Gymnema montanum improves endothelial function via inhibition of endoplasmic reticulum stress by activating Nrf2 signaling
Dornadula Sireesh, Natarajan Suganya, Suvro Chatterjee, Kunka Mohanram Ramkumar✉
1SRM Research Institute, SRM Institute of Science and Technology, Kattankulathur-603 203, Tamil Nadu, India
2Department of Biotechnology, School of Bioengineering, SRM Institute of Science and Technology, Kattankulathur -603 203, Tamil Nadu, India
3Vascular Biology Lab, AU-KBC Research Centre, Anna University, Chromepet, Chennai-600 044, Tamil Nadu, India
ABSTRACT
KEYWORDS: ER stress; Gymnema montanum; Nrf2; Endothelial cells
1.Introduction
Diabetes mellitus is a pandemic multifactorial disorder,characterized by hyperglycemia resulting from defects in insulin secretion, insulin action or both.Epidemiological studies report that chronic hyperglycemia is associated with an incremental risk of cardiovascular diseases.Endothelial dysfunction is a crucial factor in the development and progression of microvascular diseases associated with diabetes mellitus including atherosclerosis[1,2].Apoptosis of endothelial cells can be induced by a number of stimuli such as high glucose, oxidative stress, and cytokine stress,leading to endothelial dysfunction in part through the activation of endoplasmic reticulum (ER) stress[3,4].However, the mechanisms of ER stress-induced endothelial dysfunction and clinical complications remain unclear.
The endomembrane component, ER is the site for protein synthesis,protein folding and trafficking, lipid synthesis, maintenance of calcium homeostasis, and few other processes critical to metabolism[5].Under physiological conditions, cells maintain ER homeostasis by balancing the demand for protein synthesis and protein folding, whereas, disturbances in ER homeostasis due to elevated protein synthesis result in the accumulation of misfolded and unfolded proteins, imbalance in calcium flux, all adding up to elevated ER stress initiating unfolded protein response (UPR)[6-9].UPR pathway restores protein homeostasis or modulates the unfolded proteins either by halting protein translation or by producing molecular chaperones, which aid in proper protein folding.The three-major UPR signaling cascades activated by ER-resident transmembrane proteins are activating transcription factor 6 (ATF6), inositol requiring kinase 1 (IRE1) and dsRNAactivated protein kinase (PKR)-like ER kinase (PERK).A 78-kDa glucose-regulated protein (GRP78) is a chaperone that regulates these molecules.Under ER stress conditions, GRP78 dissociates from the sensing molecules to activate them.In adverse pathological conditions, the adaptive responses fail to rescue cells from ER stress and eventually apoptosis is triggered in these cells.These unresolved UPR pathways are associated with various metabolic disorders,including diabetes[6,8,9].
Nuclear factor erythroid 2-related factor 2 (Nrf2) is the regulator of an array of detoxifying and antioxidant defense gene expressions.Under physiological conditions, Kelch-like ECH-associated protein-1 (Keap1) ubiquitinates and eventually degrades Nrf2.On the other hand, under pathological conditions, Nrf2 is released from Keap1, translocates to the nucleus and binds to antioxidant responsive element (ARE), triggering a battery of genes including glutathione S-transferase (GST) heme oxygenase 1 (HO1), NAD(P)H quinone oxidoreductase 1 (NQO1) and superoxide dismutase (SOD).Hence, it is plausible to target ER stress through Nrf2 signaling cascade during the onset of diabetes[10,11].
Counteracting ER stress represents a promising therapeutic approach to prevent vascular damage thereby hindering the progression of endothelial dysfunction.Recently, plant and plant derivatives have shown a promising effect in preventing ER stress under hyperglycemia[12].Numerous studies demonstrated that natural products, as components of the daily diet or phytomedicine preparations aid in improving endothelial function[4,13].Several medicinal plants are reported to prevent the onset of diabetesmediated endothelial dysfunction including Cymbopogon citrates[14], Syzygium cumini[15], Portulaca oleracea L[16], Erigeron multiradiatus[17], and Phyllanthus emblica[18].Our earlier studies reported that quercetin, a natural polyphenol, attenuates ER stress in endothelial cells both in vitro and in vivo[19,20].
Gymnema montanum (G.montanum) Hook, an Asclepiadaceae member, is extensively used in traditional medicine against various diseases including diabetes.Antidiabetic, anti-hyperlipidemic and anti-apoptotic properties of G.montanum, have been earlier reported from our laboratory[21-23].In the current study, we investigated the effect of G.montanum leaf extract against ER stress in endothelial cells and its underlying molecular mechanism.
