Poly(propylene carbonate)-based Polymer Electrolyte with an Organic Cathode for Stable All-Solid-State Sodium Batteries
Huifang Fei , Yongpeng Liu , Chuanliang Wei , Yuchan Zhang , Jinkui Feng ,*,Chuanzhong Chen ,*, Huijun Yu
1 SDU & Rice Joint Center for Carbon Nanomaterials, Key Laboratory for Liquid Solid Structural, Evolution & Processing of Materials (Ministry of Education), School of Materials Science and Engineering, Shandong University, Jinan 250061, P. R. China.
2 Shenzhen Research Institute of Shandong University, Shenzhen 518057, Guangdong Province, P. R. China.
3 Key Laboratory of High-efficiency and Clean Mechanical Manufacture (Ministry of Education), National Demonstration Center for Experimental Mechanical Engineering Education, School of Mechanical Engineering, Shandong University, Jinan 250061,P. R. China.
Abstract: Sodium-ion batteries (SIBs) are promising candidates to replace lithium-ion batteries (LIBs) to meet the emergent requirements of various commercial applications. SIBs and LIBs are similar in many aspects, including their reduction potentials, approximate energy densities, and ionic semidiameters. Analogously, safety issues, including liquid leakage, high flammability, and explosiveness limit the usage of SIBs. All-solid-state batteries have the potential to solve the aforementioned problems. However,polycarbonates as promising solid electrolytes have been rarely exploited in allsolid-state SIBs. In addition, organic electrode materials, including nonconjugated redox polymers, carbonyl compounds, organosulfur compounds,and layered compounds, have been intensively investigated as part of various energy storage systems owing to their low cost, environmental friendliness, high energy density, and structural diversity.Nevertheless, the dissolution of small organic compounds in organic-liquid electrolytes has hindered its further applications.Fortunately, the utilization of solid polymer electrolytes combined with organic electrode materials is a promising method to prevent dissolution into the electrolyte and improve the cycling performance of SIBs. Thus, we proposed the utilization of a poly(propylene carbonate) (PPC)-based solid polymer electrolyte and cellulose nonwoven with a 3,4,9,10-perylenetetracarboxylicacid-dianhydride (PTCDA) cathode in an all-solid-state sodium battery (ASSS). The solid electrolyte significantly enhanced the safety of the SIB and was successfully synthesized via a facile method. The morphology of the as-prepared solid electrolyte was examined by electron scanning microscopy (SEM). Furthermore, the electrochemical performances of the PTCDA/Na battery with organic-liquid and solid electrolytes at room temperature were compared. The SEM results demonstrated that the solid polymer electrolyte and sodium bis(fluorosulfonyl)imide (NaFSI) were evenly distributed inside the pores of the nonwoven cellulose. The ionic conductivity of the composite solid polymer electrolyte(CSPE) at room temperature was 3.01 × 10-5 S·cm-1, suggesting that the CSPE was a promising candidate for commercial applications. In addition, the ASSS showed significantly improved cycling performance at a current density of 50 mAh·g-1 with a high capacity retention of 99.1%, whereas the discharge capacity of the liquid PTCDA/Na battery was only 24.6mAh·g-1 after 50 cycles. This indicated that the cycling performance of the PTCDA cathode in the SIB was largely improved by preventing the dissolution of the PTCDA cathode material in the electrolyte. Electrochemical impedance spectroscopy results demonstrated that the CSPE was compatible with the organic cathode electrode.
Key Words: All-solid-state battery; Poly(propylene carbonate); 3,4,9,10-perylene-tetracarboxylicacid-dianhydride;Organic cathode; High safety
1 Introduction
With the intensive endeavor to develop various energy storage system, SIBs have attracted tremendous attention in the past decades so as to meet the urgent demand of commercial application1,2. Sodium and lithium display much resemblance when it comes to reduction potential, energy densities and ionic semidiameter3–7. What’s more, SIB has been a significantly promising alternative to lithium-ion battery (LIB) as well due to the plentiful sodium nature resource and low cost1,4. However,the traditional SIBs utilizing organic liquid electrolyte have encountered several severe safety issues like liquid leakage, high flammability and explosiveness, which will definitely hinder the further development of SIBs and is supposed to be settled in the near future8,9.
