as per previous practice.The careful regular monitoring was continued until 14 days for any mortality, diarrhoea,and vomiting.
After eight week period, the 24 hours fasted HFD mice were injected with alloxan monohydrate (150 mg/kg b.w) in normal saline intraperitoneally followed by oral administration of 10% glucose solution to reduce the mortality rate.After three days of injection,blood glucose levels were measured through a glucometer by rupturing the lateral vein in mice's tail.Mice having blood glucose level ≥ 200 mg/dL were considered diabetic[22].The normal diet group served as the control group and received only normal saline as a vehicle.The HFD mice were grouped into the diabetic control group, metformin-treated group (250 mg/kg b.w), low dose (250 mg/kg b.w) and high dose (450 mg/kg b.w) of the plant extract group.The plant extract was orally administrated once in 24 h.After characterization of groups, the study was continued for further eight weeks (glycemic phase).Blood glucose level was measured on a weekly basis.
At the end of the eight-week study, mice blood was subjected to determination of haemoglobin (Hb), total cholesterol (TC), highdensity lipoproteins (HDL) and low-density lipoproteins (LDL).
2.9.Ethical statement
The animal trial was approved by the ethical committee of GC University Lahore vide approval number GCU-IIB-2205.All the animal trials were carried out under the national and international guidelines regarding animal studies.
2.10.Statistical analysis
The values were measured in triplicate and the standard deviation(±) was applied.Results were statistically analyzed and compared by computing one-way Analysis of Variance (ANOVA) by Minitab 16.0 software.A P-value less than 0.05 was considered significantly different.
3.Results
3.1.Extract yield %, TPC and TFC
The solvent system influenced the % extract yield, TPC and TFC from leaves of C.lancifolius.The maximum extract yield of (20.04± 0.06)% was obtained with 60% ethanol which was significantly different from extract yields of 80% ethanol and 40% ethanol(P<0.05).The yields by pure and 20% ethanolic extracts were compared and found statistically non-significant while the lowest extract yield was obtained in aqueous extract (Table 1).The 60%ethanol extract was the most appropriate choice for the extraction of functional metabolites from the leaves of C.lancifolius.
The 60% ethanolic extract showed the highest TPC and TFC of(349.39 ± 2.13) mg GAE/g PE and (116.95 ± 2.34) mg RE/g PE,while the minimum TPC and TFC of (93.55 ± 1.06) mg GAE/g PE and (29.54 ± 0.99) mg RE/g PE were found in the aqueous extract(Table 1).The results demonstrated that the solvent system not only influenced the extract yields but also the extraction of TPC and TFC and that 60% ethanol was shown as optimum solvent concentration to improve extraction of biologically active ingredients from leaves of C.lancifolius.
3.2.In vitro antioxidant and antidiabetic activities
The 60% ethanolic leaf extract of C.lancifolius exhibited strong antioxidant potential with a minimum IC50value of (32.87 ± 1.11)µg/mL among all extracts, which was less significant than the antioxidant activity of BHA as a standard antioxidant agent with an IC50value of (26.50 ± 0.50) µg/mL.In contrast, the aqueous extract demonstrated the lowest antioxidant activity as indicated by the IC50value of (76.50 ± 1.17) µg/mL (Table 2).
The 60% ethanolic extract was most potent to inhibit α-glucosidase activity followed by 80% ethanolic extract.The IC50value of the 60% ethanolic extract for α-glucosidase inhibition was (38.64 ±0.93) µg/mL, which was comparatively higher than that of standard drug acarbose [(29.45 ± 0.31) µg/mL].In addition, the 60% ethanolic extract showed significant in vitro α-amylase inhibition with the lowest IC50value of (44.80 ± 1.57) µg/mL compared with other extracts (Table 2).The standard drug acarbose had the IC50value of(35.50 ± 0.35) µg/mL (Table 2).
