Peanut testa extracts enhance anticancer effect of cisplatin against human cholangiocarcinoma cells via modulation of histone deacetylase inhibitory activity
Somprasong Saenglee, Gulsiri Senawong, Jarckrit Jeeunngoi, Sanun Jogloy, Albert J.Ketterman, Banchob Sripa,Thanaset Senawong,5✉
1Department of Biochemistry, Faculty of Science, Khon Kaen University, Khon Kaen 40002, Thailand
2Department of Plant Science and Agricultural Resources, Faculty of Agriculture, Khon Kaen University, Khon Kaen 40002, Thailand
3Institute of Molecular Biosciences, Mahidol University, Salaya Campus, 73170 Thailand
4Department of Pathology, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand
5Natural Product Research Unit, Faculty of Science, Khon Kaen University, Khon Kaen 40002, Thailand
ABSTRACT
KEYWORDS: Apoptosis; Caspases; Cholangiocarcinoma;Cisplatin; Natural histone deacetylase inhibitor; Peanut testa extracts
1.Introduction
Cholangiocarcinoma (CCA) is a bile duct cancer that has been reported worldwide and is non-rare in East Asia and Southeast Asia[1].Despite the relative rarity of CCA worldwide, the incidence of CCA in Thailand is the world's highest, especially in northeast Thailand[2].CCA cases in Thailand are generally intrahepatic and the Opisthorchis viverrini infection has been classified as a risk factor of the disease[3].Although specific causal factors of CCA in patients are still unclear, the changes in multiple genes through both genetic and epigenetic mechanisms may be involved[4,5].
Due to frequent late diagnosis of advanced stage CCA,chemotherapy is a common option for patients suffering from CCA.Cisplatin, epirubicin, 5-fluorouracil, gemcitabine, leucovorin,and mitomycin-C have been used to treat CCA[6], however, the 5-year survival rate of CCA patients is less than 10%[7].Moreover,the clinical application of anticancer drugs is usually associated with adverse side effects, such as anemia, neurotoxicity, and nephrotoxicity[8], as well as the development of tumor resistance[9].The combinations of different cancer drugs exhibited the potential to eliminate or delay drug resistance[10].Therefore, CCA patients are urgently in need of more effective combination chemotherapy to overcome chemoresistance and to reduce undesired side effects of chemotherapy.
Cisplatin, cisplatinum, or cis-diamminedichloroplatinum (Ⅱ), is one of the most effective cancer therapeutic drugs.It has been used for treatments of many human cancers such as bladder, lung, head and neck, ovarian, and testicular cancers.The drug target is to crosslink the N7 of the guanine base causing DNA damage, leading to stimulation of DNA repair mechanisms, and subsequently inducing apoptosis in the various cancer cell types[11].Nonetheless,drug resistance and many unpleasant side effects such as severe nephrotoxicity, hepatotoxicity, cardiotoxicity, kidney problems,allergic symptom, decreased immunity, and infections, have been observed when cisplatin was used in treatments[11].Thus,to overcome drug-resistance and to reduce toxicity for cancer therapy, combination therapies of cisplatin with other drugs have been considered.In this regard, cisplatin has been tested in combination with multiple natural compounds, including anvirzel[12],bevacizumab[13], vinblastine and bleomycin[14] in several cancer cell lines.
Herbal medicine is an inevitable alternative to reduce toxicity[15].Recently, we have demonstrated that testa extracts of two Valenciatype peanuts (Arachis hypogaea L.) (KK4 and ICG15042) inhibited growth, induced apoptosis and cell cycle arrest of several cancer cell lines, including cholangiocarcinoma, cervical, breast, colon and acute T cell leukemia cell lines[16,17].In addition, both peanut testa extracts have been shown to possess histone deacetylase (HDAC)inhibitory activity and induced apoptosis via activation of caspase activities[17].HDAC inhibitors are known to play a critical role in recovering abnormal expression of tumor suppressor genes and proto-oncogenes[18], inhibiting cell proliferation, inducing cell cycle arrest, apoptosis and/or differentiation in several cancer cell lines[19], and triggering DNA damage and repair mechanisms in cancer cells[20].Several studies have shown that HDAC inhibitors exhibited low toxicity in normal cells and demonstrated efficacy with manageable side effects in patients[21,22].To date, the U.S.Food and Drug Administration has approved several HDAC inhibitors,including suberoylanilide hydroxamic acid (SAHA), romidepsin and belinostat for the treatment of cutaneous and peripheral T-cell lymphomas[23].Our previous study demonstrated that pan HDAC inhibitors (SAHA and TSA) synergistically enhanced the antiproliferative activity of cisplatin against CCA cell lines[24].Therefore, we hypothesize that the peanut testa extracts containing natural HDAC inhibitors[17] when combined with cisplatin should be able to elicit a synergistic effect on the inhibition of CCA cell growth and reduce the dosage requirement of cisplatin.In this study, both KK4 and ICG15042 testa extracts were tested for their combined anticancer effect with cisplatin against KKU-100 and KKU-M214 cholangiocarcinoma cells to improve clinical protocols.