The new generation SIBs, called as ASSS making use of solidstate electrolyte, can totally get rid of flammable organic electrolyte and fundamentally solve the safety problems of batteries8,9. As the key component of ASSS, the solid electrolyte can be divided into two main categories, solid inorganic electrolyte and solid polymer electrolyte (SPE). Between them,the solid polymer electrolyte arouses great attention due to excellent flexibility, low chemical activity with electrodes, low cost and outstanding processability which is much more promising for large-scale commercial application10–13. For example, the superiority of poly(ethylene oxide)-based solid polymer electrolyte for ASSS has acquired widespread acknowledgement14. Nevertheless, the limited electrochemical window, poor mechanical property and low ionic conductivity of general SPE have impeded its farther development.
In an endeavor to exploit satisfactory solid polymer electrolyte, many outstanding research works have been done to enhance the integral properties of solid polymer electrolyte, like combining polymer and inorganic electrolyte, developing new polymer materials and improving the structure of existing polymer14–16. Among them, the polycarbonates demonstrate great preponderance for its high ionic conductivity at room temperature. Cui’s group proposed the poly(propylene carbonate)-based composite electrolyte which demonstrates a high ionic conductivity of 3.0 × 10-4S·cm-1at 20 °C17.However, the utilization of polycarbonates for ASSS is limited3.In addition, the advantages of the organic cathode that are ecofriendliness, low cost and structure diversity are widely acknowledged18–22. Nevertheless, the severe dissolution of the organic cathode in organic liquid electrolyte greatly hinders its application23,24.
2 Experimental section
2.1 Materials
Poly(propylene carbonate) (Mw= 50000) was supplied by Sigma-Aldrich. PTCDA (98%) and battery-grade NaFSI in this work were offered by J&K. Tetrahydrofuran (THF, anhydrous)and the filter paper (cellulose nonwoven) were commercially available. The organic liquid sodium-ion electrolyte (batterygrade) was bought from Nanjing Mojiesi Energy Technology Co. Ltd. (China), which is composed of 1 mol·L-1NaPF6in ethylene carbonate and diethyl carbonate (volume ratio, 1 : 1).
2.2 Preparation of the SPE
Firstly, 1 g PPC and 0.3 g NaFSI was added into 5 g THF. The mixture was drastically stirred at room temperature for 3 h. Then,several pre-heated cellulose nonwoven membranes with diameter of 16 mm were totally dispersed in aforementioned solution for 30 min. Finally, the as-prepared SPEs were heated in vacuum oven at 60 °C for 24 h to remove the liquid solvent.
2.3 Electrochemical test
The cathode electrode comprises 70% (mass fraction) PTCDA,20% Super P and 10% poly(vinylidene fluoride). All batteries were assembled in glove box with argon atmosphere (H2O and O2volume percent < 10-7). The PTCDA/Na battery with CSPE or organic liquid electrolyte was discharged and charged between 1.0 and 3.0 V at various current densities. The cyclic voltammogram (CV) test of PTCDA|CSPE|Na battery was carried out by electrochemical station at a scan rate of 0.1 mV·s-1and a potential range of 1.0–3.0 V. The EIS test of Na battery before or after cycling was also examined through the electrochemical station with a frequency range of 0.1–106Hz. In addition, the EIS of CSPE from 20 to 100 °C was tested by the electrochemical station at a frequency range of 1–106Hz. The ionic conductivity was calculated through the resistance (Rb), the thickness (L) and the area (S) of the CSPE membrane23.
2.4 Characterization
SU-70 field emission SEM was used to characterize the morphology of samples. The X-Ray Diffraction (XRD) patter analysis of PTCDA was carried out on a Rigaku Dmaxrc diffractometer from 5° to 40° at a scan rate of 10 (°)·min-1.
3 Results and discussion
The crystal structure and morphology of PTCDA cathode material are displayed in Fig. 1. As shown in Fig. 1a, the XRD pattern of the analyzed PTCDA reveals that the five main 2θpeaks at 9.39°, 12.33°, 24.7°, 27.46° and 27.83° are in line with the characteristic peaks for (011), (021), (042), (102) and (112)planes respectively and other weak peaks are also in great accordance with previous literature. In addition, the sharp peaks verify the high crystallinity of PTCDA cathode material. Fig. 1b exhibits the general morphology of PTCDA, suggesting that PTCDA are small distributed clusters that comprises a few bars of the same direction. The length of the rods varies from several hundred nanometers to several micrometers.