2.Materials and methods
2.1.Collection of plant material and preparation of leaf extract
Fresh leaves of G.montanum were collected from Western Ghats,Nilgiris, identified by Dr.C.Murugan with the Herbarium of Botanical Survey of India, Southern Circle, Coimbatore, India(Voucher number 32561-65).The collected leaves were air-dried in shade and made into a fine powder using a blender.Ethyl acetate extract of G.montanum (GMEt) leaves was prepared[24].For solvent extraction, 500 g of powdered leaves were loaded to the thimble and extracted with ethyl acetate solvent using a Soxhlet extractor for 16 h in the Soxhlet apparatus.The extract was then concentrated using a rotary evaporator below 50 ℃, yielded 47 g final residue and preserved at 4 ℃ in an airtight bottle until further use.
2.2.Cell culture
EA.hy926 cells were cultured in Dulbecco's Modified Eagle Medium supplemented with 10% fetal bovine serum (HyClone,Logan, UT, USA), 100 µg/mL streptomycin and 100 U/mL penicillin.The cells were maintained at 37 ℃ in a CO2incubator under a humidified atmosphere.
2.3.Determination of cytotoxicity of GMEt
The cytotoxicity of GMEt and tunicamycin (Sigma-Aldrich, USA)to EA.hy926 cells (1×104cells/well) was assessed using MTT assay.Cells were seeded in 96-well plates and subjected to treatment with different concentrations of either GMEt (0-100 µg/mL) or tunicamycin (TUN, 0-10 µg/mL) and placed in a CO2incubator at 37 ℃ for 24 h.The media were then aspirated, and MTT (5 mg/mL) was added, and further incubated for 3 h.The formazan crystals formed were completely dissolved in DMSO and OD was recorded at 570 nm using ELISA reader (Tecan, Switzerland).All experiments were performed in triplicate, and the relative cell viability (%)was measured as a fraction of control and expressed as percentage relative fold.Fifty percent of cytotoxicity was also recorded for cells.
2.4.Cytoprotective effect of GMEt against ER stress in endothelial cells
In order to study the cytoprotective role of GMEt against TUNinduced cytotoxicity, endothelial cells were seeded in a 96-well microplate, treated with different concentrations of GMEt (0-100µg/mL) and TUN (5 µg/mL) for 24 h at 37 ℃.After treatment,MTT assay was carried out and cell viability calculated as described above.All experiments were performed in triplicate, and the relative cell viability (%) was calculated as a fraction of control.
2.5.Measurement of lactate dehydrogenase (LDH) activity in TUN-induced ER stress
Endothelial cells were seeded at 1×106cells/well in 6-well plate and treated with GMEt (10 or 25 µg/mL) and TUN (5 µg/mL) for 24 h at 37 ℃.After treatment, the cells were trypsinized, and the cell pellet was collected, homogenized using ice-cold assay buffer and centrifuged at 4 ℃.To the supernatant, the reaction mixture (LDH assay buffer and LDH substrate mix) was added and the absorbance was recorded at 450 nm for 1 h at every 5 min interval.LDH activity was calculated as per the manufacturer's instructions (Abcam, UK).
2.6.Determination of malondialdehyde (MDA) levels in TUN-induced ER stress
Endothelial cells were treated with GMEt (10 or 25 µg/mL) and TUN (5 µg/mL) for 24 h at 37 ℃.After treatment, the cell pellet was collected by centrifugation.The pellet was homogenized using PBS and assessed for MDA as per the manufacturer's instructions(Cayman chemicals, UK).Briefly, the samples and standards were boiled for 1 h along with sodium dodecyl sulfate and colour reagent (provided).After incubation, the tubes were placed for 10 min on ice-cold conditions and centrifuged at 1 600×g at 4 ℃.The supernatant was collected and used to measure the MDA levels by reading absorbance at 540 nm.MDA levels were calculated by obtaining the equation against the standard curve.