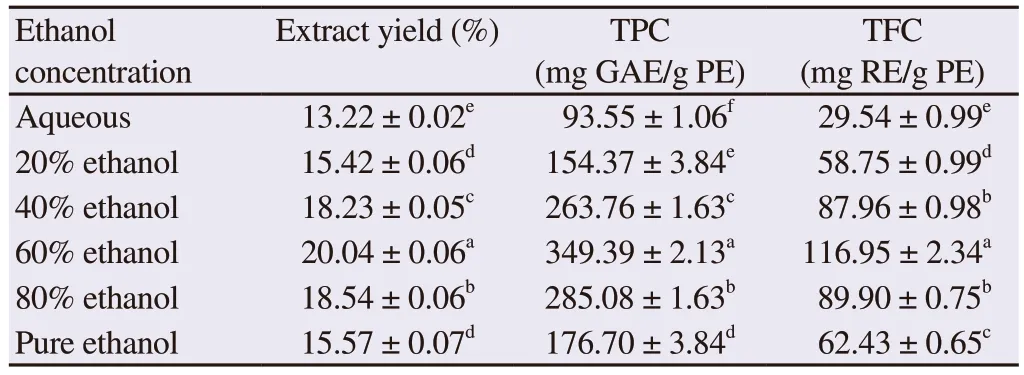
Table 1.Extract yield, TPC and TFC from leaves of Conocarpus lancifolius under different solvent compositions.
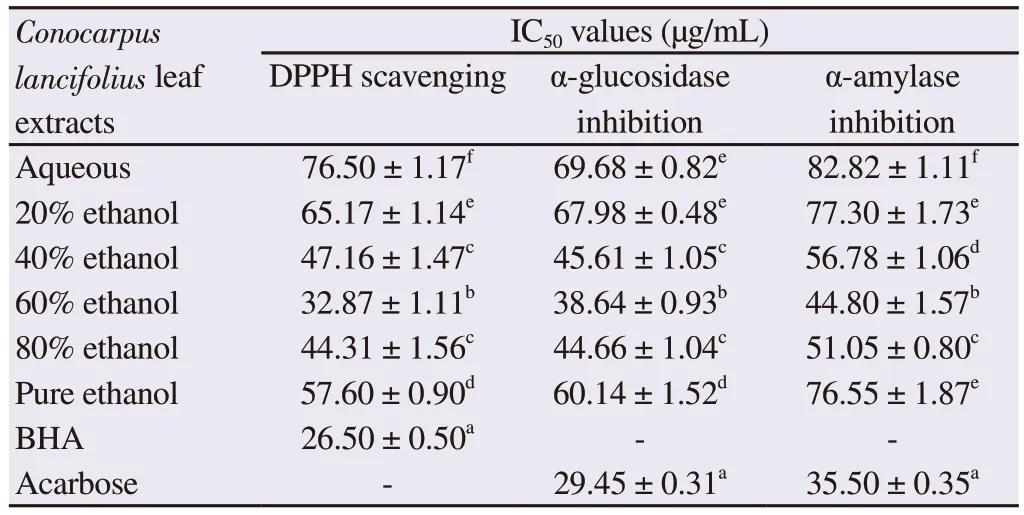
Table 2.IC50 values of Conocarpus lancifolius extracts for DPPH scavenging,α-glucosidase and α-amylase inhibition.
