Synthesis and Herbicidal Activity of Citral-based Thiosemicarbazone Compounds
HE Yun, DUAN Wengui*, LIN Guishan, CEN Bo, BU Junwen, LEI Fuhou
(1.School of Chemistry and Chemical Engineering,Guangxi University, Nanning 530004, China; 2.Guangxi Key Laboratory of Chemistry and Engineering of Forest Products, Nanning 530008, China)
Abstract: Twenty-six novel citral-based thiosemicarbazone compounds 2a-2m (thirteen pairs of (2Z, 1E)/(2E, 1E)-isomers) were designed and synthesized in an attempt to develop potent herbicidal agents. The structures of the target compounds were characterized by FT-IR, NMR, and ESI-MS. The herbicidal activities of the target compounds were preliminarily evaluated in vitro against Brassica campestris and Echinochloa crusgalli. The bioassay results indicated that compounds (2Z, 1E)-2a (R=Me), (2E, 1E)-2a (R=Me), (2Z, 1E)-2b (R=Et), (2E, 1E)-2b (R=Et), and (2E, 1E)-2c (R=i-Pr) displayed inhibition rates of 82.6%, 88.7%, 80.2%, 80.5% and 82.0%, respectively, against the root-growth of rape (Brassica campestris) at the concentration of 100 mg/L. They were much better herbicidal activity than the commercial herbicide flumioxazin. It was found that the herbicidal activity of the compounds with aliphatic groups (R) was much better than that of the compounds with aromatic groups (R), and the (2E, 1E)-isomers bearing aliphatic groups (R) exhibited better inhibition activity than corresponding (2Z, 1E)-isomers, whereas the (2Z, 1E)-isomers bearing aromatic groups (R) showed better inhibition activity than their (2E, 1E)-isomers. The electrostatic potential and the frontier molecular orbitals of the best activity compound (2E, 1E)-2a were calculated and analyzed. This implied that the thiourea moiety was critical for the bioactive performance and the small R group was favorable for bioactivity.
Key word: citral;thiosemicarbazone;stereoisomer;herbicidal activity
Citral, i. e., 3,7-dimethyl-2,6-octadienal, is a mixture of geranial (E-citral) and neral (Z-citral). It is mainly extracted from naturalLitseacubebaoil, lemongrass oil, andVerbenaoil[1-2]. Citral is commonly used as important raw materials in perfumes, foods, beverages, and soft drinks owing to its special lemon flavor[3]. And furthermore, citral demonstrated a great deal of biological activities such as antibacterial[4], antifungal[5], herbicidal[6], and antitumor[7]properties. In previous literatures, various citral derivatives were synthesized and proved to possess extensive biological activities[8]. For example, a series of novel 5,9-dimethyl- deca-2,4,8-trienoic acid amides were synthesized as efflux pump inhibitors (EPIs) againstStaphylococcusaureususing monoterpene citral as starting material[9]. Actually, individual citral isomer plays important roles in structural design, chemical synthesis, and bioactive study, due to the fundamental importance of several unsaturated groups. In recent years, the isomerization of citral aroused extensive research interests from biomedical[10]and chemical[11]fields, and a convenient method was developed to obtain geranial by acid-catalyzed kinetic resolution from citral[12]. However, highly efficient separation of geranial (E-) and neral (Z-) from citral remains a challenge to chemists. In addition, thiosemicarbazone showed a broad range of pharmacological activities, such as anticancer[13], antifungal[14], antibacterial[15], and antimalarial[16]activities. For example, a series of O-alkyl-O- [(2′-formylthiosemicarbazone) phenyl]-N-alkyl thiophosphonic amides were synthesized, and some of the compounds were found to exhibit good herbicidal activity[17].

1 Materials and Methods
1.1 Materials and instruments
Citral (GC purity 98%, isomeric mixture,m(neral) ∶m(geranial)=1 ∶1) was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Other reagents were purchased from commercial suppliers and used as received. All the plant pathogens were provided by the Bioactivity Test Room of the State Key Laboratory of Elemento-Organic Chemistry, Nankai University.