2.7.Expression analysis of GRP78 and CHOP markers using Western blot assay
In order to study the effect of GMEt against TUN-induced ER stress, the levels of ER stress markers GRP78 and CHOP were assessed by Western blot.Briefly, the cells were treated with GMEt (10 and 25 µg/mL)and TUN (5 µg/mL) for 24 h at 37 ℃.The effect of GMEt on normal endothelial cells was also studied at the fixed higher dose of 25 µg/mL.After treatment, cells were harvested, washed twice with PBS, and lyzed in ice-cold radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology) freshly added with 0.01% protease inhibitor cocktail (Biorad, PA, USA) for 30 min.Cell lysates were subjected to centrifugation at 4 ℃, 1 000×g for 10 min.The protein in the supernatant (20-30 µg) was separated on 10% SDS-PAGE and electrophoretically transferred to a PVDF membrane (Biorad, PA,USA).The blots were blocked with 3% bovine serum albumin for 1 h, followed by incubation at 4 ℃ with anti-GRP78, anti-CHOP and anti-GAPDH antibodies purchased from Santa Cruz Biotechnology,Inc.(Dallas, TX, USA).Blots were then incubated with HRPconjugated anti-rabbit or anti-mouse antibodies (Santa Cruz, USA)for 1 h at 37 ℃.Enhanced chemiluminescence (ECL; Biorad, PA,USA) detection system was used to visualize the target proteins with the GBOX documentation system (Syngene, UK).
2.8.Nrf2 activation potential of GMEt by cell-based luciferase enzyme fragment complementation assay
A complementation system of Nrf2 Keap1 was designed to investigate the activation of Nrf2 in EA.hy926 cells in the presence of GMEt.CLuc-Nrf2 and NLuc-Keap1 fusion proteins[25] were transiently transfected (500 ng) using lipofectamine in EA.hy926 cells.After 6 h, the transfection media were replaced with GMEt (0,5, 10 and 25 µg/mL) for 24 h.Cell homogenate was prepared using cell lysis reagent (Promega, USA), and dropped in the signal i.e.the activity of luciferase due to dissociation of Nrf2 from Nrf2-Keap1 complex upon GMEt treatment was measured by a luminometer(Promega, USA).The results were expressed in percentage as the relative change in the complementation system.The decreased luciferase signal was directly proportional to the activation of Nrf2.
2.9.Validation of Nrf2 activation by ARE-driven luciferase reporter assay
The effect of GMEt on Nrf2 mediated ARE downstream gene activation was assessed using cell-based reporter assay using hGST1-ARE-Luc or hNQO1-ARE-Luc reporter gene constructs.Transfection of EA.hy926 cells with the respective vector constructs was done using Lipofectamine2000 reagent.After 6 h, the medium was aspirated and fresh media were added with different concentrations of GMEt (0, 5, 10 and 25 µg/mL) and incubated for 24 h.Cell homogenate was then prepared using cell lysis reagent(Promega, USA), and luciferase activity was measured using a luminometer (Promega, USA) and expressed as percent change relative to the untreated control.Results were normalized with protein as well as with scrambled control.
2.10.Analysis of Nrf2 and its target genes expression using qPCR
The levels of Nrf2 downstream target genes were studied using qPCR.Briefly, mRNA was extracted from endothelial cells using mRNA isolation kit (Qiagen, Germany), following the manufacturer's instructions.Two µg of total RNA from each sample was subjected to treatment with DNase Ⅰ, and then was subjected to reverse transcription, using cDNA conversion kit (Qiagen, Germany).All samples were reverse transcribed under the same conditions with the same master mix.Triplicate qPCR reactions for each sample were performed (Biorad cfx connect systems, USA).To exclude the possibility of the presence of contaminating template molecules and to identify potential primer-dimer products, no template reactions were also carried out.Amplification plots were analyzed using CFX Manager Software (Biorad, USA) and expressed as relative mRNA expression.Glyceraldehyde-3-phosphate dehydrogenase (GAPDH)was used for normalizing the gene expression.Table 1 depicts the sequences of primers for the target genes.
2.11.Phosphoprotein assay using BioPlex
Phosphoproteins were analyzed using Bio-Plex Pro™Cell Signaling MAPK Panel, developed based on multiplex sandwich bead immunoassays (Bio-Rad, Hercules, CA).After treatment,proteins were extracted using the cell factor QG (provided in the kit) supplemented with protease inhibitor cocktail (Thermo Scientific, USA), and used for assay based on manufacturer's instructions.Briefly, 50 µg/mL of control and test protein samples were incubated overnight along with fluorescent capturing beads coupled to phospho antibodies (phospho-ERK1/2, phospho-JNK,phospho-MAPK, phospho-MEK) in a Bio-Plex Pro™(BioRad, CA,USA) on a platform shaker at 300 rpm at room temperature.The wells were vacuum filtered and washed, and 1 µL of biotinylated detection antibodies were added, vortexed and incubated for 30 min.After additional vacuum filtration and washing of the wells,0.5 µL streptavidin-phycoerythrin (100×) was added to each well and allowed to incubate for 10 min.The wells were again vacuum filtered and washed, and 125 µL of re-suspension buffer was added and incubated for 30 s.Finally, the data was acquired by cytometric imaging using Bio-plex 200 system (BioRad, CA, USA) and analysed using Bio-Plex Manager™software 6.1 (Bio-Rad, CA,USA).Phosphoprotein levels were determined from standard curves of each analyte and recorded as mean fluorescence intensities.
