Sesamum indicum (sesame) enhances NK anti-cancer activity, modulates Th1/Th2 balance, and suppresses macrophage inflammatory response
Amin F.Majdalawieh, Jenna F.Farraj, Ronald I.Carr
1Department of Biology, Chemistry, and Environmental Sciences, Faculty of Arts and Sciences, American University of Sharjah, Sharjah, P.O.Box 26666, United Arab Emirates
2Department of Microbiology and Immunology, Faculty of Medicine, Sir Charles Tupper Medical Building, Dalhousie University, Halifax, Nova Scotia, Canada B3H 4R2
ABSTRACT
KEYWORDS: Sesamum indicum; Anti-cancer; Inflammation;Immunomodulation; Macrophages; NK cells
1.Introduction
Historically, plants have been incorporated in natural remedies for the treatment of a series of diseases due to their potent therapeutic effects[1].Various plants and their derivatives have been traditionally used in diets and food products because of their well-known health benefits and nutritional value[1].Following the advancement in evidence-based research practices, many plant-derived constituents have been shown to exert immunomodulatory effects, which play an important role in the development of modern medicine[2].Recently, the use of natural products has regained great interest as opposed to the use of chemically-synthesized drugs to treat various medical conditions.While some natural products have been found to enhance several immune responses, others have been shown to suppress various immunological processes, striking a balance in terms of overall immunity.
Sesame seeds are obtained from the herbaceous flowering plantSesamum indicum(S.indicum), a traditional oilseed crop belonging to the Pedaliaceae family and theSesamumgenus.S.indicumis widely grown and cultivated in Africa, India, China, and South America[3].S.indicumthrives in subtropical and tropical climates but is capable of adapting to harsh environments as well.It could be cultivated in a wide range of soil conditions where many plants fail to survive, making it an easily accessible crop.It is considered one of the most important and oldest oilseed crops, cultivated on millions of hectares of cropland with an annual production of several million tons ofS.indicumseeds.This makes it the “queen of oilseeds”[4].S.indicumis known to promote nutrition because of its balanced amino acid composition and its high source of essential macromolecules including unsaturated fatty acids, proteins,carbohydrates, oils, and vitamins[5].
Despite its significance in traditional natural medicine, studies performed on extracts ofS.indicumseeds and their constituents are relatively scarce, and the mechanisms by which they exert their immunomodulatory effects are not fully understood.Yet, severalin vitroandin vivostudies have demonstrated anti-cancer, antioxidant, anti-microbial, and anti-inflammatory effects ofS.indicumconstituents on various immune cells.Sesamin, sesamol, sesamolin,and sesaminol are the main active lignans found inS.indicumand are valuable for combating a wide range of health problems.Sesamin was shown to exert cholesterol-lowering activity[6],suppress inflammatory reactions[7], enhance macrophage cholesterol efflux[8], and show anti-cancer activity[9].It was found that sesamolin enhanced natural killer (NK) cell lysis against Burkitt’s lymphoma cells[10].Other studies demonstrated that sesame oil enhances macrophage cholesterol efflux[11], decreases plasma cholesterol levels, reduces hypertension, and alleviates diseases related to lipid metabolism because of its high content of polyunsaturated fatty acids[12].FermentedS.indicumseeds have been shown to exert potent anti-allergic effects in HaCaT cells[13].
Cancer immunity refers to the potential of various immune cells to selectively target and kill cancer cells without affecting normal and healthy cells[14].The transformation of healthy cells into cancer cells is accompanied by the expression of novel cell surface markers and aberrant expression of normal cellular proteins[14],which is advantageous for the organism as cancer cells become easily recognizable by specific immune cells that identify the novel and aberrantly expressed proteins.Cancer immunotherapeutic approaches focus on boosting the ability of immune cells to selectively recognize, target, and kill cancer cells[14,15].This involves the use of artificial immune cells, antibodies, or other immunological components to help eradicate cancer cells[14,15].Usually, cancer immunotherapeutic methods target specific markers expressed on the surface of cancer cells such as tumor-associated antigens using monoclonal antibodies[15].Among the prime examples of successfully employed monoclonal antibodies are anti-CD20(rituximab), anti-CD52 (alemtuzumab), anti-CD137 (urelumab and utomilumab/PF-05082566), anti-CTLA4 (ipilimumab), and anti-PD1 (nivolumab)[15].
While there is considerable data regardingS.indicumand its respective lignans, the number of studies related to its immunomodulatory effects remains limited.In this study, an aqueous extract ofS.indicumseeds was investigated in light of its ability to modulate key immunological processes related to splenocyte proliferation, Th1/Th2 balance, inflammatory effects of macrophages, and anti-cancer effects of NK cells.
2.Materials and methods
2.1.Materials and reagents
Brewer’s thioglycollate broth was purchased from DIFCO (Detroit,USA).Concanavalin A (ConA) and lipopolysaccharide (LPS) were purchased from Sigma-Aldrich (St.Louis, USA).Fetal bovine serum(FBS), RPMI-1640 media,L-glutamine, and penicillin-streptomycin antibiotic cocktail were purchased from Invitrogen (Ontario,Canada).IFNγ was purchased from Pepro-Tech (Rocky Hill,USA).[3H]-thymidine was purchased from Amersham Biosciences(Buckinghamshire, England).The fiberglass filter paper was purchased from Skatron Instruments (Lier, Norway).Scintillation fluid was purchased from Beckman/PerkinElmer (Ohio, USA).IBD OptEIATMELISA kits were obtained from BD Pharmingen (Ontario,Canada).Nalgene 0.22 μm filters were purchased from Thermo Fisher Scientific (Rochester, USA).YAC-1 tumor cells (mouse lymphoma cells) were obtained from ATCC (Rockville, USA).