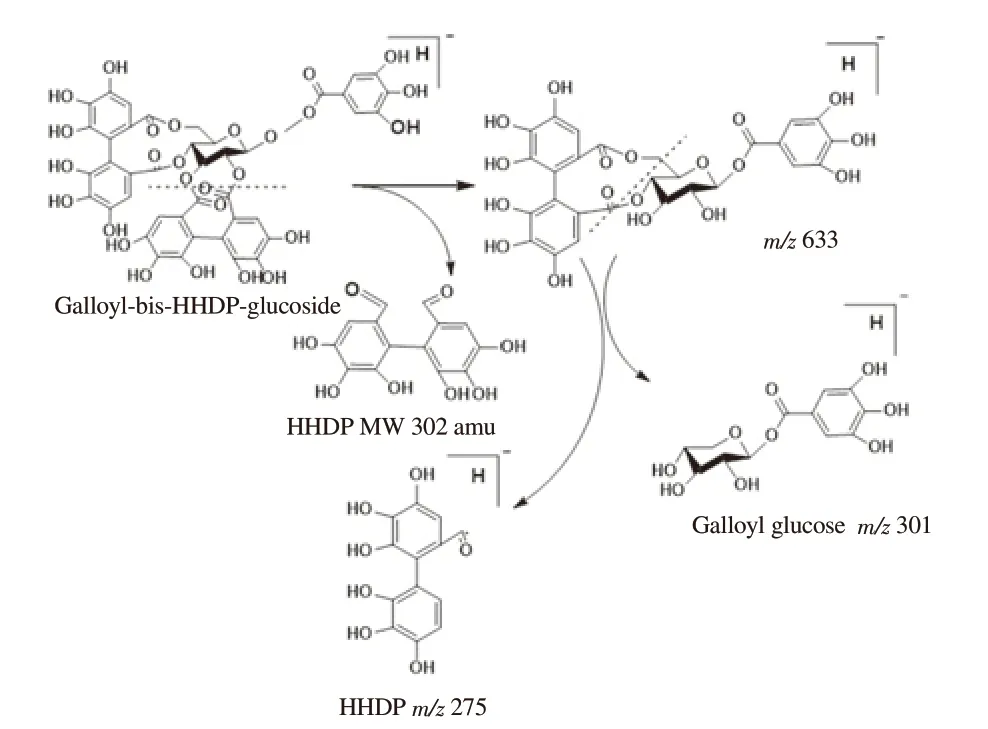
Figure 1.Fragmentation pattern of galloyl HHDP glucoside identified in 60% ethanolic leaf extract of Conocarpus lancifolius with their characteristic mass to charge ration (m/z).
3.3.UHPLC-Q-TOF-MS/MS analysis
Major identified compounds in 60% ethanolic leaf extract were gallic acid, corilagin, galloyl-3-O-glucoside isomers and its derivative, kaempherol-3-O-rutinoside, terflavin B, ellagic acid derivative, caffeic acid derivative as well as isorhamnetin.The fragmentation details of identified compounds along with retention time (Rt), and molecular formula are given in Table 3.
Gallic acid was confirmed on the basis of its characteristic peaks at m/z 169 [M-H]-, m/z 125 [M-CO2-H]-and m/z 79 [M-CO2-H2O-H]-.Compounds at serial number 2, 5, 6 and 7 were identified as galloyl HHDP-glucoside isomers probably differing in position of galloyl group on glucose.All these compounds exhibited characteristic galloyl HHDP-glucoside parent ion peak at m/z 935.The fragment ion peak m/z 783 was appeared due to removal of galloyl moiety of 152 amu.Removal of HHDP (MW 302) resulted in the formation of peak m/z 633 which further split to generate m/z 301 by releasing galloyl glucose (MW 332 amu) (Figure 1).The peak at Rt 5.849 with m/z 633 appeared due to the possible removal of hydroxyl group.The fragment ion peak at m/z 481 [M-OH-169 amu]-was due to removal of galloyl moiety.The compound was identified as corilagin.Ellagic acid was recorded at Rt 9.454 with parent ion peak at m/z 301.The collision energy produced fragment ions with specific m/z.The peaks at m/z 284 [M-OH-H]-, m/z 229 [M-CO2-CO-H]-, m/z 185[M-2CO2-CO]-confirmed the presence of ellagic acid.Isorhamnetin was detected at Rt 12.546 with parent ion peak at m/z 315 which further fragmented to generate ions at m/z 300 and m/z 271 due to removal of methyl and H+CO from parent ion, respectively.The fragmentation pattern confirmed the presence of isorhamnetin which is flavonoid with bitter taste.Peak for kaempferol 3-O-rutinoside was recorded at Rt 9.708 min with m/z 593.The typical fragmented aglycone moieties were appeared at m/z 285, m/z 284, m/z 255 and m/z 245.
Terflavin B is hydrolysable ellagitannin common in plants.It was detected at Rt 6.758 min with m/z 783.The high energy collision generated fragment ions at m/z 301 corresponded to ellagic acid and m/z 481 for remaining molecular species.Peak at Rt 10.205 with characteristic peak at m/z 179 indicated the caffeic acid derivative.
3.4.In vivo determination of antidiabetic potential
Obesity development was monitored by observing considerable increase in body weights.The HFD significantly increased the body weights.After eight weeks, a periodic increase of 91.34% in body weights of HFD mice was observed which was higher than that of normal diet-fed mice for which the increase was 50.19%.The increased body weights of HFD mice reflected the development of obesity (Figure 2).