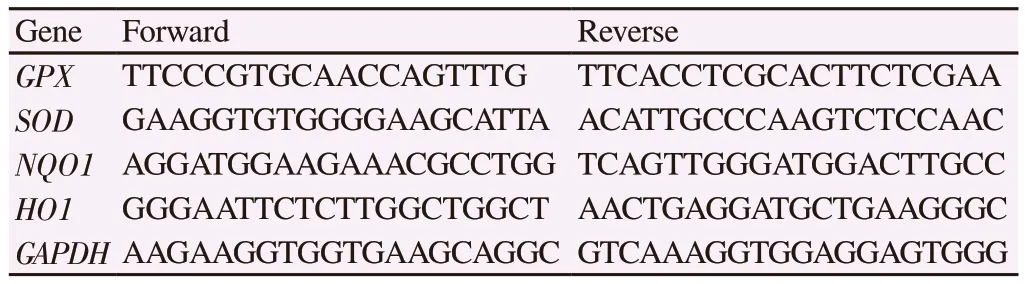
Table 1.Primer sequences used in the study.
2.12.Statistical analyses
All studies were carried out in three independent experiments and results were represented as mean ± SD.Statistical significance was calculated based on one-way ANOVA, followed by Tukey's post hoc test; P < 0.05 was considered significant between groups.
3.Results
3.1.Effect of GMEt on TUN-induced cytotoxicity in EA.hy926 cells

Figure 1.Cytotoxicity of EA.hy926 cells against (A) tunicamycin, (B) GMEt, and (C) effect of GMEt on tunicamycin-induced cytotoxicity assessed by MTT assay.Data are presented as mean ± SD of three independent experiments.Statistical analysis was performed by one-way ANOVA, followed by Tukey's post hoc test.*P<0.05, compared with the untreated control; #P<0.05, compared with the tunicamycin-exposed control.GMEt: Ethyl acetate extract of Gymnema montanum.
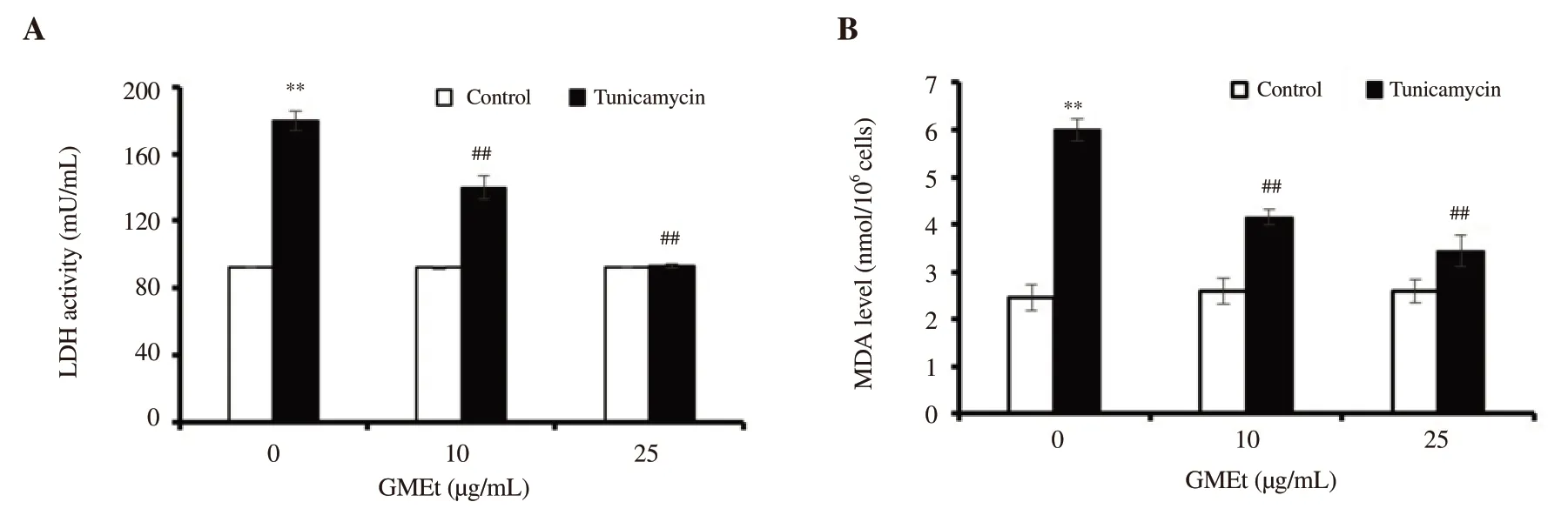
Figure 2.Effect of GMEt on the levels of (A) LDH and (B) MDA on tunicamycin-induced cells.Data are presented as mean ± SD of three independent experiments.Statistical analysis was performed by one-way ANOVA, followed by Tukey's post hoc test.**Significance compared with the untreated control;##Significance compared with the tunicamycin-exposed control; ** & ##P<0.01.