2.2.Mice
BALB/c and C57BL/6 mice (age-matched, 6-8 weeks old) were obtained from Jackson Labs (Bar Harbor, USA).Mice were fed chow diet and housed on a 12 h light/dark cycle at the Carleton Animal Care Facility in Dalhousie University.Splenocytes and peritoneal macrophages were isolated after sacrificing mice by euthanasia using an overdose of sodium pentobarbital (Somnitol) as per the approved guidelines.
2.3.Ethical statement
All animal experiments were performed according to procedures(protocol number 09-112) approved by the institutional/ethical Animal Care Committee at the Carleton Animal Care Facility at Dalhousie University (Halifax, NS, Canada) [July 2010].All guidelines were followed as clearly defined by the Canadian Council on Animal Care (CCAC).
2.4.Preparation of S.indicum aqueous extract
S.indicumseeds were obtained from a local grocery market in Halifax (Nova Scotia, Canada).The seeds were washed three times with phosphate buffered saline (PBS) and left to air dry.Afterwards,the dried seeds were ground in liquid nitrogen using pestle and mortar.Subsequently, 10 mL ddH2O was added to the 20 g ground seeds and stirred overnight to allow extraction.The crude extract was centrifuged for 15 min at 10 000×gat room temperature.The supernatant was subjected to evaporation using a rotatory evaporator.Upon complete evaporation, a stock concentration of 20 mg/mL was prepared using ddH2O and the aqueous extract was filter-sterilized using Nalgene 0.22 μm filters.
2.5.Isolation and culture of splenocytes
Splenocytes were isolated from BALB/c and C57BL/6 mice and cultured as previously described[16].Briefly, spleens were cut into small pieces and gently squashed.For further dispersion of clumped tissue, the cell suspension was passed through a 19-G needle and further filtered through a 200-μm nylon mesh patch.The cell suspension was then subjected to centrifugation for cell collection.ACK lysis buffer (0.15 mol/L NH4Cl, 1 mM KHCO3, and 0.1 mM Na2EDTA) was used to lyse erythrocytes.The isolated splenocytes were washed three times and subsequently cultured in RPMI-1640 medium in the presence of 10% heat-inactivated FBS, 1% penicillinstreptomycin antibiotic cocktail, 50 μM β-mercaptoethanol, and 10 mM HEPES.Cell counting by trypan blue exclusion confirmed>98% cell viability.
2.6.Splenocyte proliferation assay
Splenocyte proliferation assay was performed as previously described[17].Briefly, 2×105splenocytes were cultured for 48 and 72 h in the presence of the vehicle, 1 μg/mL ConA, 10 ng/mL LPS,or various doses ofS.indicumextract.After treatment, splenocytes were labeled with [3H]-thymidine (1 μCi/well) for 16 h and subsequently harvested using a multi-well pipettor.Splenocytes were then lysed and the cell lysates were transferred onto small pieces of filter paper.After drying, the filter paper pieces were transferred to vials containing 1.5 mL scintillation fluid, and [3H]-thymidine incorporation was assessed using a scintillation counter (LKB Wallac, Finland).
2.7.Isolation and culture of peritoneal macrophages
Peritoneal macrophages were obtained from BALB/c as previously described[18].Briefly, mice were injected intraperitoneally with 3 mL sterile 3% Brewer’s thioglycollate medium.After 5 d, mice were sacrificed and peritoneal lavage was performed to obtain peritoneal cells, which were then subjected to centrifugation.ACK lysis buffer(0.15 M NH4Cl, 1 mM KHCO3, and 0.1 mM Na2EDTA) was used to lyse erythrocytes.After centrifugation, the isolated macrophages were washed three times and subsequently cultured in RPMI-1640 medium in the presence of 10% heat-inactivated FBS, 1% penicillinstreptomycin antibiotic cocktail, 50 μM β-mercaptoethanol, and 10 mM HEPES.Cell counting by trypan blue exclusion confirmed>98% cell viability.
2.8.Assessment of nitric oxide (NO) production by macrophages (Griess assay)
Griess assay was performed to assess NO production by macrophages as previously outlined[19].Briefly, 2×105peritoneal macrophages were cultured in the presence of the vehicle, 10 ng/mL LPS, 2 U/mL IFNγ, a combination of LPS plus IFNγ, or various doses ofS.indicumextract with or without IFNγ and/or LPS for 48 h.Next, 100 μL supernatant samples and serial dilutions of NaNO2standard solution were transferred into 96-well plates.Griess reagent(0.1% naphthyl ethylenediamine dihydrochloride, 1% sulfanilamide,2.5% H3PO4) was added to the samples.Using Emax Precision Microplate Reader (Molecular Devices, San Jose, USA), the optical density was determined at 550 nm.A standard curve was used to determine the amount of accumulated nitrite.
2.9.Analysis of cytokine secretion by ELISA
To evaluate the secretion of IL-4, IL-10, and IFNγ by splenocytes,2×105splenocytes were treated with vehicle, 10 ng/mL LPS, 1 μg/mL ConA, or various doses ofS.indicumextract in the presence or absence of 1 μg/mL ConA for 48 h.To evaluate the release of TNFα and IL-6 by macrophages, 2×105peritoneal macrophages were treated with vehicle, 10 ng/mL LPS, 2 U/mL IFNγ, a combination of LPS plus IFNγ, or various doses ofS.indicumextract in the presence or absence of LPS and IFNγ for 12 and 48 h (TNFα and IL-6, respectively).Supernatants were collected, and cytokine concentration was assessed using BD OptEIATMELISA kits and Emax Precision Microplate Reader (Molecular Devices, San Jose,USA).