The findings of the oral glucose tolerance test revealed that glucose intake resulted in high blood glucose levels within a very short time period.Blood glucose levels of all groups rosed to the maximum level within the first 60 min of glucose intake.The blood glucose level started to decrease and reached normal blood glucose level(<120 mg/dL) after 120 min.No death or unusual behaviour was observed for mice under acute toxicity test which suggested that the median LD50dose for mice was considered to be greater than 2 000 mg/kg b.w, so 450 mg/kg b.w of extract dose was within a safe range to check its impact on hypoglycemic and hypolipidemic activities.
After the development of obesity (completion of initial phase comprising of 8 weeks), diabetes was induced in the obese mice by injecting alloxan monohydrate.The diabetic mice were subjected to blood glucose measurement on a weekly basis for further 8 weeks.The diabetic control group showed a high level of blood glucose starting from the 1st week.Both 250 and 450 mg/kg b.w dose of plant extracts markedly reduced the blood glucose level.The effect of plant extract at 450 mg/kg b.w was comparable to that of metformin as a standard drug (Figure 3).
3.5.Effect of treatments on blood biochemistry
The effect of plant extract on Hb, TC, HDL and LDL is represented in Table 4.The Hb of treated mice was determined in blood samples at the end of experiment.It was observed that induction of diabetes adversely affected the Hb level of mice.
The average Hb of mice for the normal control group was (9.13± 0.01) g/dL which reduced to (5.98 ± 0.02) g/dL due to diabetes.However, the Hb level of mice treated with the plant extracts was relatively higher than that of diabetic and metformin groups (P<0.05).The plant extract at 450 mg/kg b.w.exhibited much improved Hb level of (8.44 ± 0.02) g/dL which was comparable to that of the normal control group.
The diabetic group lifted the TC from (44.67 ± 1.09) mg/dL to(146.78 ± 1.93) mg/dL.Both metformin and plant extract considerably reduced the TC and there was no significant difference in TC between metformin and plant extract group (450 mg/kg b.w.) (P>0.05).It was observed that metformin and plant extract treated groups significantly reduced HDL and LDL compared with the diabetic group.
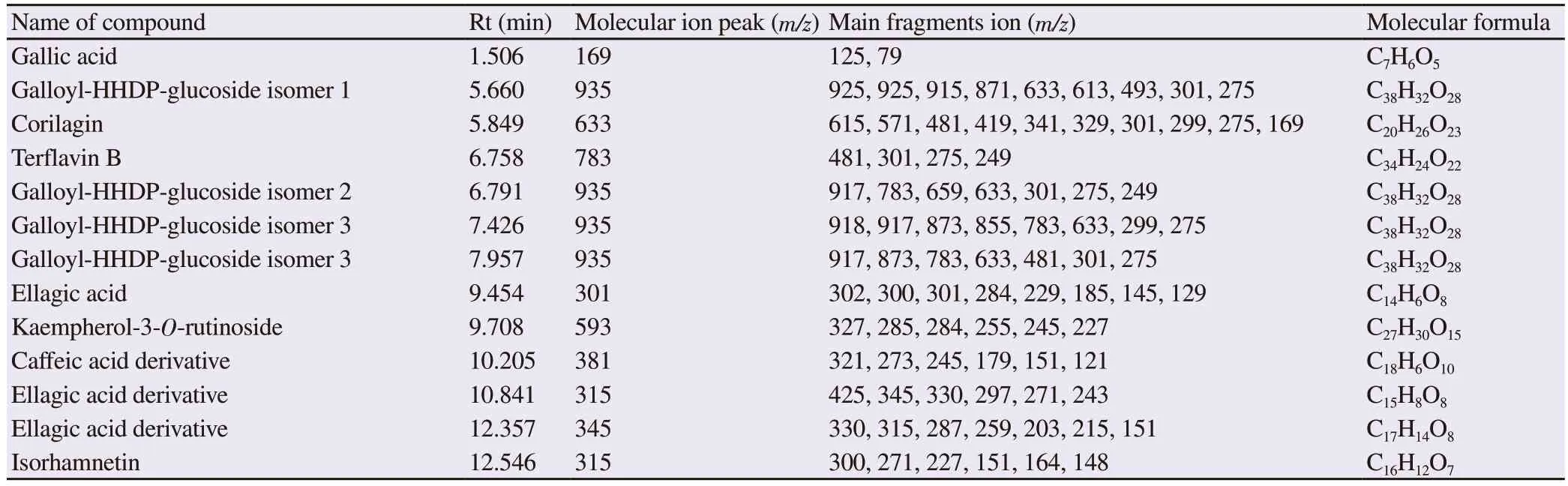
Table 3.Chemical composition of 60% hydroethanolic leaf extract of Conocarpus lancifolius analyzed by UHPLC-Q-TOF-MS/MS.