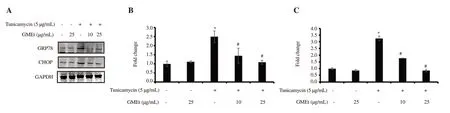
Figure 3.Effect of GMEt against tunicamycin-induced ER stress in endothelial cells using Western blot analysis.(A) Representative blots of GRP78 and CHOP; (B) Level of GRP78; (C) Level of CHOP.Data are presented as mean ± SD of three independent experiments.Statistical analysis was performed by one-way ANOVA, followed by Tukey's post hoc test.*Significance compared the untreated control; #Significance compared with the tunicamycin-exposed control; P<0.05.ER: endoplasmic reticulum.
Preliminary cytotoxic effect of GMEt (0-100 µg/mL) and TUN(0-10 µg/mL) on endothelial cells, EA.hy926, was assessed using MTT assay.LD50of TUN was recorded as 5 µg/mL (Figure 1A) and GMEt did not cause any significant cytotoxicity up to 50 µg/mL(Figure 1B).To evaluate the cytoprotective effect of GMEt against TUN-induced toxicity, endothelial cells were exposed to different concentrations of GMEt (0-100 µg/mL) and TUN (5 µg/mL) for 24 h.The viability of cells decreased in the presence of TUN to 57%,but in the presence of GMEt, the viability increased to 71% and 75%at 10 µg/mL and 25 µg/mL, respectively, (Figure 1C) showing the protective effect of GMEt against ER stress.From this result, the GMEt concentrations for further experiments were taken as 10 and 25 µg/mL.
3.2.Effect of GMEt on LDH and MDA levels in TUN-induced EA.hy926 cells
In order to understand the involvement of free radicals in ER stress,the levels of LDH and MDA were analyzed.Figure 2 shows that a significant elevation in levels of LDH and MDA was observed upon TUN treatment compared with the untreated control, whereas GMEt treatment significantly reduced the levels of both LDH and MDA.
3.3.Effect of GMEt on ER stress-induced protein expression in EA.hy926 cells
TUN exposed cells showed an increase in the levels of ER stress markers including GRP78 (2.5 fold, P<0.05) and CHOP (3.24 fold,P<0.05) when compared to the control cells, confirming the induction of ER stress by TUN exposure (Figure 3).Treatment with GMEt(10 and 25 µg/mL) resulted in reduced levels of GRP78 and CHOP compared with TUN treated cells, indicating dose-dependent protection of GMEt against TUN.The GMEt (25 µg/mL) alone did not result in any significant change (Figure 3).
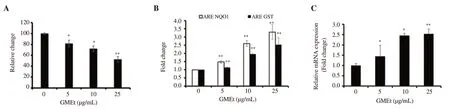
Figure 4.Effect of GMEt on (A) activation of Nrf2 using cell-based luciferase enzyme fragment complementation assay, (B) ARE driven hNQO1-Luc and hGST-Luc gene constructs for its downstream genes and (C) activation of Nrf2 using qPCR.Data are presented as mean ± SD of three independent experiments.Statistical analysis was performed by one-way ANOVA, followed by Tukey's post hoc test.*Significance compared with the untreated control,*P<0.05; **P<0.01.