2.Materials and methods
2.1.Chemicals
Cisplatin and propidium iodide (PI) were obtained from Sigma-Aldrich Corporation (St.Louis, MO, USA).The kernels of KK4 and ICG15042 Valencia-type peanut, the 2017 crop (October 2017 to February 2018), were kindly supplied from the KKU Field Crop Research Station, Thailand.The preparations of peanut testae were done as described previously[25].The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from Invitrogen, Molecular Probes products (Eugene, Oregon, USA),while Annexin V-FITC was from Biolegend (San Diego, CA, USA).The primary antibodies against cleaved caspase 3 (9661), procaspase 3 (9663), cleaved caspase 8 (9748), procaspase 8 (9746), cleaved caspase 9 (9501), procaspase 9 (9508), Ac-H3 (9671S), p21 (2946S),p53 (2524S), Bcl-2 (2872S), Bax (2772S), pERK1/2 (4377S),ERK1/2 (9107S), β-actin (3700S) and all secondary antibodies including HRP-linked anti-mouse IgG (7076S) and HRP-linked antirabbit IgG (7074S), were obtained from Cell Signaling Technology(Beverly, MA, USA).Colorimetric assay kits for caspases 3, 8 and 9 were obtained from Biovision Incorporated (Milpitas, CA, USA).
2.2.Cell lines and culture conditions
KKU-100 and KKU-M214 cells were previously derived from CCA patients with poorly- and moderately-differentiated adenocarcinoma, respectively, residing in opisthorchiasis endemic areas of Northeastern Thailand[26].H69 cell line is a human SV40 immortalized biliary epithelial cell line derived from normal liver and requires a hormone-supplemented medium for optimal growth.KKU-M214 and KKU-100 cells were obtained from Prof.Dr.B.Sripa, Khon Kaen University, Thailand and grown in RPMI-1640 medium supplemented with 10% fetal bovine serum, penicillin (100 U/mL) and streptomycin (100 µg/mL) (Gibco-BRL).H69 cells were obtained from Prof.Dr.D.Jefferson, Tufts University, Boston, MA,USA and grown in Dulbecco's minimum essential medium (DMEM;Gibco, Invitrogen Corporation) as previously described[24].
2.3.Sample preparation
Peanut testa extract was prepared as described previously[16].In brief, peanut testae were thoroughly ground in an agate mortar to obtain fine powder.The extracts were prepared by mixing 1 g of ground peanut testae with 40 mL ethanol and continued stirring at room temperature using a magnetic stirrer for 48 h.Samples were centrifuged and the supernatant was then filtered through a Whatman No.4.The filtrate was evaporated using a rotary evaporator until the remaining solution was around 2 mL and the remaining solvent was dried under a gentle stream of nitrogen gas.Testa extracts were stored at -20 ℃ until further use.
2.4.Cell viability and drug combination assay
The MTT assay was used to assess cell viability as described in a previous study[17].Briefly, KKU-M214, KKU-100, and H69 cells were seeded (8 × 103cells/well) in 96-well plates and incubated in a CO2incubator for 24 h.Then, various concentrations of KK4 and ICG15042 testa extracts, cisplatin and vehicle control (0.1%DMSO) were added in each well and incubated for 24, 48 and 72 h.The half-maximal inhibitory concentrations (IC50values) of singledrug treatments were determined.For combination treatments, a subtoxic concentration (the concentration required to cause ~20%growth inhibition; IC20) of cisplatin was used to treat KKU-M214(10, 7, 5 µM for 24, 48 and 72 h, respectively), KKU-100 (7, 5, 3µM for 24, 48 and 72 h, respectively) and H69 (20, 7, 5 µM for 24,48 and 72 h, respectively) cells.KK4 and ICG15042 peanut testa extracts with varying concentrations were used in combination with cisplatin for the treatments.At definite time intervals, the culture medium was removed and replaced with fresh culture medium containing 1.2 mM MTT and incubated for 2 h at 37 ℃.The absorbance (Abs) was determined at 550 nm using a Spectramax M5 microplate fluorometer (Molecular Devices Cooperation, Sunnyvale,CA, USA) and the optical density at 655 nm was used to subtract optical density of cell debris.Cell viability was then calculated as a percentage using the following equation: % Cell viability = [Sample Abs/ Control Abs] × 100.
The combination index (CI) theorem of Chou and Talalay[27]was used to determine drug interaction between two peanut testa extracts and cisplatin.The CI values were evaluated by the following equation for mutually non-exclusive modes of drug action:
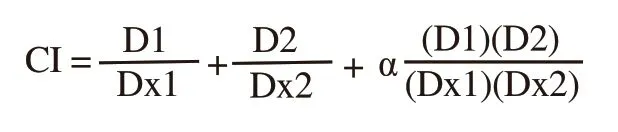
Where, D1 is dose of drug 1 (peanut testa extract) to produce 50%growth inhibition in combination; D2 is dose of drug 2 (cisplatin)to produce 50% growth inhibition in combination; Dx1 is dose of drug 1 to produce 50% growth inhibition alone; Dx2 is dose of drug 2 to produce 50% growth inhibition alone; α = 1 for mutually non-exclusive modes of drug actions.A CI value < 1.00 indicates synergism; 1.00 indicates additivity, and > 1.00 indicates antagonism.The dose reduction index (DRI) signifies the number of dose reduction (fold) that is allowed in combination for a given level of effect as compared to the dose of a single agent.DRI values were calculated by the following equation[27]:
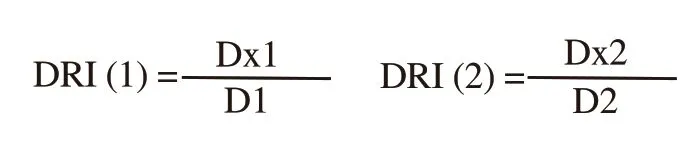
2.5.Analysis of cell cycle phase distribution
Cell cycle progression was analyzed using PI staining and flow cytometry as described in a previous study[17].In brief, KKU-M214 cells were seeded in a culture dish at 1 × 106cells/dish and treated with peanut testa extracts (40 µg/mL) and cisplatin (10 µM) alone and in combination for 24 h.The cells were harvested, washed twice in phosphate-buffered saline (PBS) and permeabilized in ice-cold 70% ethanol at 4 ℃ for 1 h.The cell pellets were then washed twice with PBS and incubated for 1 h in PBS containing 0.2 mg/mL of RNase A at 37 ℃.To stain the cells, 50 µg/mL of PI was added and incubated for 45 min at room temperature in the dark.Cell cycle phases were analyzed by flow cytometry (Becton Dickinson, San Jose, CA, USA).