The GC analysis was conducted on an Agilent 6890 GC (Agilent Technologies Inc., Santa Clara, CA, USA) equipped with a HP-1 column (30 m×0.530 mm×0.88 μm) and an FID. IR spectra were recorded on a Nicolet iS50 FT-IR spectrometer (Thermo Scientific Co., Ltd., Madison, WI, USA) using the KBr pellet method. NMR spectra were recorded on a Bruker Avance III HD 600 MHz spectrometer (Bruker Co., Ltd., Zurich, Switzerland) using CDCl3as the solution. The chemical shifts are expressed inδdownfield relative to TMS as an internal standard. MS spectra were obtained by means of the electrospray ionization (ESI) method on TSQ Quantum Access MAX HPLC-MS instrument (Thermo Scientific Co., Ltd., Waltham, MA, USA). Melting points were determined on MP420 automatic melting point apparatus (Hanon Instruments Co., Ltd., Jinan, China) and the thermometer were not calibrated before use.
1.2 Synthesis of (2Z, 1E)- and (2E, 1E)-citral-based thiosemicarbazones (2a-2m)
As illustrated in Fig. 1, the substituted thiosemicarbazides were prepared by a two-step reaction involving the reaction of substituted isothiocyanates witht-Boc protected hydrazine and their deprotection[25]. Then, a series of novel citral-based thiosemicarbazones (2a-2m) were synthesized by the condensation reaction of citral with the substituted thiosemicarbazides. In a typical procedure: A solution of thiosemicarbazide (4 mmol) in anhydrous ethanol (10 mL) was added in small portions to the solution of citral (0.46 g, 3 mmol) in anhydrous ethanol (10 mL) containing five drops of glacial acetic acid under the protection of nitrogen. The reaction was refluxed for 2 h at 78 ℃[26]. Upon completion, the mixture was concentrated under reduced pressure, dissolved in ethyl acetate, washed with deionized water, and dried over MgSO4. The residue was filtered and purified by silica column chromatography using a mixed solvent (V(petroleum ether) ∶V(EtOAc)=10 ∶1) as eluent to afford (2Z, 1E)/(2E, 1E)-citral-based thiosemicarbazone compounds (2a-2m), as white solid, in about 45% yield for each isomer.
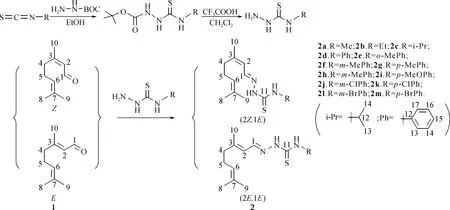
Fig.1 Synthesis of (2Z, 1E)- and (2E, 1E)-citral-based thiosemicarbazones 2a-2m
1.3 Herbicidal activity test
1.3.1Inhibition of the root-growth of rape (B.campestris) This test was carried out according to the literature[27]. The tested compounds were made into emulsions using Tween-80 as emulsifying agent to aid dissolution at the concentrations of 10 mg/L and 100 mg/L. Groups of 15 seeds of rape (B.campestris) were placed on a 5.6 cm filter paper that was in 6 cm Petri dishes containing 2 mL of compound solutions. Equal volume of distilled water was used as control. Petri dishes were placed in darkness at (28±1) ℃ for 72 h. The radicle lengths of seedlings were measured. All experiments had three replicates. The inhibition percent of average length to control was used to describe the activity of compounds. And the current commercial herbicide flumioxazin was used as a positive control.
1.3.2Inhibition of the seedling growth of barnyard grass (E.crusgalli) This test was performed according to the literature[27]. The compounds to be evaluated were made into emulsions by using TW-80 as emulsifying agent to aid dissolution, at concentrations of 10 mg/L and 100 mg/L. Groups of 10 ger minated seeds of barnyard grass (E.crusgalli) were placed on a filter paper that was in a 50 mL beaker containing 6 mL of compound solutions. Equal volume of distilled water was used as control. Beakers were placed at (28±1)℃ (3 000 lx) for 72 h. The heights of seedlings were measured. All experiments had three replicates. The inhibition percent of average height to control was used to describe the activity of compounds, and the current commercial herbicide flumioxazin was used as a positive control.
2 Results and Discussion
2.1 Characterization of target compounds
Compounds2a,2b,2c,2g, and2jwere characterized as examples, and the characterization data of the other compounds were shown in the auxiliary materials.