2.10.Assessment of NK cytotoxic activity (JAM assay)
NK cytotoxic activity was assessed by JAM assay as previously described[20].In brief, YAC-1 tumor cells were cultured for 4 h in RPMI-1640 medium in the presence of 5 μCi/mL [3H]-thymidine.After washing, [3H]-thymidine-labeled YAC-1 tumor cells were cultured in 96-well culture plates (V-bottom) in the presence or absence of C57BL/6-derived splenocytes (containing NK cells) at three ratios of effector:target (E:T) (200:1, 100:1, and 50:1).Cocultured YAC-1 tumor cells were treated with vehicle or various doses ofS.indicumextract.After 4 h, YAC-1 tumor cells were collected using a multi-well pipettor.[3H]-thymidine radioactivity was measured using a scintillation counter (LKB Wallac, Finland).% Cytotoxicity was determined as per the following formula: %cytotoxicity = [(vehicle-treated YAC-1 cells- targeted-YAC-1 cells)/vehicle-treated YAC-1 cells] × 100.
2.11.Statistical analysis
Statistical analysis was performed using Prism (GraphPad) software(version 5.01).Data are expressed as mean ± SEM.The error bars in the figures represent SEM values.Statistical significance for unpaired observations was determined using the student’st-test.Statistical significance was set atP< 0.05.
3.Results
3.1.S.indicum increases splenocyte proliferation
The potential ability ofS.indicumextract to modulate splenocyte proliferation was examined.BALB/c splenocytes were cultured in the presence of the vehicle, ConA (T lymphocyte mitogen), LPS(B lymphocyte mitogen), orS.indicumextract at 1, 10, 50, and 100 μg/mL for 48 and 72 h.Subsequently,in vitroproliferation assays were conducted on cultured splenocytes using [3H]-thymidine incorporation.As shown in Figure 1A,S.indicumextract at all doses significantly increased splenocyte proliferation in a dose-dependent manner 72 h post-treatment, with the 100 μg/mL dose showing the best result (~19 folds) (Figure 1A).Although all doses led to a significant increase in splenocyte proliferation 48 h post-treatment,the effect was less pronounced compared to the 72 h treatment (data not shown).
To test the possibility that the stimulatory effects ofS.indicumextract on splenocyte proliferation may be due to LPS contamination,splenocyte proliferation was examined in the presence of a potent inhibitor of LPS, polymyxin B.Specifically, BALB/c-derived splenocytes were cultured in the presence of the highest dose(100 μg/mL) ofS.indicumextract, LPS and vehicle with or without 1 μg/mL polymyxin B for 72 h, andin vitrosplenocyte proliferation assay was performed.As expected, polymyxin B led to a significant reduction in LPS ability to stimulate splenocyte proliferation.However, polymyxin B had no noticeable inhibitory effect on the potential ofS.indicumextract to induce splenocyte proliferation (Figure 1B).Thus, the significant increase in splenocyte proliferation caused byS.indicumextract cannot be attributed to LPS contamination.
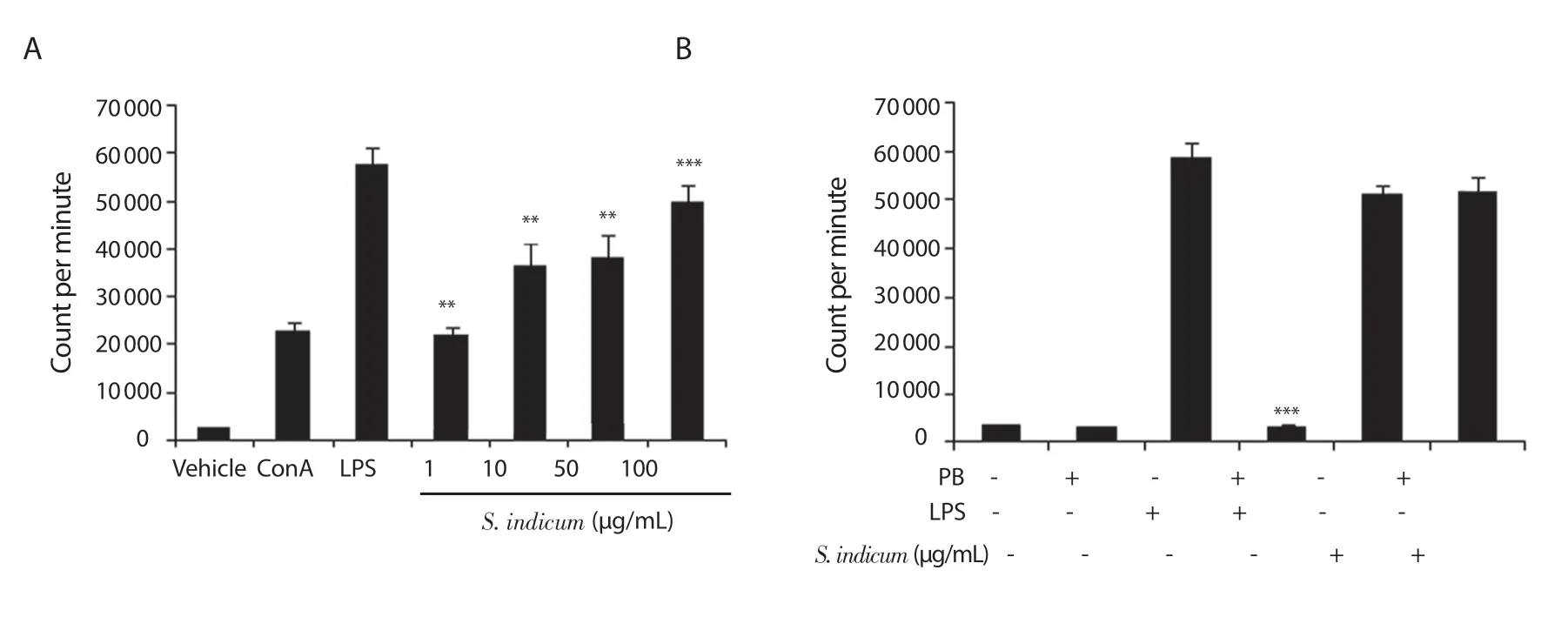
Figure 1.(A) Stimulation of splenocyte proliferation by Sesamum indicum (S.indicum) extract (1-100 μg/mL) (72 h treatment).Statistical significance was determined in comparison to vehicle-treated splenocytes (n = 6); (B) Stimulation of splenocyte proliferation by S.indicum extract (100 μg/mL) (72 h treatment)in the presence of polymyxin B (PB).For each sample where PB was used, statistical significance was determined in comparison to the corresponding sample without PB treatment (n = 6).Data are expressed as mean ± SEM.**P < 0.01 and ***P < 0.001.The error bars represent SEM values.ConA: concanavalin A,LPS: lipopolysaccharide.