2.6.Analysis of apoptosis
Apoptosis induction was determined using Annexin V-FITC and PI staining and analyzed by flow cytometry as described previously[17].In brief, KKU-M214 cells were cultured for 24 h in a 5.5-cm dish at a density of 1 × 106cells/dish and treated as described in the analysis of cell cycle phase distribution.Cells were harvested, rinsed twice with ice-cold PBS, resuspended in the Annexin-binding buffer (100 mL), and incubated in the dark with Annexin V- FITC and PI for 15 min.Apoptotic cells were analyzed using BD FACSCantoII Flow Cytometer.
2.7.Caspase 3, 8 and 9 activity assay
Activities of caspases 3, 8 and 9 were determined using the Caspase 3, 8 and 9 Colorimetric Assay Kit (BioVision, Milpitas, CA, USA).Briefly, KKU-M214 cells were seeded into the culture dish (1 × 106cells/dish) and incubated for 24 h.Cells were treated as described in the analysis of cell cycle phase distribution and the activity assay was performed as described previously[17].
2.8.Western blotting assays
KKU-M214 cells (1 × 106cells/dish) were seeded in a 5.5-cm dish and cultured for 24 h before treatment as described in the analysis of cell cycle phase distribution.The treated cells were harvested and lysed in RIPA lysis buffer.Total protein (60 µg) was separated through 12% SDS-PAGE (15 mA for 1.30 h), followed by blotting of the proteins onto a PVDF membrane.The blots were incubated overnight with specific primary antibodies after blocking with 5%skim milk for 1 h.The following antibodies were used: anti-p21,anti-p53, anti-Bax, anti-acetylated H3, anti-pERK1/2, anti-ERK1/2,anti-Bcl-2, anti-cleaved caspase 3, anti-procaspase 3, anti-cleaved caspase 8, anti-procaspase 8, anti-cleaved caspase 9, anti-procaspase 9 and anti-β-actin.Subsequently, the membrane was incubated with horseradish peroxidase (HRP)-conjugated secondary antibody at room temperature for 2 h.The protein bands were detected using ClarityTMWestern ECL substrate (Bio-Rad) and X-ray films (GE Healthcare Bio-Sciences, USA).
2.9.Statistical analysis
The IBM SPSS Statistic version 20.0 (SPSS Corporation, Chicago,IL, USA) for mac was used for analyzing the data.Statistical analysis was performed using One-way ANOVA, followed by Duncan's post hoc test.The criterion for the significant difference was set at P<0.05.Data were expressed as mean ± standard deviation(SD) from three independent experiments.
3.Results
3.1.Antiproliferation of CCA cells by peanut testa extracts and cisplatin
To establish combination treatments, single-drug treatments were required to determine the IC50values of individual drugs.Exposure of KKU-M214 and KKU-100 cells to ICG15042 and KK4 testa extracts resulted in growth inhibition of cancer cells in a dose- and time-dependent manner as shown in terms of IC50values (Table 1).KKU-M214 cells were more sensitive to both ICG15042 and KK4 testa extracts than KKU-100 cells.The KK4 testa extract exhibited significant cytotoxic effect against KKU-M214 and KKU-100 cells.ICG15042 testa extract also inhibited the proliferation of KKUM214 and KKU-100 cells in a similar fashion.Cisplatin significantly suppressed the growth of KKU-M214 and KKU-100 cells with IC50values of (12.35 ± 0.21) and (4.20 ± 0.85) µM respectively after 72 h exposure.In addition, the non-cancer H69 cells were found less sensitive to both peanut testa extracts than KKU-M214 cells for all exposure times.However, KKU-100 cells were more resistant to the peanut testa extracts than H69 cells for all exposure times.
3.2.Antiproliferation of CCA cells by peanut testa extracts and cisplatin in combinations
The combined effects of KK4 and ICG15042 peanut testa extracts and cisplatin on the proliferation of CCA cells are shown in Figure 1.The combination of cisplatin and the peanut testa extracts decreased cell viability more significantly than either agent alone.KK4 peanut testa extract in combination with cisplatin decreased KKU-M214 cell viability to (2.01 ± 1.31)%, whereas KK4 peanut testa extract alone decreased cell viability to (40.20 ± 0.40)% for 72 h exposure.Cisplatin alone at sub-toxic concentrations (IC20values)decreased KKU-M214 cell viability to (66.05 ± 1.42)% for 72 h exposure (Figure 1A).Similarly, ICG15042 peanut testa extract in combination with cisplatin reduced KKU-M214 cell viability to(0.96 ± 0.32)%, while ICG15042 peanut testa extract alone decreased cell viability to (24.13 ± 6.47)% for 72 h exposure.In KKU-100 cells, the percentage of cell viability after combination treatment of each peanut testa extract (KK4 and ICG15042) and cisplatin was not different from single-drug treatment for 24 h exposure (Figure 1B).However, cell viabilities were slightly reduced to (40.99 ± 3.65)%and (20.22 ± 2.90)% after treatment with KK4 and cisplatin for 48 and 72 h exposures, respectively, while cell viabilities after KK4 alone treatment were (72.92 ± 3.65)% and (44.19 ± 2.90)% for 48 and 72 h exposures, respectively.Cisplatin alone decreased KKU-100 cell viabilities to (50.29 ± 4.71)% and (66.57 ± 1.78)% for 48 and 72 h exposures, respectively.Combination of cisplatin and ICG15042 testa extract reduced KKU-100 cell viabilities to (31.00 ±1.01)% and (28.89 ± 9.96)% for 48 and 72 h exposures, respectively,whereas ICG15042 alone decreased cell viabilities to (65.08 ±6.59)% and (46.36 ± 0.27)% for 48 and 72 h exposures, respectively.In contrast, non-cancer H69 cells were more resistant to the peanut testa extracts in combination with cisplatin than CCA cells for 48 and 72 h exposures (Figure 1C).