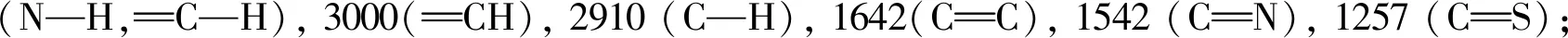




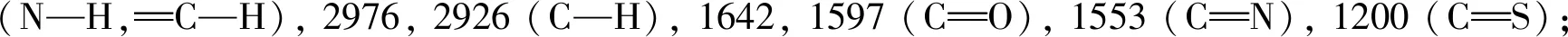

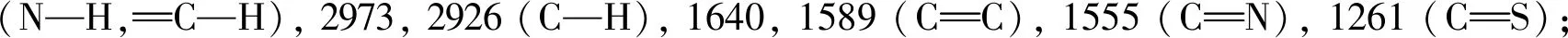



2.2 Herbicidal activity
The herbicidal activities of the target compounds2a-2mwere evaluated by the rape petri dish method and the barnyard grass beaker method against the root-growth of rape (B.campestris) and the seedling-growth of barnyard grass (E.crusgalli) at 10 mg/L and 100 mg/L, respectively[41]. Only the herbicidal data of compounds2a,2b,2c,2gand2jwere listed in Table 1, and the rest were shown in the auxiliary materials. It was found that, at 100 mg/L, eleven target compounds exhibited better herbicidal activity with 64.4%-88.7% inhibition rates than that of the commercial herbicidal flumioxazin (positive control) with inhibition rate of 63.0%, against the root-growth of rape (B.campestris). Among them, compounds (2Z, 1E)-2a(R=Me), (2E, 1E)-2a(R=Me), (2Z, 1E)-2b(R=Et), (2E, 1E)-2b(R=Et), and (2E, 1E)-2c(R=i-Pr) held growth inhibition rates of 82.6%, 88.7%, 80.2%, 80.5%, and 82.0%, respectively (A-class activity level). In general, the herbicidal activity was improved after the modification of citral, and the herbicidal activity of the compounds with aliphatic groups (R) was much better than that of the compounds with aromatic groups (R). Interestingly, it was also found that, against the root-growth of rape (B.campestris), the (2E, 1E)-isomers bearing aliphatic groups (R) exhibited better inhibition activity than corresponding (2Z, 1E)-isomers, whereas the (2Z, 1E)-isomers bearing aromatic groups (R) showed better inhibition activity than corresponding (2E, 1E)-isomers. However, the target compounds showed almost no inhibition activity against the seedling-growth of barnyard grass (E.crusgalli).
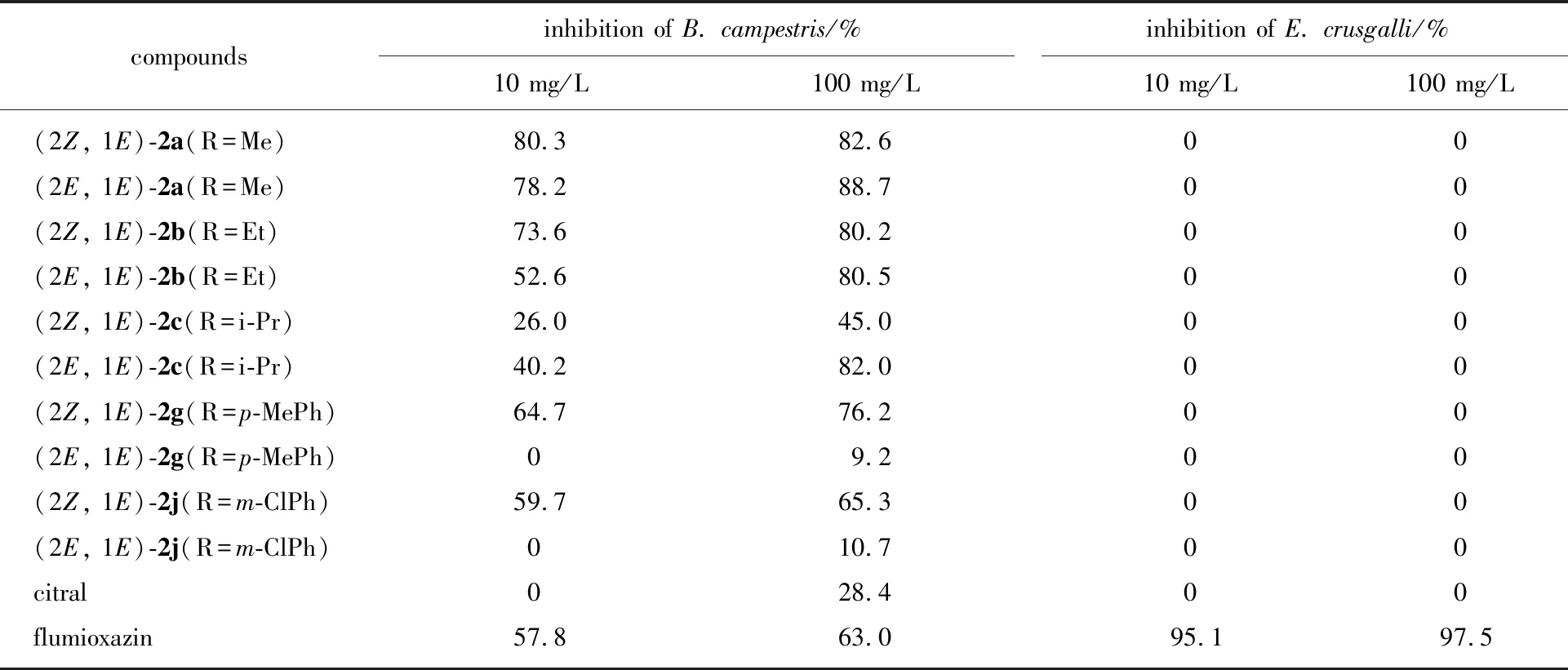
Table 1 Herbicidal activity of some of the target compounds at 10 mg/L and 100 mg/L1)
1)activity grade: A-class: ≥80%; B-class: 60%-79%; C-class: 40%-59%; D-class: ≤39%
2.3 Theoretical calculation and analysis
In view of the knowledge of an effective drug that must be complementary with receptor in both spatial structure and charge distribution[28], the electrostatic potential (ESP) and the frontier molecular orbitals of the best activity compound (2E, 1E)-2a(R=Me) were calculated by means of DFT/B3LYP/6-31G (d, p)[29]in Gaussian 09 package[30]on the Computer Supercomputing Platform at Guangxi University. And the result was viewed by Gauss View 5 software[31]. The molecular conformation was optimized before the calculation (Fig.2 (a)). As showed in Fig. 2 (b) for the ESP, the highest negative