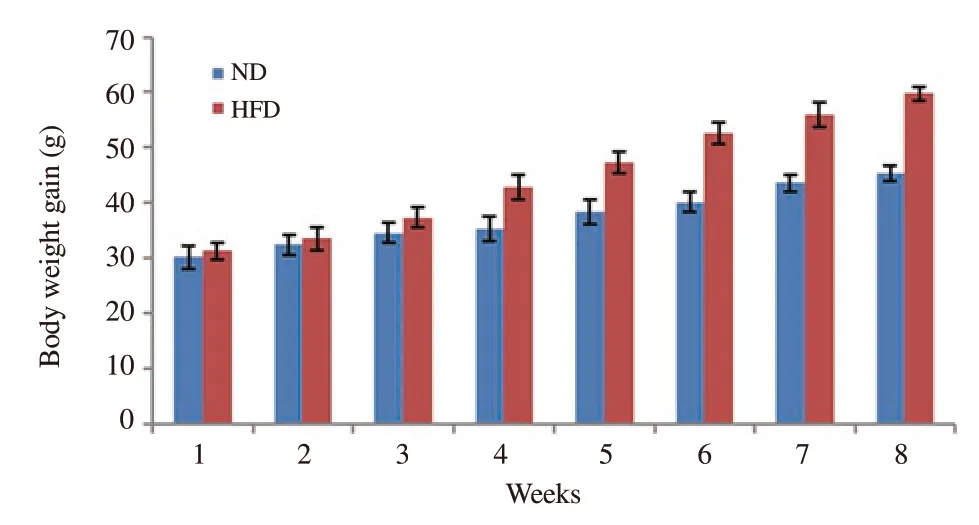
Figure 2.Bodyweight changes (g) of high-fat diet-fed (HFD) and normal diet-fed (ND) mice.Standard deviations were applied to body weights to quantify the dispersion of data set values from mean.
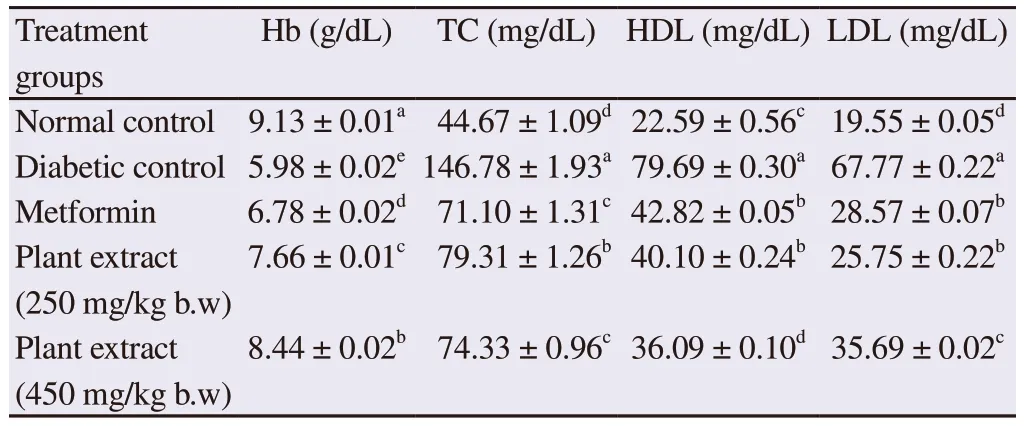
Table 4.Impact of 60% ethanolic leaf extract of Conocarpus lancifolius on Hb, TC, HDL and LDL of diabetic mice.