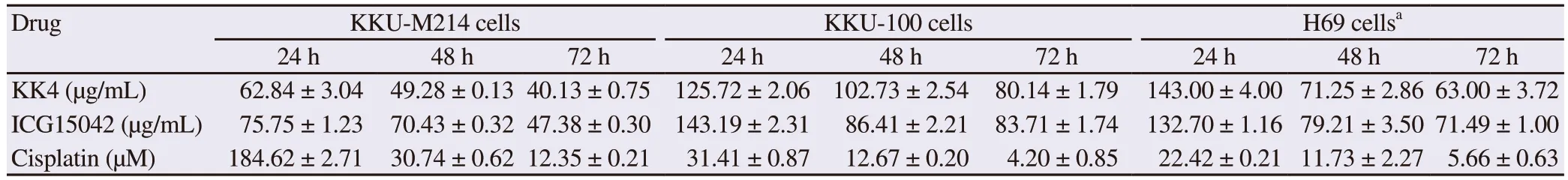
Table 1.Half maximal inhibitory concentrations (IC50) for the antiproliferative action of cisplatin, KK4 and ICG15042 testa extracts after 24 h, 48 h and 72 h exposures to CCA cell lines.
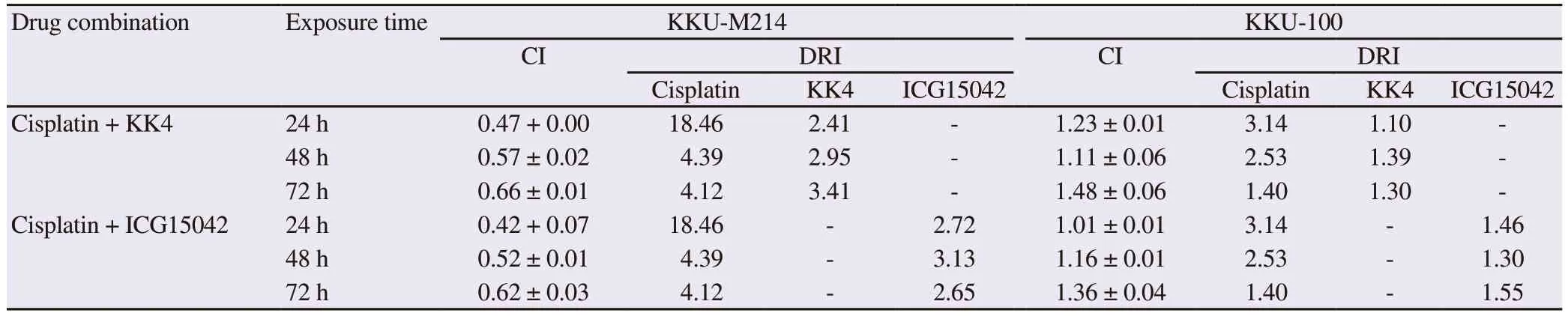
Table 2.Combination index (CI) and dose reduction index (DRI) of peanut testa extracts (KK4 and ICG15042) and cisplatin in cholangiocarcinoma cells.
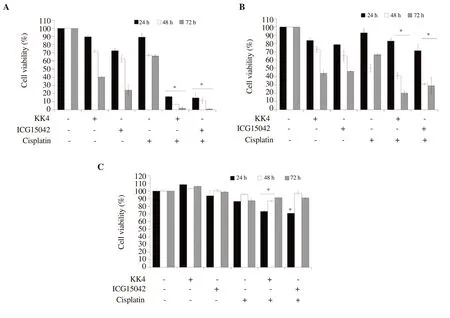
Figure 1.Antiproliferative activity of peanut testa extracts in combination with cisplatin against CCA and H69 cells.(A) KKU-M214 cells were treated with 10, 7,5 µM (IC20) of cisplatin for 24, 48 and 72 h, respectively.(B) KKU-100 cells were treated with 7, 5, 3 µM (IC20) of cisplatin for 24, 48 and 72 h, respectively.(C) H69 cells were treated with 20, 7, 5 µM (IC20) of cisplatin for 24, 48 and 72 h, respectively.The concentration of both KK4 and ICG15042 testa extracts used for combination treatment was 40 µg/mL.The data are presented as the mean (± standard deviation, SD) of three independent experiments.Asterisk “*”indicates a significant inhibition (P<0.05) compared to single-drug treatment.
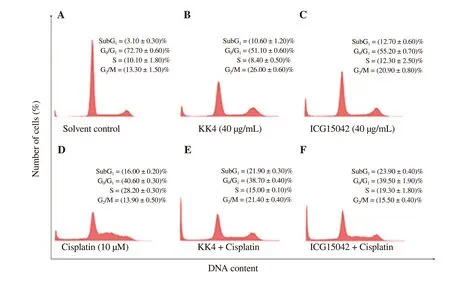
Figure 2.DNA histograms representing cell cycle profiles of (A) solvent-, (B) KK4 alone-, (C) ICG15042 alone-, (D) cisplatin alone-, (E) KK4 + cisplatin- (F)ICG15042 + cisplatin-treated KKU-M214 cells.Cells were treated with DMSO (0.03%) as a solvent control, cisplatin (10 µM), KK4 testa extract (40 µg/mL)and ICG15042 testa extract (40 µg/mL) alone or in combination for 24 h.Stained cells were analyzed by flow cytometry.Data shown are mean ± SD of three independent experiments.Sub-G1 is a cell population with DNA content < 2n.