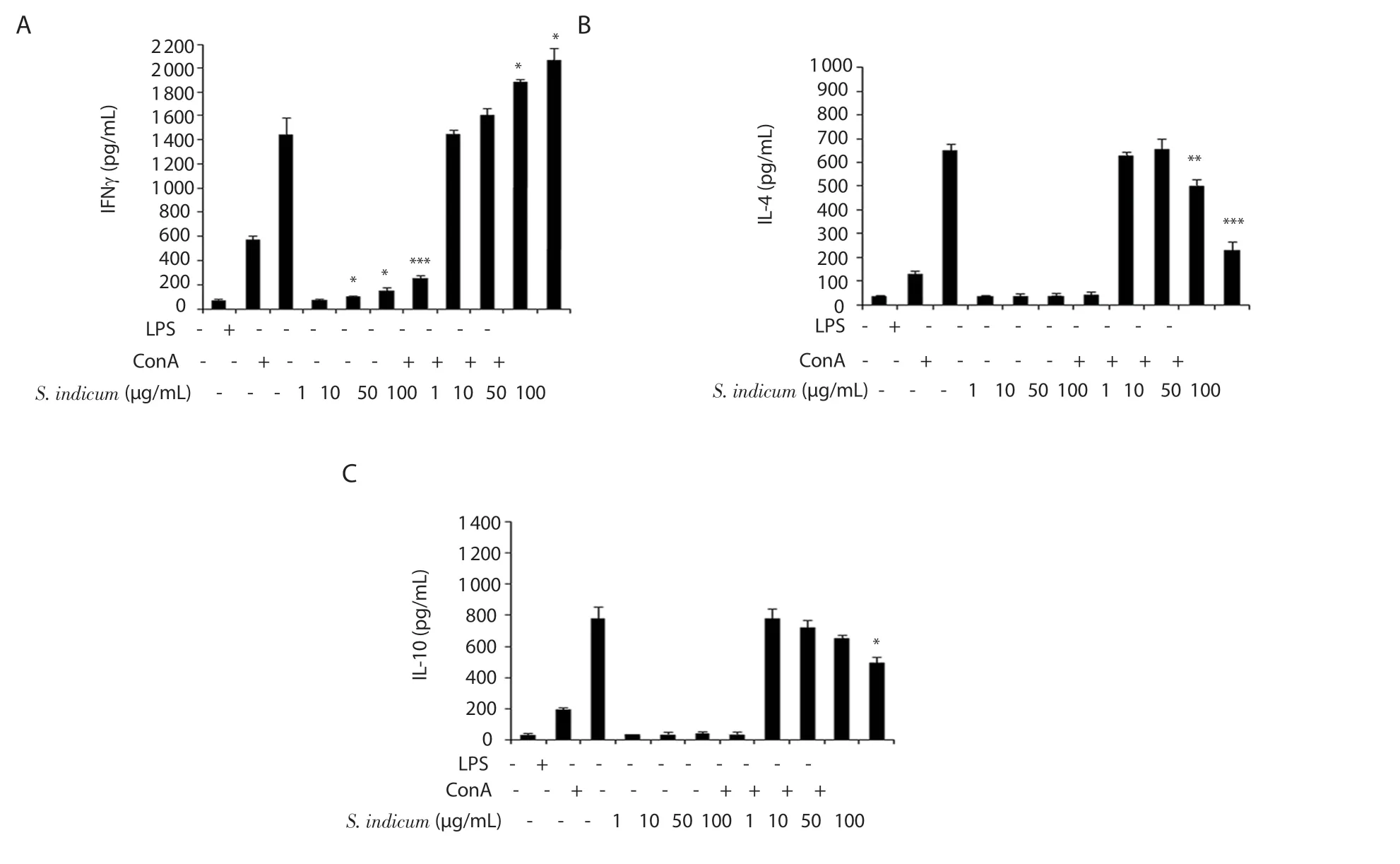
Figure 2.Modulatory effects of S.indicum extract (1-100 μg/mL) on the secretion of IFNγ (A), IL-4 (B), and IL-10 (C) by splenocytes assessed by ELISA.For S.indicum extract-treated splenocytes that were cultured in the absence of ConA, statistical significance was determined in comparison to vehicle-treated splenocytes.For S.indicum extract-treated splenocytes that were cultured in the presence of ConA, statistical significance was determined in comparison to ConA-treated splenocytes (n = 6).Data are expressed as mean ± SEM.*P < 0.05, **P < 0.01 and ***P < 0.001.The error bars represent SEM values.