4.Discussion
Emerging role of medicinal plants to control T2DM expansion emphasizes to explore plants for substantial and scientifically proved evidences rather than simply believing the traditional use.The side effects associated with synthetic treatments of T2DM and divine antidiabetic potential of plants are the key factors behind the scope of work on natural remedies.The antioxidant, anti-αglucosidae and anti-α-amylase activities of plants are mainly due to phenolics and flavonoids present in them.Their extraction is a major contributor towards optimal biological activities.The modern techniques like ultrasonication have solved the issues of low yields and lesser bioactive amounts by extraction.The biological activities and medicinal potentials of plants may be enhanced by proper extraction covering the accumulation of active natural compounds in the extracts.Multi-dimensional factors including solvent polarity and ultrasonication may be good strategies to cope with the low yield of extracts.Better yields are only possible by considering the knowledge of structural properties of compounds to be extracted.In current study, the polarity governed by the components of solvent system designed for extraction played a key role to enhance the vitality associated with extraction of bioactive components from plant leaves.The efficient working of ethanol based binary solvent system for enriched extract preparation with improved antioxidant potential was also supported by some previous investigations on plants.Similarly, DPPH based strong antioxidant activity of 60%ethanolic plant extracts was also supported by previous research[15].The excellent antioxidant activity of C.lancifolius extracts was most probably due to the existence of bioactive molecules in respective extracts.The improvement in DPPH radical scavenging with changing solvent concentration was noticed and was associated with the enhanced functional ability of extracts.The findings for inhibition of α-glucosidase activity were comparable and even better than those reported by some recent reports covering similar aspects of other plants[23,24].In terms of α-glucosidase and α-amylase inhibitory activities, improved functional attributes of the 60%ethanolic extracts were probably due to the discriminative amount of biologically active ingredients including TPC and TFC contained in these extracts.