3.3.CI and DRI
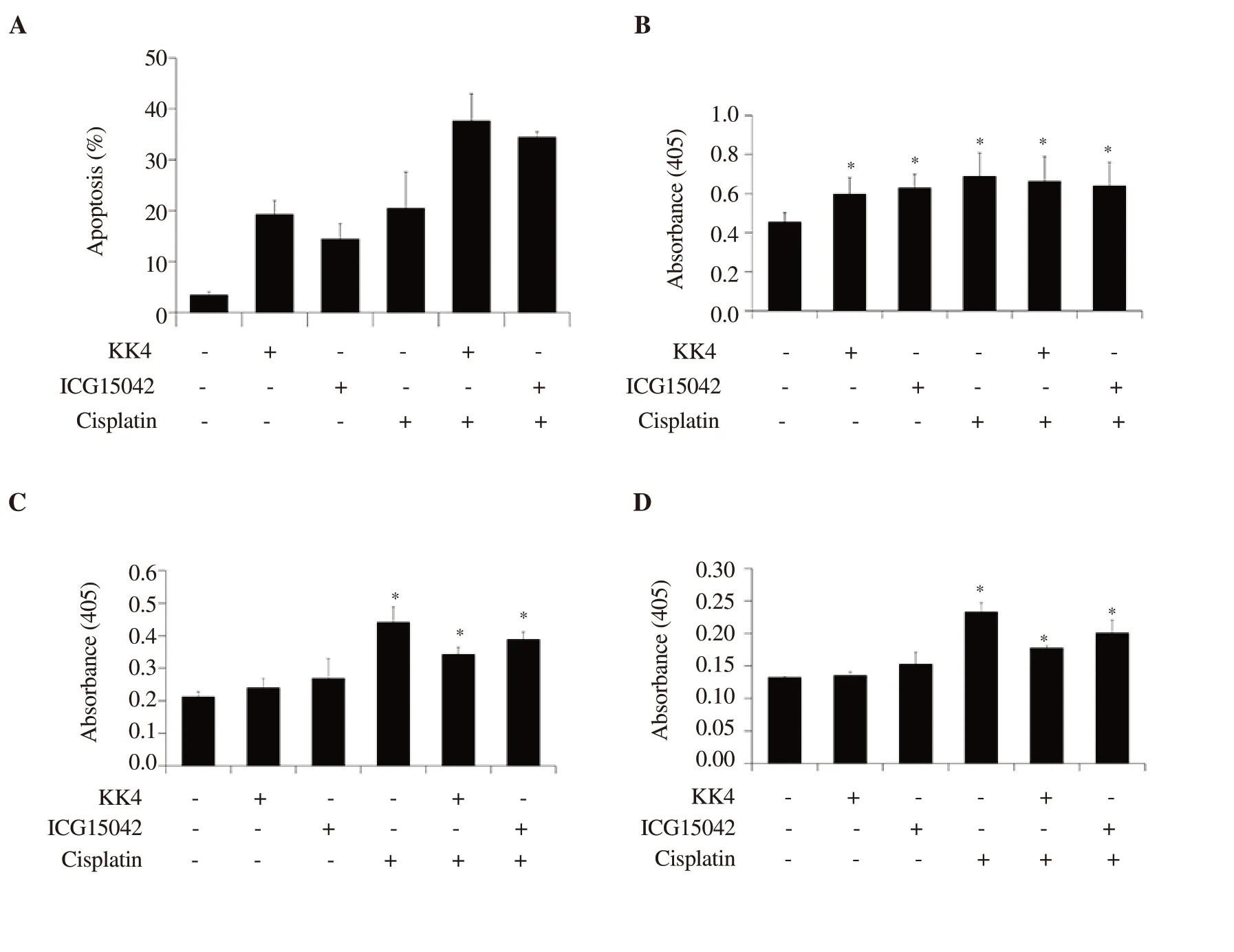
Figure 3.Apoptosis induction and caspase activities induced by cisplatin and peanut testa extracts alone or in combination in KKU-M214 cells.Cells were treated with DMSO (0.03%) as control, cisplatin (10 µM), KK4 testa extract (40 µg/mL) and ICG15042 testa extract (40 µg/mL) alone or in combination for 24 h.(A) Percentages of apoptotic cells induced by cisplatin, KK4 and ICG15042 peanut testa extracts alone or in combination.Stained cells were analyzed by flow cytometry.(B) caspase-3, (C) caspase-8 and (D) caspase-9 activities were determined by colorimetric caspase activity assay.Data shown are mean ± SD of three independent experiments.Asterisk “*” indicates a significant difference (P<0.05) compared to a solvent control treatment.