3.2.S.indicum increases Th1 cytokine profile and suppresses Th2 cytokine profile
We examined the ability ofS.indicumextract to regulate Th1 and Th2 immune responses by assessing the release of Th1 and Th2 cytokines from splenocytes.BALB/c-derived splenocytes were cultured in the presence of vehicle, LPS, ConA, orS.indicumextract at four doses (1, 10, 50, and 100 μg/mL) with or without ConA.The secretion of IFNγ (Th1 cytokine) as well as IL-4 and IL-10 (Th2 cytokines) was assessed by ELISA.Our findings demonstrate thatS.indicumextract had no significant effect on IFNγ secretion by splenocytes at the lowest dose (1 μg/mL) in comparison to vehicletreated splenocytes in the absence of ConA (Figure 2A).However,IFNγ level was significantly elevated at the three higher doses (10,50, and 100 μg/mL) ofS.indicumextract without ConA (Figure 2A).Interestingly, in the presence of ConA,S.indicumextract caused a dose-dependent increase in IFNγ level (Figure 2A).ConA-stimulated splenocytes exhibited ~1.3-fold and ~1.4-fold significant elevation in IFNγ level at 50 μg/mL and 100 μg/mL doses of the extract, respectively (Figure 2A).As shown in Figure 2B and C,S.indicumextract had no significant effect on IL-4 and IL-10 secretion at any dose in the absence of ConA.Nevertheless, at the two higher doses (50 and 100 μg/mL) ofS.indicumextract, and in the presence of ConA, IL-4 level was significantly reduced (Figure 2B).Moreover, IL-10 level was significantly reduced at the highest dose(100 μg/mL) ofS.indicumextract in ConA-stimulated splenocytes(Figure 2C).Collectively,S.indicumextract increased Th1 cytokine profile, while suppressing Th2 cytokine profile, in ConA-stimulated splenocytes.
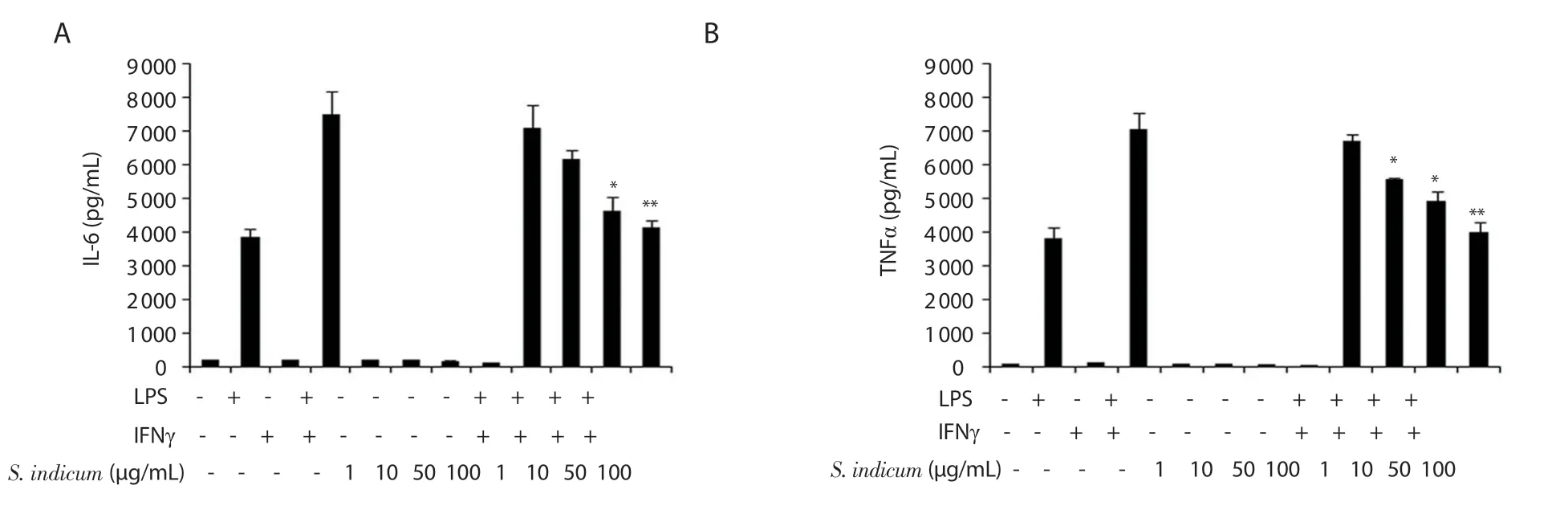
Figure 3.Suppression of IL-6 (A) and TNFα (B) secretion by peritoneal macrophages by S.indicum extract (1-100 μg/mL) assessed by ELISA.For S.indicum extract-treated macrophages that were cultured in the absence of LPS plus IFNγ, statistical significance was determined in comparison to vehicle-treated macrophages.For S.indicum extract-treated macrophages that were cultured in the presence of LPS plus IFNγ, statistical significance was determined in comparison to LPS- and IFNγ-treated macrophages (n = 6).Data are expressed as mean ± SEM.*P < 0.05 and **P < 0.01.The error bars represent SEM values.
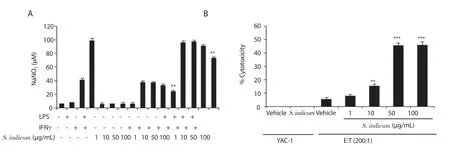
Figure 4.(A) Inhibitory effect of S.indicum extract (1-100 μg/mL) on nitric oxide production by peritoneal macrophages assessed by Griess Assay.Statistical significance was determined in comparison to the corresponding control samples (n = 6).(B) Effect of S.indicum extract (1-100 μg/mL) on NK cytotoxic activity against YAC-1 tumor cells assessed by JAM assay.An effector:target (E:T) ratio of 200:1 was used.For S.indicum extract-treated cells, statistical significance was determined in comparison to the corresponding vehicle-treated cells (n = 6).Data are expressed as mean ± SEM.**P < 0.01 and ***P < 0.001.The error bars represent SEM values.