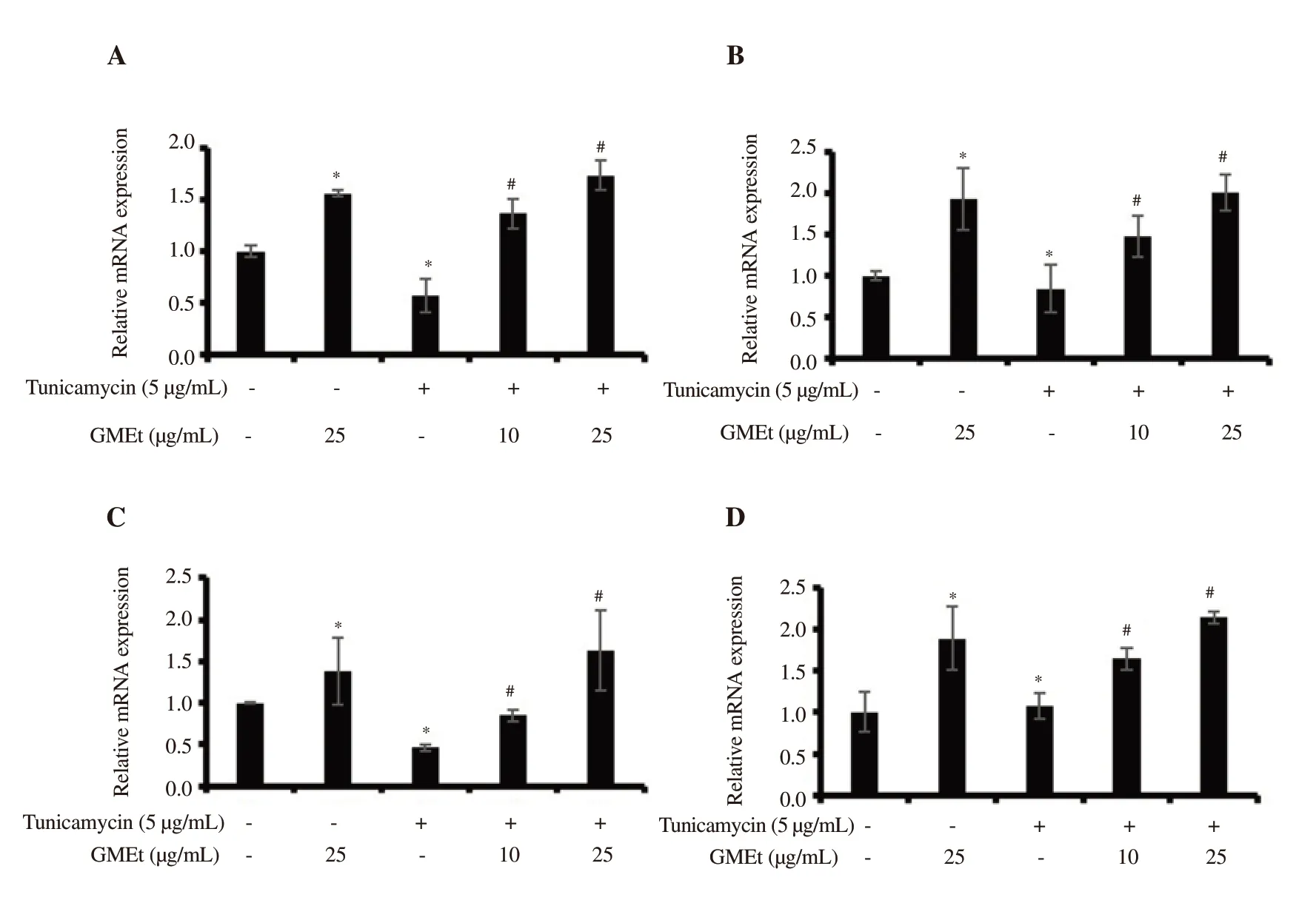
Figure 5.Effect of GMEt on Nrf2 downstream genes including (A) GPx, (B) SOD, (C) NQO1, and (D) HO-1 assessed by qPCR in tunicamycin-induced cells.Data are presented as mean ± SD of three independent experiments.Statistical analysis was performed by one-way ANOVA, followed by Tukey's post hoc test.*Significance compared with the untreated control; #Significance compared with the tunicamycin-exposed control; P<0.05.