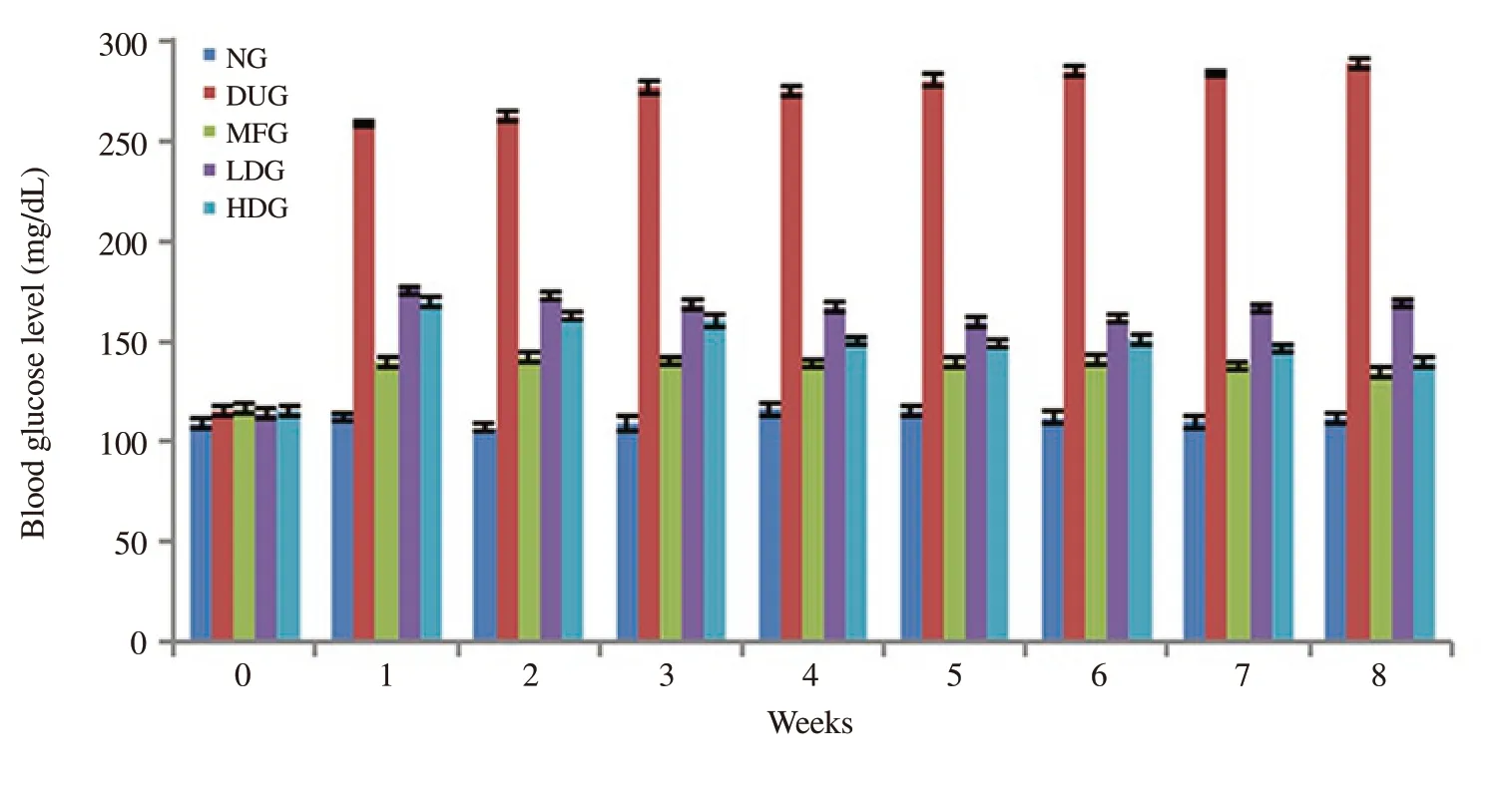
Figure 3.Effect of metformin and hydroethanolic leaf extract of Conocarpus lancifolius on blood glucose level of normal and diabetic mice.Standard deviations(±) were applied to quantify the dispersion of data set values from mean.Normal mice group (NG), diabetic untreated group (DUG), metformin treated group(MFG), low plant extract dose group (LDG) and high dose group (HDG).
The mice developed obesity upon consuming HFD and diabetes was induced by a single injection of alloxan.Obesity and diabetes both are interlinked and obesity is one of the leading causes of T2DM.The risk factor to have T2DM increases under obese conditions.The obese mice were tested through a series of experiments to evaluate the hypoglycemic and hypolipidemic activities of the 60% ethanolic C.lancifolius leaf extracts.The efficiency of the plant extracts to minimize the increase of glucose concentration in blood was dose-dependent as 450 mg/kg was proved significantly effective.A previous study reported 64 plant species used for the treatment of DM in Pakistan, but most lacked identification regarding phytotherapeutics and nutraceuticals[25].Another scientific report indicated that the extract of Ajugaremota benth at a dose of 500 g/kg body weight of rats efficiently controlled the blood glucose level in rat model.The antidiabetic potential was probably due to the presence of saponins, tannins, phenolics and flavonoids in plant extracts[26].The hypoglycemic impact of C.lancifolius was more pronounced as compared with antihyperglycemic effect of closely related species Conocarpus erectus in alloxan induced diabetic mice.The variation in attenuation of blood glucose for both plants was probably due to the different concentrations of phytochemicals[27].