3.3.S.indicum suppresses macrophage inflammatory response
WhetherS.indicumextract exerts pro-inflammatory or antiinflammatory effects in macrophages was evaluated by assessing the secretion of IL-6 and TNFα, key pro-inflammatory cytokines.BALB/c-derived peritoneal macrophages were cultured in the presence of vehicle, LPS, IFNγ, a combination of LPS plus IFNγ,orS.indicumextract at four doses (1, 10, 50, and 100 μg/mL)with or without LPS plus IFNγ.The supernatants were harvested 12 and 48 h post treatment to assess the level of TNFα and IL-6,respectively, by ELISA.S.indicumextract alone had no significant modulatory effect on IL-6 or TNFα secretion by macrophages at any dose in comparison to vehicle-treated macrophages (Figure 3A and B).However, in the presence of LPS and IFNγ,S.indicumextract caused a significant dose-dependent decrease in IL-6 and TNFα levels (Figure 3A and B).Treatment of macrophages with the highest dose (100 μg/mL) ofS.indicumextract as well as LPS and IFNγ inhibited the release of IL-6 and TNFα by ~1.5-fold and ~1.7-fold, respectively (Figure 3A and B).
3.4.S.indicum diminishes macrophage-derived NO production
The immunoregulatory effects ofS.indicumextract on macrophage function were further investigated by assessing NO production by macrophages cultured in the presence ofS.indicumextract.BALB/c-derived peritoneal macrophages were cultured in the presence of vehicle, LPS, IFNγ, a combination of LPS plus IFNγ, orS.indicumextract at four doses (1, 10, 50, and 100 μg/mL) with or without LPS and/or IFNγ.Supernatants were harvested 48 hours post treatment and subjected to Griess assay to determine NaNO2concentration.As shown in Figure 4A, macrophages released 6, 9, 40, and 100 μM NaNO2in the presence of the vehicle, LPS, IFNγ, and a combination of LPS plus IFNγ, respectively.At all doses,S.indicumextract alone had no significant effect on NO production by macrophages in comparison to vehicle-treated macrophages.However, in the presence of IFNγ,S.indicumextract caused a dose-dependent inhibition of NO production compared to IFNγ-treated macrophages with significant difference only found at the highest dose (100 μg/mL).In addition, 100 μg/mLS.indicumextract led to ~1.2-fold significant suppression of NO production under LPS plus IFNγ stimulatory condition (Figure 4A).
3.5.S.indicum enhances NK cytotoxic activity
NK cells are known to exert potent cytotoxic effects against tumor cells.Using JAM assay, we further examined the potential immunomodulatory roles ofS.indicumextract by evaluating its ability to modulate NK cytotoxic activity against YAC-1 tumor cells (mouse lymphoma cells).YAC-1 tumor cells were grown with vehicle orS.indicumextract at four doses (1, 10, 50, and 100 μg/mL)in the presence of effector cells (NK cells) at different effector:target(E:T) ratios (200:1, 100:1, and 50:1).As negative controls, YAC-1 tumor cells were grown in the presence of vehicle or 100 μg/mL ofS.indicumextract without effector cells (NK cells).In the vehicletreated sample, NK cells exhibited ~5% cytotoxic activity against YAC-1 tumor cells at E:T ratio of 200:1.Intriguingly, the three higher doses (10, 50, and 100 μg/mL) ofS.indicumextract elevated NK cytotoxic activity (~2.9, ~9.0, and ~9.0 folds, respectively),compared to the vehicle-treated sample.In contrast, in the absence of NK cells,S.indicumextract caused no direct cytotoxicity against YAC-1 tumor cells (Figure 4B).
4.Discussion
Natural products have been used in the treatment of a variety of diseases over the years.Studies have shown that different plants and their respective constituents have varying immunomodulatory effects on the immune system, which may be immuno-suppressing or immune-enhancing.S.indicumhas been traditionally used as a food source in the prevention of health risks and is recently being studied for its anti-allergic immune reactions[13].Previously, we underscored thein vitroimmunomodulatory effects of black seed[21], black pepper[22], and cardamom[22].Herein, we explored the potential ofS.indicumextract to regulate major immunological processes including Th1/Th2 balance, splenocyte proliferation, macrophage-derived inflammation, and NK anti-cancer activity.
Our experimental findings clearly demonstrate thatS.indicumextract modulates the function of various immune cellsin vitro.Based on trypan blue extraction and MTT assays, we observed that theS.indicumextract (1-100 μg/mL) had no toxic effects on splenocytes, macrophages, or YAC-1 cells (data not shown).TheS.indicumextract was used at the dose range of 1-100 μg/mL for all experiments because this dose range was shown to have no toxicity and was used in previous studies by us and other investigators.Our findings clearly indicate thatS.indicumextract dose-dependently enhances splenocyte proliferation, similar to the extracts of black seed[21], black pepper[22], and cardamom[22].Importantly, such an increase in splenocyte proliferation is not due to LPS contamination,but rather a direct effect ofS.indicumextract.Of note, anin vivostudy by Kumar and colleagues suggests that intraperitoneal injection with 100 mg/kg sesamol 30 min prior to γ-irradiation protects γ-irradiated C57BL/6 mice against diminished splenocyte proliferation[23].