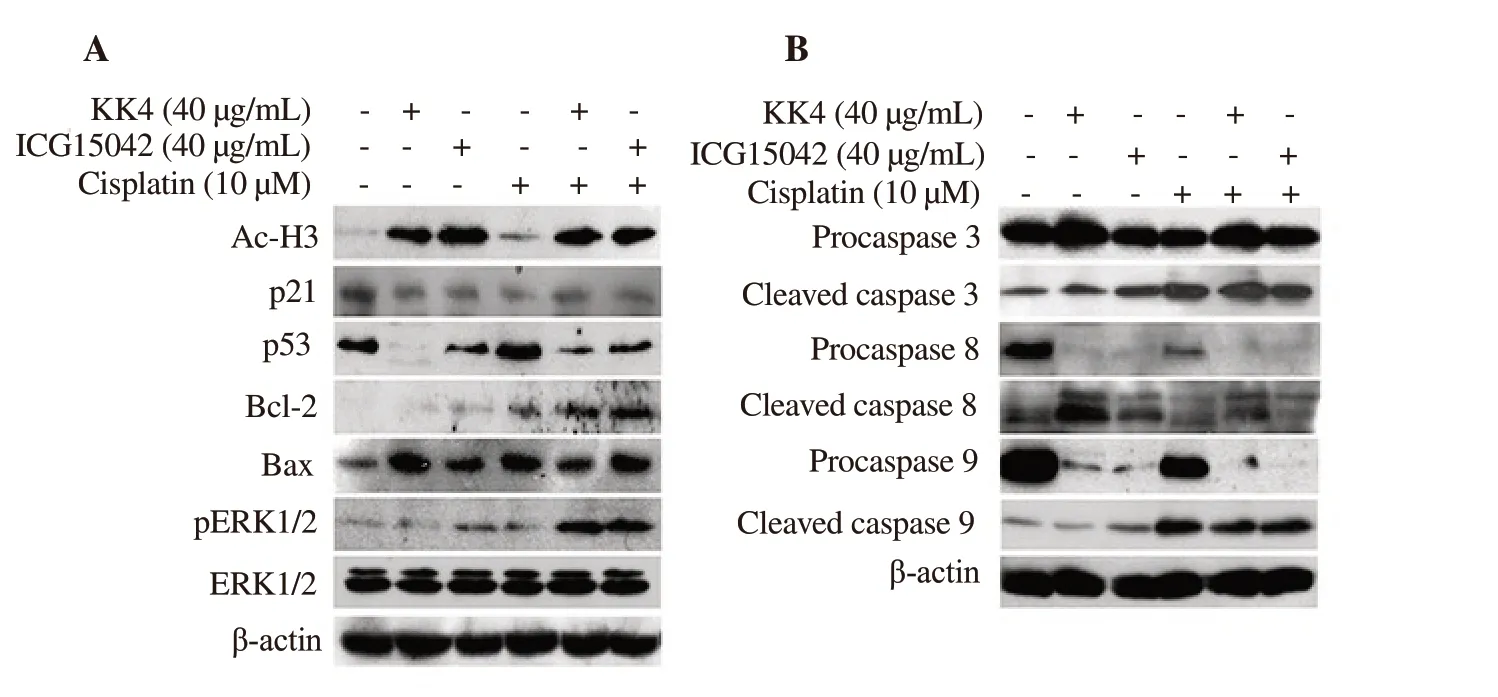
Figure 4.Effect of cisplatin and peanut testa extracts alone or in combination on expression of acetylated histone and apoptotic proteins in KKU-M214 cells.(A) The protein expression level of acetylated histone H3, p53, p21, Bcl-2, Bax and pERK1/2 was shown.(B) The protein expression level of pro- and cleaved caspases was shown.Cells were treated with DMSO (0.03%) as a solvent control, cisplatin (10 µM), KK4 testa extract (40 µg/mL) and ICG15042 testa extract (40µg/mL) alone or in combination for 24 h.Total proteins were separated on SDS-PAGE and blotted onto PVDF membrane.β-actin was used as a loading control in Western blotting analysis.
As shown in Table 2, the combinations of both peanut testa extracts and cisplatin produced synergistic cytotoxic effects in KKUM214 cells (CI < 1.00) but not in KKU-100 cells (CI > 1.00).The synergistic cytotoxic effect in KKU-M214 cells resulted in an advantageous dose reduction of both cisplatin and peanut testa extracts.Cisplatin and peanut testa extracts yielded propitious DRIs ranging from 4.12-18.46-fold and 2.41-3.41-fold, respectively.
3.4.Induction of cell cycle arrest in KKU-M214 cells by combination of peanut testa extracts and cisplatin
According to the above results, the synergistic effect of cisplatin and the extracts was observed only in KKU-M214 cells.Therefore,we further investigated if the induction of cell cycle arrest was associated with the potentiation of cisplatin-induced cytotoxicity by both peanut testa extracts in KKU-M214 cells.As shown in Figure 2, KK4 peanut testa extract in combination with cisplatin caused G2/M and S arrests with (21.40 ± 0.40)% and (15.00 ± 0.10)%,respectively, compared with the untreated control.Similarly, the combination of ICG15042 peanut testa extract and cisplatin caused both G2/M and S phase arrests with (15.50 ± 0.40)% and (19.30± 1.80)%, respectively.Comparing with cisplatin alone treatment[(13.90 ± 0.50)%], combination treatments caused more percentage of cells at the G2/M phase.Comparing with KK4 and ICG15042 alone treatments [(8.40 ± 0.50)% and (12.30 ± 2.50)%], combination treatments caused more percentage of cells at S phase.These results demonstrated that cell cycle arrest was not associated with cytotoxic potentiation caused by combination treatments in KKU-M214 cells (G2/M + S = 42.1% for cisplatin alone, G2/M + S = 36.4%for cisplatin + KK4, G2/M + S = 34.8% for cisplatin + ICG15042).However, more sub-G1populations [(21.90 ± 0.30)% for cisplatin+ KK4, (23.90 ± 0.40)% for cisplatin + ICG15042)] were observed in combination treatments when compared with control and single agents, suggesting that the cytotoxic potentiation caused by combination treatments was dependent on induction of apoptosis in KKU-M214 cells.