The morphology of bare cellulose nonwoven and CSPE is vividly shown in Fig. 2. As revealedviaSEM in Fig. 2a, the cellulose nonwoven membrane is composed of randomly oriented micrometer-scale fibers. These fibers tangle and interlock with each other, forming a three-dimension and tough framework which will offer adequate mechanical strength to support the solid polymer electrolyte. In addition, the intercommunicating pores inside these fibers can carry sufficient SPE. Fig. 2b shows the surface morphology of CSPE and the inset picture is the cross-section morphology. As it exhibits in the pictures, the solid polymer electrolyte totally penetrated into the framework of cellulose nonwoven and fulfilled the interconnecting pores. Furthermore, the EDS mapping was carried out to identify the chemical composition of CSPE. Fig.2c reveals the element distribution of the selected area, which implies the even distribution of C, O, S, N and F element. The S,N and F element belong to NaFSI, indicating the existence and uniform distribution of Na salt. To further understand the characteristics of CSPE, the ionic conductivity of CSPE is examined. Fig. 3 shows that the ionic conductivity of CSPE at room temperature (20 °C) is 3.01 × 10-5S·cm-1and the ionic conductivity at 100 °C reaches 1.72 × 10-4S·cm-1. Furthermore,the temperature dependency of the ionic conductivity is in well accordance with the Arrhenius equation23.
To examine the utilization of the as-prepared CSPE in PTCDA/Na battery, the electrochemical performance was tested.The schematics in Fig. 4 propose inserting/stripping mechanism of Na+in PTCDA cathode in accordance with previous literature.For the verification of the inserting/stripping behavior of Na+,the CV measurement was carried out from 1.0 to 3.0 V at a scan rate of 0.1 mV·s-1. As exhibited in Fig. 5a, the CV curves present two pairs of redox peaks which are located at 2.18 and 2.46 V for the anodic scan and 1.98 and 2.16 V for the cathodic scan. At the end of the anodic scan, two Na+had reacted with the carbonyl groups of PTCDA and the final production, Na2PTCDA, formed.In turn, two Na+extracted from the cathode electrode during the cathodic scanning process. For further investigation of the extraction/intercalation of sodium-ion in PTCDA cathode, Fig.5b displays the discharge/charge curves from 1.0 to 3.0 V at a current density of 50 mA·g-1. In the discharging process, there are two apparent working plateaus located at about 2.2 and 2.0 V, respectively. In addition, two slope plateaus at approximate 2.5 and 2.2 V were identified. The results of the charge-discharge curves agree with CV test.
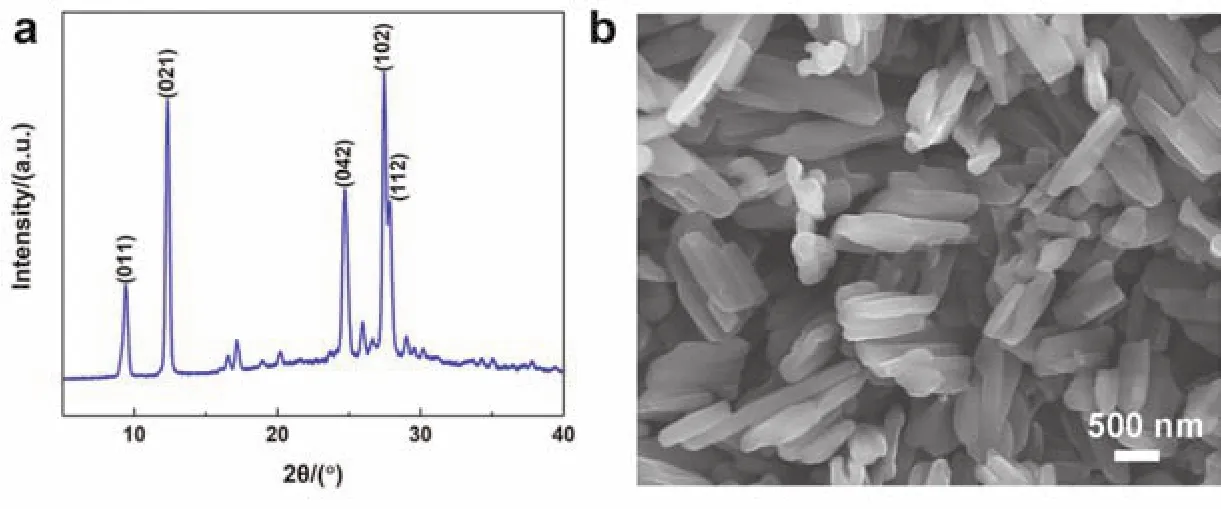
Fig. 1 (a) XRD pattern and (b) SEM image of PTCDA cathode material.