Upon exposure to specific antigens, helper T cells differentiate into one of the two main subsets: Th1 and Th2.Differentiation into either subset is mainly controlled by signature cytokines that promote one subset while inhibiting the other, consequently polarizing the differentiation[24-26].IFNγ and IL-12 are the key cytokines that induce Th1 differentiation while inhibiting Th2 differentiation.On the contrary, IL-4, IL-5, and IL-13 induce differentiation into Th2 cells while inhibiting the development of Th1 cells[24-26].Th1 and Th2 cells are usually distinguished based on the cytokines they release, which are the same cytokines that led to their differentiation in the first place.The enhancement of Th1 response and Th2 response is shown to have effects on cellular and humoral immunity,respectively[24-26].Our findings demonstrate thatS.indicumextract stimulates the release of a key Th1 cytokine, IFNγ, while suppressing the release of IL-4 and IL-10, as key Th2 cytokines by splenocytes in a dose-dependent manner, which suggests thatS.indicumextract can exert a potent regulatory role on the Th1/Th2 balance of various immune-related processes.Notably,S.indicumextract plays a very similar role as black pepper extract, which we have previously shown to favor Th1 cytokine profile[22].Interestingly, Ghazavi and Mosayebi demonstrated in anin vivostudy using C57BL/6 mice that sesame oil is effective in treating Th1 cell-mediated experimental autoimmune encephalomyelitis through the reduction of IFNγ secretion[27].Moreover, the same study revealed that IL-10 secretion by splenocytes isolated from sesame oil-treated mice was enhanced.This study suggests that sesame oil contributes to the control of the Th1/Th2 balance of immune responses through reducing Th1 response and enhancing Th2 response, which is inconsistent with our findings.It is likely that the discrepancy between thisin vivostudy and ourin vitrostudy is largely due to different experimental conditions including doses, strains, detection methods,etc.
Given thatS.indicumextract favors Th1 response by enhancing IFNγ secretion and that Th1 immune response is crucial in the elimination of intracellular pathogenic infections[25], our study suggests thatS.indicumextract and its constituents may be effective in exerting immune-enhancing effects on immune cells involved in combating intracellular pathogens such as viruses, bacteria (e.g.Mycobacterium tuberculosis,Listeria monocytogenes,etc), and parasites(e.g.Leishmania majorandToxoplasma gondii).On the other hand,Th2 cells are critically involved in immune responses against helminths and other extracellular pathogens as well as immediate hypersensitivity (allergic) responses (e.g.asthma, conjunctivitis,atopic dermatitis, rhinitis,etc).Th2 cytokines are known to play fundamental roles in promoting humoral immunity, leading to the production of antibodies that help fight extracellular pathogens[25,26].Therefore, our findings demonstrate thatS.indicumextract and its constituents can exert stimulatory effects on cell-mediated immune responses while exerting inhibitory effects on humoral immune responses and immediate hypersensitivity reactions.In agreement with our results, a recent study by Jung and colleagues demonstrated that fermentedS.indicumseeds exert an anti-allergic response by suppressing the expression of various cytokines and chemokines that are critically involved in mediating hypersensitivity reactionsviablockade of NF-κB and STAT1 signaling[13].In anin vivostudy,sesamin, a key lignan inS.indicumseeds, was shown to exert similar effects on allergen-induced Th2 responses by means of suppressing the secretion of key Th2 cytokines IL-4, IL-5, and IL-13, which consequently reduced symptoms of asthma in BALB/c mice[28].Similar to our findings, sesamin has been shown to increase IFNγ levels and reduce inflammation in a BALB/c mouse model with allergic asthma by means of suppressing NF-κB activity[29].It is noteworthy that Th1 and Th2 immune cells may have antagonistic effects on each other, which suggests that IFNγ secreted by Th1 cells can block the increase in Th2 cells, and IL-4 or IL-10 secreted by Th2 cells can block the generation of Th1 cells from naive T cells[25].Hence,S.indicumextract and its constituents may directly suppress the release of Th2 cytokines, IL-4 and IL-10, or indirectlyviaenhanced secretion of IFNγ, a key Th1 cytokine.
Our study also suggests thatS.indicumextract can exert potent antiinflammatory effects in macrophages.A dose-dependent reduction was observed in the levels of key pro-inflammatory mediators, IL-6 and TNFα, secreted by macrophages treated with theS.indicumextract in the presence of LPS and IFNγ.Furthermore,S.indicumextract significantly reduced the release of NO in LPS- and IFNγtreated macrophages in a dose-dependent manner.Consistent with these findings, a recent study suggests that a sample of fermentedS.indicumseeds inhibits the production of pro-inflammatory cytokines(IL-1β and IL-6), chemokines (TARC and MDC), and adhesion molecule (ICAM-1) by means of suppressing NF-κB and STAT1 signaling[13].In addition, strong experimental evidence indicates that sesamin plays variousin vitroandin vivoanti-inflammatory roles, mainly through the down-regulation of pro-inflammatory mediators[7,30].Another majorS.indicumlignan, sesamol, has been shown to exert significant and dose-dependent anti-inflammatory activity in LPS-stimulated RAW 264.7 macrophagesviadownregulation of NF-κB signaling pathway[31].Similar findings have been reported suggesting that sesamol mediates its anti-inflammatory effects through upregulating AMPK and NRF2 pathways and blocking NF-κB and MAPK signaling pathways[32].Moreover, a study performed by Hsu and colleagues demonstrated that sesame oil has a therapeutic effect on monosodium urate monohydrate crystalinduced acute inflammation in rats[33].Indeed, sesame oil was shown to decrease IL-1β, IL-6, and TNFα levels and monosodium urate monohydrate crystal-induced total cell counts in rats.The same researchers further showed that sesame oil attenuates the acute inflammatory effects that accompany endotoxemia and sepsis in rats due to its ability to reduce the levels of various mediators that promote inflammation and oxidative stress[34,35].Sesame oil was further shown to significantly reduce oxidative stress by inhibiting NO production in rats challenged with LPS[36].Interestingly, the constituents of sesame oil are similar to those of other extracts ofS.indicumseeds which include sesamin and sesamolin[13,37],suggesting that the reported anti-inflammatory effects of sesame oil and other extracts ofS.indicumare most likely due to their common active constituents.Similar to these anti-inflammatory effects ofS.indicumextract, we have previously demonstrated that the aqueous extracts of black seed[21] and cardamom[22] also significantly inhibit the release of IL-6, TNFα, and NO by primary BALB/c-derived macrophages in a dose-dependent manner.