3.5.Induction of apoptosis by combination of peanut testa extracts and cisplatin in KKU-M214 cells
Based on the increased sub-G1populations of the cell cycle by combination treatment, apoptosis induction of cisplatin combined with peanut testa extract in KKU-M214 cells was further evaluated.Both combination and single-drug treatments induced apoptosis in KKU-M214 cells.The percentages of apoptotic cells induced by combination treatments were greater than single drug treatments(Figure 3A), which were consistent with the increased sub-G1populations observed in cell cycle profiles (Figure 2E and F).
We determined whether extrinsic (death receptor-mediated) or intrinsic (mitochondria-mediated) pathway contributes to apoptosis induced by KK4 and ICG15042 testa extracts in combination with cisplatin.In this study, activities of caspases 3, 8 and 9 were evaluated using chromophore p-nitroaniline coupled to peptide substrates specific for each of the caspases.Combination treatments of cisplatin and both testa extracts induced apoptosis in KKUM214 cells through increasing caspase-3, -8 and -9 activities (Figure 3B-D).However, single-drug treatments with both testa extracts were less effective at inducing apoptosis than the treatment with cisplatin as demonstrated by the corresponding caspase-8 and -9 activities.
3.6.Effect of combination of peanut testa extracts and cisplatin on expression of apoptotic proteins in KKU-M214 cells
Acetylated histone H3 level was increased in both single and combination treatments of peanut testa extracts and cisplatin except for cisplatin single drug treatment.Additionally, combination treatments caused a decrease in p53 and p21 levels in KKU-M214 cells when compared to vehicle control (Figure 4A).We observed increased expressions of Bax, Bcl-2, and p-ERK1/2 after both single and combination treatments when compared to the control (Figure 4A).In KKU-M214 cells, the levels of cleaved caspases 3, 8 and 9 were clearly increased after treatment with both testa extracts in combination with cisplatin when compared to the solvent control.Moreover, increased cleaved caspases 3 and 8 were observed in all single-agent treatments while increased cleaved caspase 9 was found in treatment with ICG15042 testa extract or cisplatin alone but not with KK4 testa extract.However, procaspase 9 was significantly decreased in the treatments of both testa extracts.Accordingly,observation of caspase activities and cellular levels of caspases 3, 8 and 9 indicated that both extrinsic and intrinsic apoptotic pathways were involved in inhibition of the growth of KKU-M214 cells(Figure 4B).
4.Discussion
Drug combinations have been proposed as a promising therapeutic strategy to reduce drug resistance and improve the efficacy of monotherapy regimens in cancer.The use of cisplatin treatment is often effective, but drug resistance or side effects can be uncontrolled.Our previous studies have shown that peanut (KK4 and ICG15042) testa extracts induce apoptosis in several cancer cell lines such as cholangiocarcinoma, colon, cervical, T-leukemia,and breast cancer cell lines[28,29].Our previous study demonstrated that KK4 and ICG15042 peanut testa extracts functioned as histone deacetylase inhibitors to inhibit the growth of several cancer cells[17].In this study, we evaluated in vitro drug combination treatments of cisplatin with peanut (KK4 and ICG15042) testa extracts in order to overcome drug-resistance and to reduce the toxicity of cisplatin for cancer therapy in the future.
The IC50value is enough to evaluate the efficiency of a single drug on inhibition of cell proliferation.However, in a combination treatment, the CI and DRI values are both important parameters as quantitative indicators of the cancer treatment modality.These parameters are important to identify whether the combination of two drugs has additive, synergistic or antagonistic cytotoxic effects in cancer cells.Combination treatments of cisplatin and both testa extracts significantly inhibited the growth of both cholangiocarcinoma cell lines (KKU-M214 and KKU-100)more efficiently than single-agent treatments.Intriguingly, the combinations of cisplatin and peanut testa extracts yielded favorable DRIs.Combination treatments produced an 18-fold dose reduction of cisplatin in KKU-M214 cells for 24 h compared to single treatment.The dose reduction effect may be due to the natural HDAC inhibitors in peanut testa extracts affecting the regulation of DNA unwinding which is tightly controlled by the balance of activities of histone acetyltransferase and HDAC enzymes.A possible explanation is that the natural HDAC inhibitors in peanut testa extracts caused hyperacetylation facilitating the binding of cisplatin to DNA and interfering with DNA repair mechanisms,ultimately leading to cell death.A dose reduction of cisplatin can lead to a reduction in cytotoxicity and drug resistance in chemotherapy.However, the synergistic effects of drug combination between the peanut testa extracts and cisplatin were observed in only KKU-M214 cells but not in KKU-100 cells.Poorly- (KKU-100) and moderately- (KKU-M214) differentiated CCA cell lines seemed to respond differently to both single-agent and combination treatments.Poorly differentiated CCA (KKU-100) was more aggressive than the moderately differentiated CCA (KKU-M214) cells.In addition,KKU-100 cells are a type of CCA that is quick growing, more resistant and difficult to be completely removed[30].KKU-M214 cells were more sensitive than KKU-100 cells to both single-agent and combination treatments.In contrast to KKU-100 cells, the noncancer H69 cells were more sensitive to single-agent treatments of peanut testa extracts.Therefore, we focused on the synergistic effects of drug combination and mechanism of cisplatin and peanut (KK4 and ICG15042) testa extracts against KKU-M214 cells.
We previously demonstrated that peanut (KK4 and ICG15042) testa extracts suppressed the growth of CCA cells through the induction of apoptosis[17].Here, we demonstrated that acetylated histone H3 was increased in KKU-M214 cells after treatment with peanut(KK4 and ICG15042) testa extracts alone and in combination with cisplatin.Consistent with this observation, we previously identified three phenolic acids, p-coumaric, ferulic and sinapinic acids, in the testa extracts that exhibited HDAC inhibitory, antiproliferative and apoptosis induction activities in cervical, breast and colon cancer cell lines[28,29].