3.4.Effect of GMEt on Nrf2 activation in endothelial cells
In order to confirm the Nrf2 activation potential of GMEt, cellbased luciferase enzyme fragment complementation assay and ARE-driven luciferase reporter assay were used.GMEt treatment resulted in a dose-dependent decrease in the luciferase signal in the complementation system, reflecting the activation of Nrf2 (Figure 4A).The ARE-dependent transcriptional activation of Nrf2 was studied using hNQO1-ARE-Luc and hGST-ARE-Luc reporter gene constructs.As shown in Figure 4B, GST and NQO1 reporter luciferase signals increased in a dose-dependent manner in GMEt treated cells, indicating that GMEt stimulated the activation of Nrf2.GMEt treatment also significantly increased the expression of Nrf2 as assessed by qPCR (Figure 4C).
3.5.Effect of GMEt on Nrf2 downstream target genes by qPCR analysis
GMEt treatment increased the expression of downstream target genes including GPx, HO-1, NQO1, and SOD, thereby showing protective effect against ER stress induced by TUN exposure (Figure 5).
3.6.Effect of GMEt on phosphoproteins in ER stress-induced EA.hy926 cells
In order to analyze the effect of TUN on upstream kinases and modulatory effect of GMEt in the induction of apoptosis, the levels of phosphoproteins were determined using Cell Signaling MAPK Panel.Exposure of the EA.hy926 cells to TUN led to a significant(P<0.05) increase in the levels of p-ERK1/2, p-JNK, p-MAPK,and p-MEK expression.No significant changes in the levels of phosphoproteins were observed in endothelial cells treated with GMEt alone except p-ERK1/2.GMEt treatment significantly decreased the expressions of all phosphoproteins induced by TUN(Figure 6).
4.Discussion
One of the early events in the development of various vascular diseases, including diabetes, hypertension, coronary and peripheral artery diseases is endothelial dysfunction[26,27].Numerous factors assault the functioning of endothelial cells including hyperglycemia,accumulation of advanced glycation end products, oxidative stress, production of vasodilators such as NO, endothelin, etc[4].All these factors affect ER homeostasis, leading to ER stress.Several clinical and preclinical studies emphasize the association of diabetes and ER stress, and this occupies the center stage in the progression of diabetes and its complications.Hence, restoring ER homeostasis appears to be a plausible therapeutic approach to impede the progression of diabetes.The current study was aimed at understanding the role of GMEt in TUN-induced ER stress in endothelial cells, and also to explore the possible molecular mechanism of GMEt.Currently, several treatment strategies are being explored to enhance endothelial function from various attacks.Over the past decade, attention was focused on the attenuation of ER stress using naturally available resources through a “mechanismbased approach” that can target various damages induced by oxidative, ER and inflammatory stress.
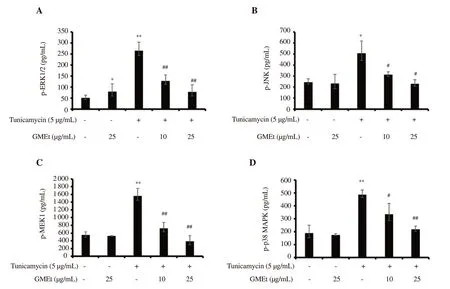
Figure 6.Effect of GMEt on upstream phospo-kinases including (A) p-ERK1/2 (B) p-JNK (C) p-MEK1 and (D) p-p38 MAPK against ER stress.Data are presented as mean ± SD of three independent experiments.Statistical analysis was performed by one-way ANOVA, followed by Tukey's post hoc test.*Significance compared with the untreated control; #Significance compared with the tunicamycin-exposed control; *&#P<0.05; **&##P<0.01.
It is well known that sustained or uncontrolled ER stress leads to inflammation, cell injury and apoptosis, resulting in endothelial dysfunction mediated vascular diseases in diabetes.The signaling cascade involves various molecules, including MAP kinases, caspases,chaperones, and ER stress markers viz., CHOP and GRP78, which play a critical role in ER stress-induced apoptosis[28,29].MAPKs primarily activate and trigger the expression of various cellular response proteins that regulate multiple functions; hence the degree of phosphorylation of MAP kinases including ERK, JNK, MEK, and p38 is important in TUN-induced ER stress[27,29].Our data is in line with Dai et al., who reported cell death mediated by activation of ERK during chronic ER stress[30].Activation of MAPK/ERK and MEK/ERK pathways by TUN that causes induction of GRP78 and in turn leads to cell death has been previously reported[31].Results obtained in the present study are consistent with these reports.According to the previous study, U0126-induced inhibition of MEK reduced the levels of GRP78 and blocked ER stress[31].The finding of the present study which showed GMEt treatment suppressed the levels of GRP78 induced by TUN is also in agreement with these findings.