Although studies related to the anti-cancer effects ofS.indicumextracts are scarce, several lignans ofS.indicum(e.g.sesamin,sesamol, and sesaminol) have been documented to exert anti-cancer activities bothin vitroandin vivo.Sesamin was shown to trigger apoptosis in a dose-dependent manner in various cancer cell lines including HL-60, U937, and Molt 4B cells[38].Similarly, sesamin had anti-proliferative effects on HepG2[3] and induced apoptosis in HepG2 cells[39].Likewise, sesamol induced similar effects in HepG2 cells by means of DNA fragmentation[40] and impairment of mitochondria functions[41].Thein vitroandin vivoanti-cancer effects of sesamin[42] and sesamol[43] and the molecular mechanisms involved have recently been reviewed.Sesamolin inhibited growth and induced apoptosis in human lymphoid leukemia cells by DNA fragmentation depending on the concentration of sesamolin and time of exposure to the cancer cell[44].In addition, experimental evidence suggests that sesame oil exerts anti-proliferative and apoptotic effects[45].
Our data providein vitroexperimental evidence suggesting that NK cytotoxic activity against YAC-1 tumor cells is significantly enhanced byS.indicumextract (100 μg/mL).Importantly,S.indicumextract does not cause direct cytotoxicity against YAC-1 tumor cells, which indicates thatS.indicumextract promotes the killing of YAC-1 tumor cellsviaits ability to augment NK activity rather than provoking an immediate cytotoxic effect.Of note,S.indicumextract-mediated enhancement of NK cytotoxic activity against YAC-1 tumor cells positively correlates to the E:T ratio (data not shown).Together,these findings strongly suggest thatS.indicumextract can potently improve the intrinsic anti-cancer activity of NK cells against tumor cells.S.indicumextract leads to ~46% cytotoxicity compared to ~5%cytotoxicity in the vehicle-treated YAC-1 cell population (i.e.~9.2-fold enhancement) at a dose of 100 μg/mL and E:T ratio of 200:1.Compared to our previous findings,S.indicumextract seems to exert more potent effects (~46% cytotoxicity) with regard to NK cytotoxic potential compared to those of black seed[21] and black pepper[22],which lead to ~25% and ~35% cytotoxicity, respectively.S.indicumextract exerts slightly more potent effects on NK cytotoxic activity compared to cardamom[22], which leads to ~45% cytotoxicity under the same experimental conditions.A study performed by Kim and Lee demonstrated that sesamolin promotes NK cytotoxic activity against cancer cells by upregulating the expression of NKG2D ligands on the surface of Burkitt’s lymphoma cellsviaenhanced ERK signaling.However, sesamin did not have any cytotoxic effects on NK cell activity on the same cancer cell lines, which may be due to differences in their chemical structures[10].Our study substantiates the potent anti-cancer effects ofS.indicumviaits ability to enhance NK cytotoxic activity against cancer cells, rather than exerting direct cytotoxic effects.
Although there have not been substantial studies focusing on the immunomodulatory effects ofS.indicumextract, there is enough data onS.indicumlignans to support our results on the modulatory effect ofS.indicumextract on the immune system through the enhancement of some immune cells.S.indicumextract can potently augment splenocyte proliferation in a dose-dependent manner and stimulate immune cells responsible for combating intracellular pathogens and cellular immune responses through the enhancement of Th1 response.Our results also suggest thatS.indicumextract has immuno-suppressing effects with regard to Th2 cytokine expression and consequently, the allergic response.Similarly, the dosedependent reduction of macrophage pro-inflammatory mediators(IL-6, TNFα, and NO) suggests thatS.indicumextract suppresses macrophage-derived inflammation.In summary,S.indicumextract promotes splenocyte proliferation, favors Th1 cytokine profile,triggers anti-inflammatory functions in macrophages, and plays anti-cancer rolesviaprovoking NK cytotoxic activity against tumor cells.Thus,S.indicumextract may serve to combat a variety of health issues depending on whether they require immune-enhancing or immuno-suppressing outcomes of the immune system, due to its potent immunomodulatory effects.S.indicumextracts and its active constituents may be employed as potential therapeutic agents in controlling key immunological processes implicated in the development of various infectious and non-infectious conditions including, but not limited to, cancer.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgments
We are thankful to Dr.Fredrick Palmer (Dalhousie University,Halifax, Canada) for allowing us to use the rotatory evaporators for the preparation of the aqueous extract ofS.indicum.We also thank Hana James, Bruce Musgrave, Wendy Hughes, and Jillian Tarrant for their technical assistance.
Authors’ contributions
AFM and RIC designed the experiments and supervised their execution.AFM performed all experiments and assays.AFM and JFF performed data analyses of all experimental findings.AFM, JFF,and RIC contributed to manuscript writing and preparation.
 Asian Pacific Journal of Tropical Biomedicine2020年7期
Asian Pacific Journal of Tropical Biomedicine2020年7期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Anticancer effect of Psidium guajava (Guava) leaf extracts against colorectal cancer through inhibition of angiogenesis
- Ferruginol alleviates inflammation in dextran sulfate sodium-induced colitis in mice through inhibiting COX-2, MMP-9 and NF-κB signaling
- Antibiofilm activity of alpha-mangostin loaded nanoparticles against Streptococcus mutans
- Anti-proliferative potential of sodium thiosulfate against HT 29 human colon cancer cells with augmented effect in the presence of mitochondrial electron transport chain inhibitors