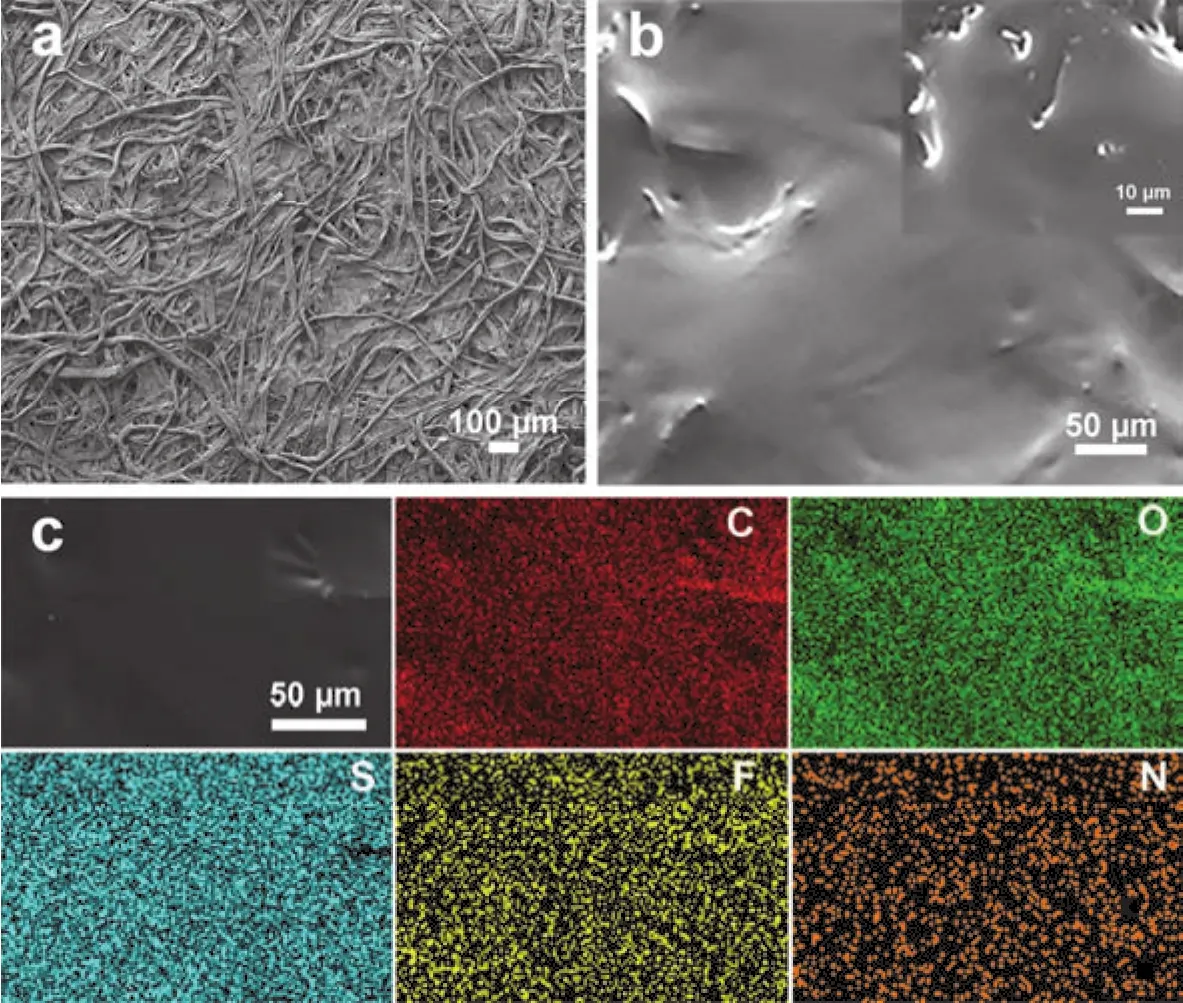
Fig. 2 Physical characterization of membranes.