The decline in blood glucose availability by the 60% ethanolic extract of C.lancifolius might be attributed to the presence of secondary metabolites as identified by UHPLC-Q-TOF-MS/MS.Some medicinally important metabolites found in this plant extract were gallic acid, ellagic acid, ellagic acid derivative, corilagin,caffeic acid derivative, galloyl-HHDP-glucoside, its derivative and isomers, terflavin B, kaempferol-3-O-rutiniside and isorhamnetin.It was reported that additional hydroxyl groups on gallic acid added structure-based antidiabetic functionalities in this molecule and its presence in plant extracts was responsible for inhibition of α-glucosidase[28].A previous in-vivo investigation elaborated the antidiabetic impact of caffeic acid in diabetic mice.Caffeic acid reduced the hepatic outflow of glucose and improved glucose uptake by adipocytes through enhanced expression of hepatic glucose 4 transporter.Caffeic acid esters were also reported to have excellent antidiabetic potential in murine insulin-sensitive cells by activating adenosine 5'-monophosphate-activated protein kinase[29].An Indian fruit Emblica officinalis containing gallic acid, ellagic acid,and corilagin as major bioactive constituents was reported to have the antihyperglycemic potential[30].Similarly, kaempferol-3-Orutinoside which is a structural analogue of kaempherol and rutin has been reported to have 8-times high anti-α-glucosidase activity than that of standard drug acarbose[31].Isorhamnetin is a common flavanol in plants and is associated with potent medicinal properties including antihyperglycemic.The discussed compounds are of potential nutraceutical importance and have the privilege to alter disease physiology due to various multiple target-oriented modes of actions.The most probable route to control the rapid increase in blood glucose level was the inhibitory action of bioactive ingredients present in leaf extracts against α-glucosidase, thus delaying the degradation of carbohydrates.The slow rate of degradation led to low carbohydrate absorption which in turn resulted in a declined blood glucose level.The antioxidant nature of these secondary metabolites might be associated with the potential to reduce the level of oxidative stress and to improve metabolic functions.Possibly, there was an obvious linkage between antioxidant activity and the antidiabetic effect of plant extract.From the lowering of blood glucose level in diabetic mice to a considerable extent, it was established that the phytotherapeutic agents present in leaf extract were directly responsible for the antidiabetic potential of C.lancifolius.Biochemical analysis revealed that reduction in blood Hb was observed for diabetic mice.The reduction in Hb level under diabetic conditions could be possibly ascribed to its non-enzymatic glycosylation which resulted in conversion to HbA1C.As a major biomarker, HbA1C and its variations in concentration reflected the physiological alterations in blood glucose level over a specific time period.The elevated TC and HDL concentrations represented the detrimental impact of high blood glucose level on lipid profiles of the mice.However, high extract dose significantly reduced the TC by modulating the LDL and HDL.Similar findings were previously reported highlighting the impact of T2DM on lipid profile and emphasizing the role of plant extracts to improve biochemical indicators[32].The improvement in lipid profile might be due to the activation of AMPK and glucose transporter type 4 with an increase in insulin secretion and glucose uptake by cells[33].The synergic effect of medicinally important compounds present in the particular extract may be responsible for changes in biochemical indicators of mice[34,35].The consumption of diet enriched with C.lancifolius leaf extract would be useful to treat T2DM along with haematological improvements.The study also supports the notion of the efficacy of plants to cope with chronic ailments like T2DM.The C.lancifolius possessing antihyperglycemic and antihyperlipidemic attributes may serve as an appropriate and cost-effective choice to treat the T2DM along with associated biochemical amelioration for a considerable reduction in socioeconomic burden.
In conclusion, the work explored the promising antioxidant and antidiabetic potential of C.lancifolius leaf extracts.The 60% ethanol was established as the best solvent in order to accumulate substantial amounts of bioactive ingredients in extract.The leaves were proven to be a rich source of high-value metabolites of functional nature.The outcomes of the study provided an efficient and eco-friendly way to manage T2DM.This plant could be used in the development of nutraceuticals for the treatment of diabetes.
Conflict of interest statement
Authors declare that they have no conflict of interests among them regarding the publication of this paper.
Authors' contributions
SAR performed the extraction, antioxidant activity, α-glucosidase and α-amylase inhibition assays.ARC conducted the phytochemical analysis.MWM designed the study and supervised the work.AA reviewed the contents and interpreted data.HM and MTA were involved in animal study and data analysis.
 Asian Pacific Journal of Tropical Biomedicine2020年8期
Asian Pacific Journal of Tropical Biomedicine2020年8期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Vector-borne diseases: Mosquito holobiont and novel methods for vector control
- Gymnema montanum improves endothelial function via inhibition of endoplasmic reticulum stress by activating Nrf2 signaling
- Peanut testa extracts enhance anticancer effect of cisplatin against human cholangiocarcinoma cells via modulation of histone deacetylase inhibitory activity
- Immunosuppressive and antibacterial activities of dihydromorin and norartocarpetin isolated from Artocarpus heterophyllus heartwoods
- Isolation and characterization of five novel mini-Mconotoxins from the venom of mollusk-hunter snail Conus bandanus