It has been reported that, during ER stress, activation of JNK and p38 results in cell death[28].Hao et al.revealed that berberine treatment suppressed the levels of apoptosis markers such as JNK, caspase-3 under ER stress environment[32].TUN induced ER stress, in the present study, resulted in the upregulation of p-JNK and p-p38, however, GMEt treatment counteracted this effect by reducing the levels of p-JNK.Based on the aforementioned data, it can be ascertained that GMEt protects cells by modulating the MAP kinases during ER stress.
In vitro studies on endothelial cells revealed that GMEt protected endothelial cells from TUN-induced ER stress via the suppression of ER stress markers including GRP78 and CHOP.Few other studies also highlighted that plant extracts and their derivatives such as purple perilla extracts, bitter melon extract, propolis extract, and quercetin attenuate ER stress-induced damages[33,34].Our earlier in vivo studies on GMEt confirmed that administration of the extract maintains the glucose homeostasis, protects pancreatic beta cells and also attenuates oxidative stress-mediated disturbances in the pancreas[21,35,36].Few studies on extracts of Euphorbia pekinensis, Glychirrhiza uralensis, Magnolia officinalis, Pueraria lobata, etc., have proven their protective effects on the pancreatic beta-cell via suppression of ER stress markers[37].
To understand the molecular mechanisms involved in GMEt mediated protection, the expression of Nrf2 was analyzed.Nrf2 has been reported to be a promising therapeutic target for diabetes and its complication.GMEt treatment resulted in increased expression of Nrf2 in a dosedependent manner as assessed using enzyme split complementation assay.Furthermore, it also enhanced the expressions of ARE mediated downstream target genes including Nrf2, GST and NQO1.The activation of Nrf2 is critical for the protection of cells against various types of oxidative stress factors.These findings are consistent with results for Rubia philippinensis[38], Garcinia vilersiana[39], Psidium guajava pomífera L.[40], which reported improvement of the antioxidant status through Nrf2 activation.Increased expressions of Nrf2 target genes, such as NQO1, HO-1, GPX and SOD were observed upon GMEt treatment to endothelial cells.
We have previously reported the chemical constituents of leaves of G.montanum which contain 11.57% w/w of carvacrol, 6.77% of erythritol, 4.58% of gallic acid and 3.09% of quercetin[35,41].The presence of these constituents may be responsible for the beneficial activity of the extract.Moreover, many polyphenols present in plant extracts have been reported for their Nrf2 activation potential[10].The cytoprotective effect of G.montanum reported in the present study is due to the activation of Nrf2, thereby triggering its downstream targets including antioxidant and detoxifying enzymes.
In conclusion, our results demonstrate that G.montanum inhibited ER stress-induced endothelial apoptosis through the activation of the Nrf2 signaling pathway.Further studies will be required in different cell types and in animal models to explore the efficacy of G.montanum in the development and progression of conditions associated with ER stress.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors gratefully acknowledge the facilities provided by“SRMDBT Partnership Platform for Contemporary Research Services and Skill Development in Advanced Life Sciences Technologies” (BT/PR12987/INF/22/205/2015).
Funding
This study was supported by the Indian Council of Medical Research(grant no.59/57/2011/BMS/TRM), Government of India.
Authors' contributions
DS, NS, SC and KMR designed the work.DS and NS conducted the work, collected and analyzed the data.DS, NS, SC and KMR drafted the manuscript and revised it critically.All authors agree to be accountable for all aspects of the work.
 Asian Pacific Journal of Tropical Biomedicine2020年8期
Asian Pacific Journal of Tropical Biomedicine2020年8期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Vector-borne diseases: Mosquito holobiont and novel methods for vector control
- Peanut testa extracts enhance anticancer effect of cisplatin against human cholangiocarcinoma cells via modulation of histone deacetylase inhibitory activity
- Immunosuppressive and antibacterial activities of dihydromorin and norartocarpetin isolated from Artocarpus heterophyllus heartwoods
- Metabolite profiling and antidiabetic attributes of ultrasonicated leaf extracts of Conocarpus lancifolius
- Isolation and characterization of five novel mini-Mconotoxins from the venom of mollusk-hunter snail Conus bandanus