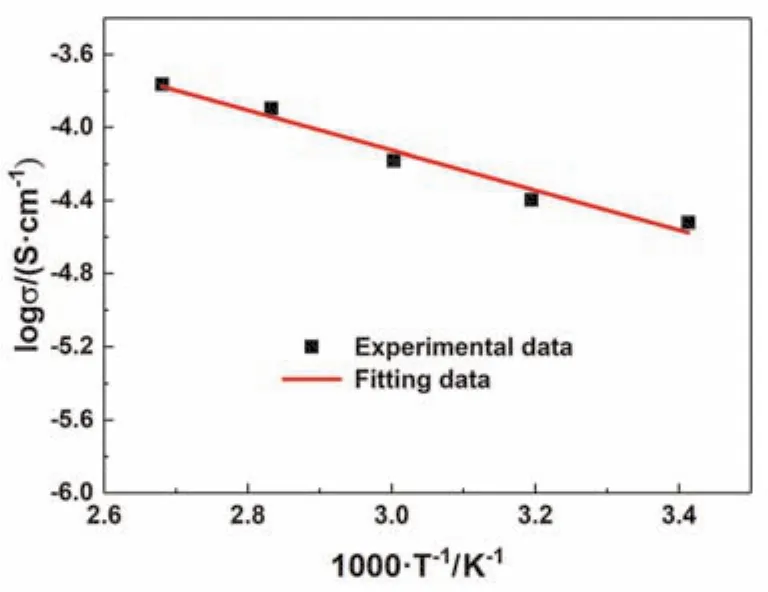
Fig. 3 The ionic conductivity of CSPE from 20 to 100 °C.
Fig. 5c shows the comparison of cycling performance with CSPE and organic-liquid electrolyte at room temperature. After activation of four cycles at a current density of 20 mA·g-1, the PTCDA|CSPE|Na battery shows a much stable cycling performance. For the 1st activation cycle, the discharge capacity of the solid-state battery is 125.6 mAh·g-1which is quite close to 132 mAh·g-1of the liquid battery. Afterwards, when the current density increased to 50 mA·g-1, the discharge capacity of the PTCDA|CSPE|Na battery descends to 76.5 mAh·g-1.However, the capacity of the liquid battery only decreased a little. With larger current density, the abrupt reduction of discharge capacity of PTCDA|CSPE|Na battery may result from the comparatively lower ionic conductivity of CSPE when compared to that of the organic-liquid electrolyte. However,after the activation, the PTCDA|CSPE|Na battery exhibits a quite stable cycling performance and keeps a high capacity retention of 99.1% for 50 cycles at a current density of 50 mA·g-1,demonstrating the good compatibility of the organic cathode with the solid polymer electrolyte. Furthermore, the battery with the organic-liquid electrolyte suffered from a severe capacity loss which arouse from the solubility of the organic cathode material in the organic-liquid electrolyte. The rate performance in Fig. 5d of PTCDA|CSPE|Na battery is also passable. As shown in Fig. 5d, the discharge capacities of the solid-state battery are 125.4, 93.7, 69.2 and 56.8 mAh·g-1with current densities of 20, 40, 60 and 80 mA·g-1, respectively. In addition,when the current density gets back to 20 mA·g-1, the discharge capacity is 115.0 mAh·g-1, demonstrating the excellent reversible cycling performance.

Fig. 4 The schematics of the proposed inserting/stripping mechanism of Na+ in PTCDA cathode.
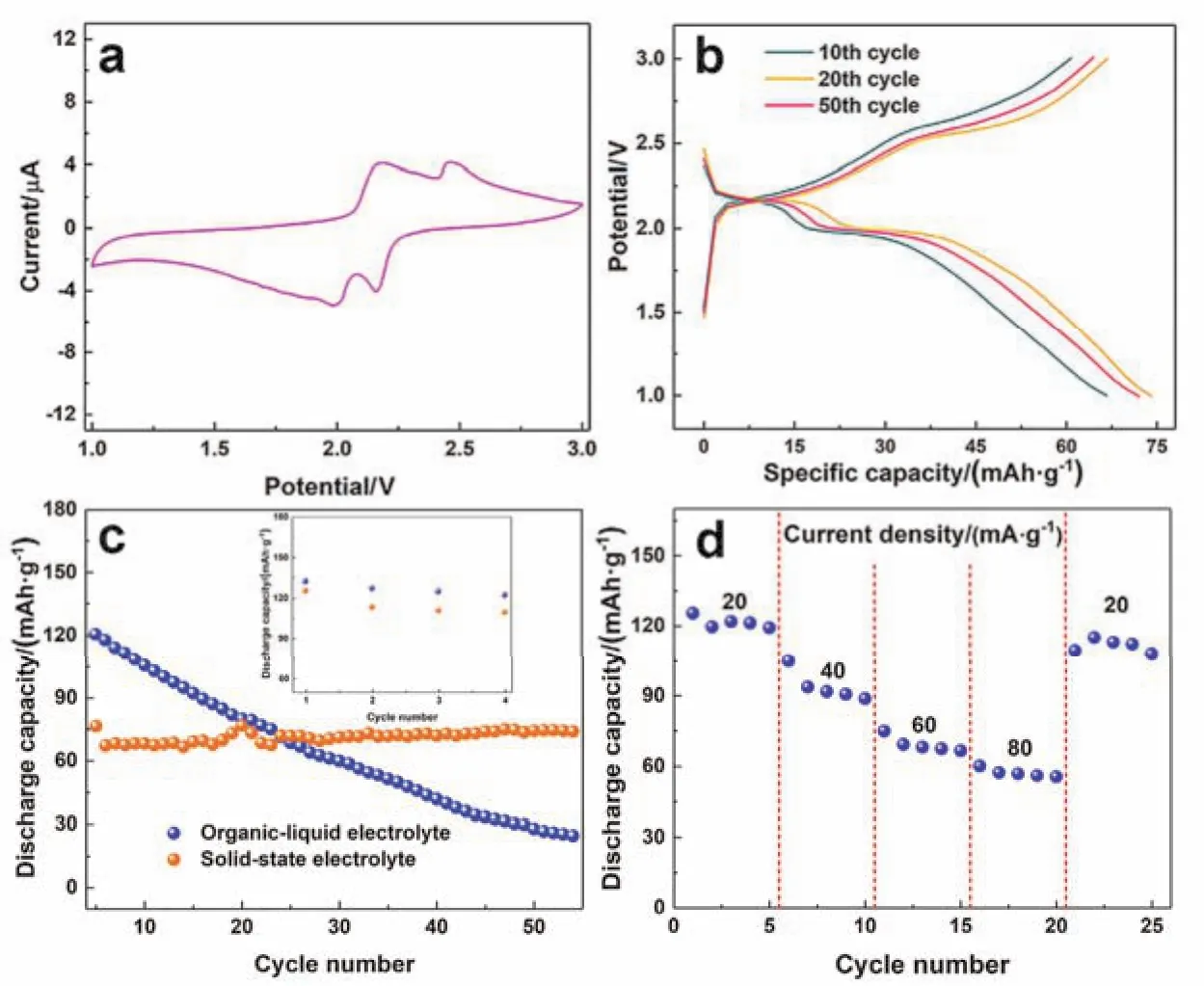
Fig. 5 Electrochemical performance of PTCDA/Na battery with CSPE or organic-liquid electrolyte.