potential energy (showed in red) was located on the sulfur atom, and the highest positive potential energy (showed in blue) on the two nitrogen atoms in thiourea moiety. This implied that this sulfur atom contributed negative field, and these nitrogen atoms contributed positive sites. Their negative and positive positions were important for the interaction a drug with receptor by the means of week forces such as dipole-ion or dipole-dipole forces. Meanwhile, the HOMO and LUMO mainly distributed on the thiourea group (Fig. 2 (c) and (d)). This indicated that the thiourea group possessed the potential to contribute the interaction with receptor by the electronic transition. In summary, the thiourea moiety was the key for the bioactive performance.

Fig.2 The optimized conformation (a), ESP (b), HOMO (c), and LUMO (d) maps of the compound (2E, 1E)-2a
The influential factors to thiourea group included electronic effect and steric effect. Obviously, the steric effect showed more important, and the small bulk R group was favorable for bioactivity. The target compounds bearing small aliphatic R group had higher inhibition activity than that of big aromatic R group. And the closer to nitrogen atom the substituents in the aromatic R group, the weaker activity the corresponding compound showed.
3 Conclusions
Twenty-six novel (2Z, 1E)/(2E, 1E)-citral-based thiosemicarbazone compounds were synthesized and screened for their herbicidal bioassay. Compounds (2Z, 1E)-2a(R=Me), (2E, 1E)-2a(R=Me), (2Z, 1E)-2b(R=Et), (2E, 1E)-2b(R=Et), and (2E, 1E)-2c(R=i-Pr) displayed excellent inhibition rates of 82.6%, 88.7%, 80.2%, 80.5% and 82.0%, respectively, against the root-growth of rape (Brassicacampestris) at 100 mg/L. This showed that they had much higher herbicidal activity than the commercial herbicide flumioxazin (positive control). The herbicidal activity of the compounds with aliphatic groups (R) was much better than that of the compounds with aromatic groups (R). On the other hand, the (2E, 1E)-isomers bearing aliphatic groups (R) exhibited better herbicidal activity than the corresponding (2Z, 1E)-isomers, whereas the (2Z, 1E)-isomers bearing aromatic groups (R) showed higher herbicidal activity than the corresponding (2E, 1E)-isomers. The electrostatic potential and the frontier molecular orbitals of the best activity compound (2E, 1E)-2awere calculated and analyzed, implying that the thiourea moiety was critical for the bioactive performance and the small R group was favorable for bioactivity.
Auxiliarymaterialscould be found on the website of this journal(http:∥www.cifp.ac.cn).