A key process linked to apoptosis is caspase activation.The combination treatments enhanced apoptosis induction by increasing cleaved caspase-3, -8 and -9 activities as demonstrated by caspase activity assay and Western immunoblotting.The extrinsic apoptotic pathway is activated by the binding of death ligand to death receptor which stimulates the assembly of death-inducing signaling complexes comprised of the adaptor molecule, procaspase 8,procaspase 10 and the caspase-8/10 regulator c-FLIP[31].The cleaved caspase 8 then activates the executioner caspases 3, 6, 7 causing the breakdown of several intracellular proteins[32].The intrinsic apoptotic pathway is involved in caspase 9 activation via the formation of an active apoptosome complex, including adaptor Apaf-1, followed by activation of the apoptotic effector caspases 3 and 7[33].However,crosstalk between the intrinsic and extrinsic pathways can occur through caspase 8 cleavage of BH3 interacting domain death agonist following the decrease of Bcl-2 expression and release of cytochrome c into the cytosol[33].Our results indicate that both peanut testa extracts in combination with cisplatin induce apoptosis via both intrinsic and extrinsic pathways.Conversely, the amount of Bcl-2, an anti-apoptotic protein, was increased after combination treatments of both peanut testa extracts and cisplatin.Perhaps, enhancing Bcl-2 expression in cancer cells may be related to an attempt to escape apoptosis by up-regulation or down-regulation of expression of antior pro-apoptotic genes[34,35].The expression of the pro-apoptotic protein Bax was up-regulated after treatment with both peanut testa extracts and cisplatin alone and combination.Bax is generally associated with intracellular stress (DNA damage or oxidative stress)and required for cytochrome c release.Cytochrome c release could further provide the expression of some caspase genes (caspases 2,7, 8, and 9), which is transcriptionally regulated in response to p53 or E2F1[36].Nonetheless, apoptosis can occur through either p53-dependent or independent pathways[37].Our results showed that the p53 level in KKU-M214 cells was reduced after combination treatments compared with cisplatin alone.Our previous study also showed that peanut testa extracts down-regulated expression of p53 and induced apoptosis in KKU-M214 cells[17].Alternatively,p53-independent apoptosis may occur via direct up-regulation of some genes including Apaf-1 and p73[38].Apaf-1 and cytochrome c are assembled leading to the formation of the apoptosome that catalyzes caspase 9, which successively activates pro-apoptotic effector caspases including caspase 3.This is of interest because in the present study, we observe the activation of both caspase 3 and caspase 9 in the presence of cisplatin.In addition, pERK1/2 levels in KKU-M214 cells increased after exposure to peanut testa extracts in combination with cisplatin.Although pERK1/2 is generally found to promote cell survival, proliferation, differentiation and apoptosis inhibition, increased pERK1/2 can also function in a pro-apoptotic manner[39].It has been reported that cisplatin-induced elevated pERK1/2 levels coincided with the induction of apoptosis and cell cycle arrest and were involved in the response to DNA damage or oxidative stress[40].p21 up-regulation inhibited TRAIL-mediated extrinsic apoptosis, contributing to a resistance to SAHA in acute myeloid leukemia cells[41].However, in the present study, the p21 level was not up-regulated in either single agent or combination treatments in KKU-M214 cells.Possibly, apoptosis rather than cell cycle arrest may be associated with synergistic effects of peanut testa extracts and cisplatin.
The effective killing of cancer cells and better tolerance for normal cells are the goal of designing a better regimen for combinative chemotherapy in cancer treatment.In this study, we demonstrated that the growth of KKU-M214 cells was synergistically inhibited by combination treatments of cisplatin and both peanut (KK4 and ICG15042) testa extracts, whereas KKU-100 cells exhibited antagonistic responses.KKU-M214 cells underwent apoptosis in the presence of peanut testa extracts and cisplatin through activation of caspases 3, 8 and 9, suggesting that apoptosis was stimulated via both the intrinsic and extrinsic apoptotic pathways.The combination of cisplatin with peanut testa extracts may improve the therapeutic efficacy on CCA with minimum side effects.Further researches to determine the synergistic components of the peanut extracts are needed in the future.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgments
We are thankful to the Postdoctoral Training Program, Graduate School, Khon Kaen University for providing a fellowship to Dr.Somprasong Saenglee, and to the Royal Golden Jubilee PhD Training Program, Thailand Research Fund, for providing a scholarship to Jarckrit Jeeunngoi.
Funding
This research was supported by the Thailand Research Fund for providing financial support through the Senior Research Scholar Project of Prof.Dr.Sanun Jogloy (Project no.RTA6180002),and also partially supported by the National Research Council of Thailand through Khon Kaen University, Thailand.
Authors' contributions
TS conceived and designed the experiments, analyzed the data,contributed reagents/materials/analysis tools, and wrote the paper.SS performed the experiments, analyzed the data and wrote the first draft of the manuscript.GS analyzed the data and wrote the paper.JJ performed the antiproliferation experiments.SJ contributed reagents/materials/analysis tools.AJK and BS contributed to the final version of the manuscript.
 Asian Pacific Journal of Tropical Biomedicine2020年8期
Asian Pacific Journal of Tropical Biomedicine2020年8期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Vector-borne diseases: Mosquito holobiont and novel methods for vector control
- Gymnema montanum improves endothelial function via inhibition of endoplasmic reticulum stress by activating Nrf2 signaling
- Immunosuppressive and antibacterial activities of dihydromorin and norartocarpetin isolated from Artocarpus heterophyllus heartwoods
- Metabolite profiling and antidiabetic attributes of ultrasonicated leaf extracts of Conocarpus lancifolius
- Isolation and characterization of five novel mini-Mconotoxins from the venom of mollusk-hunter snail Conus bandanus