Fig. 6 (a) EIS spectrum of the solid-state cell before cycling and after cycling for 50 cycles at 50 mA·g-1.(b) The digital image of 0.1 g PTCDA powder dissolving in 10 mL organic-liquid electrolyte.
To further understand the better electrochemical performance of PTCDA|CSPE|Na battery, the EIS measurement of the cell before cycling and after cycling for 50 cycles at 50 mA·g-1was performed as demonstrated in Fig. 6a. As Fig. 6a shows, the typical Nyquist plots comprise a compressed semicircle at high and middle frequencies and an oblique line at low frequencies.The sloping line is associated with the diffusion of Na-ion in the electrode materials while the intercept of the semicircle with the horizontal axis ascribes to the total impedance of the cell,containing interfacial impedance and charge-transfer impedance. Note that the resistance of the solid-state cell after cycling for 50 cycles at a current density of 50 mA·g-1is 3.3 kΩ which is smaller than that (4.3 kΩ) of the cell before cycling. The decreased impedance after cycling demonstrates the good compatibility of CSPE with the electrodes which may guarantee the passable electrochemical performance. What’s more, Fig. 6b displays the digital image of 0.1 g PTCDA powder dissolving in 10 mL organic-liquid electrolyte, implying that the organic compound, PTCDA, has high solubility in the organic-liquid electrolyte. This phenomenon well explains the poor cycling performance of PTCDA/Na battery with the organic-liquid electrolyte and indirectly verifies that the CSPE can prevent PTCDA from dropping off the cathode electrode.
4 Conclusions
In summary, the PPC-based CSPE has been successfully synthesizedviafacial method and used in all-solid-state sodiumion battery, which significantly enhances the safety of sodium battery. In addition, the cycling performance of solid-state cell at room temperature is greatly improved by prohibiting the dissolution of PTCDA cathode material in the organic-liquid electrolyte and good compatibility of CSPE with the organic electrodes.

