新型碳材料质子交换膜燃料电池Pt催化剂载体的研究进展
罗燚, 冯军宗, 冯坚, 姜勇刚, 李良军
新型碳材料质子交换膜燃料电池Pt催化剂载体的研究进展
罗燚, 冯军宗, 冯坚, 姜勇刚, 李良军
(国防科技大学 空天科学学院, 新型陶瓷纤维及其复合材料重点实验室, 长沙 410073)
质子交换膜燃料电池(PEMFC)具有能量转换效率高、功率密度大、室温启动快、噪音低和零污染等特点, 有望减少二氧化碳排放量, 缓解能源危机, 在轨道交通、航空航天等领域具有广阔的应用前景。催化剂是PEMFC的关键材料, Pt催化氧还原反应活性和稳定性好, 是广泛使用且很难被取代的电催化剂。然而Pt储量低、价格昂贵, 导致PEMFC成本较高, 使用Pt载体可减少PEMFC的Pt负载量, 提高Pt利用率。碳材料具有成本低廉、比表面积大、孔结构丰富、电导率和表面性质可调等特性, 是广泛应用的Pt载体。商用的炭黑载体对Pt的利用效率低, 抗电化学腐蚀性较差。为了进一步提高PEMFC的性能和持续性, 需要研发能够均匀负载Pt、高效利用Pt、抗电化学腐蚀性强且导电性好的碳载体, 进而实现PEMFC的大规模应用。炭气凝胶、碳纳米管和石墨烯等新型碳载体具有独特的结构和性质, 可以提高PEMFC性能和寿命, 引起了研究者的广泛关注。本文对近年来PEMFC新型碳材料Pt载体的研究进展进行了较为详细的综述, 并对其发展趋势作出了适当评论。
质子交换膜燃料电池; 炭气凝胶; 碳纳米管; 石墨烯; 综述
面对化石能源日益减少的现状, 人类社会迫切需要开发绿色、环保和高效的新能源。质子交换膜燃料电池(PEMFC)具有能量转换效率高、功率密度大、室温启动快、噪音低和零污染等特点, 在轨道交通、航空航天等领域具有广阔的应用前景[1-4]。PEMFC系统的核心部件为膜电极, 由气体扩散层、催化剂层和质子交换膜组成[5-6], 如图1所示。Pt催化氧还原反应活性和稳定性好, 是在PEMFC中被广泛使用且很难被取代的电催化剂。然而, Pt在地壳中的储量稀少, 阻碍了PEMFC的大规模生产应用。催化剂载体能够降低PEMFC中Pt的用量[7], 是实现PEMFC商业化应用的途径之一。

图1 PEMFC的组成结构示意图[6]
PEM: Proton exchange membrane; MEA: Membrane electrode assembly; GDL: Gas diffusion layer; CL: Catalyst layer
载体是PEMFC中非常重要的组成部分, 直接影响催化剂的粒径大小、分布、电化学活性比表面积、稳定性和利用率, 最终影响PEMFC的性能和寿命[8-9]。PEMFC中反应物、产物、电子以及质子传输效率和速度也与载体性质密切相关[10-13], 并且载体与催化剂协同效应能够提高催化剂性能[14-15]。好的载体主要具备[16-20]: (1)高的活性比表面积, 能够均匀负载催化剂; (2)合适的孔结构, 能提供高活性三相反应界面; (3)较强的稳定性, 在高湿、高电压等苛刻条件下, 长时间不发生严重电化学腐蚀; (4)高电导率。以上条件之间存在矛盾或依赖的关系: (1)比表面积与孔结构有关, 电导率与结晶度有关; (2)高电导率载体的稳定性好; (3)中孔结构有利于负载催化剂, 但大量中孔结构的载体, 稳定性往往较差。理想载体能够搭建高效电催化反应界面、锚定催化剂纳米粒子、减缓催化剂失活, 延长PEMFC寿命、提升性能、降低成本。
炭黑是商品PEMFC的主要载体。美国Cabot公司生产的 Vulcan XC性能较好, BET 比表面积约为250 m2∙g–1, 中孔和大孔达 54% 以上, 电导率2.77 S/cm, 基本满足电催化剂载体对比表面积和导电性的要求, 是目前应用最为广泛的商品载体。
然而, 炭黑的缺点之一是结构中微孔含量较高[21]。Thommes等[22]研究表明, 导电高聚物无法进入微孔, 陷入炭黑微孔中的Pt实际上没有参与电催化反应过程。因而, 炭黑负载Pt利用率较低。同时, Maillar等[23]研究发现, 分散在炭黑表面的Pt虽能够参与反应, 但在催化过程中容易发生溶解、团聚和位置转移等失活行为, 如图2所示。炭黑抗电化学腐蚀性也较差[24], 炭黑的常温电化学腐蚀热力学电压(0.203 V. NHE)低于PEMFC的工作电压(>0.6 V. NHE)。提高炭黑的石墨化程度虽能够降低电化学腐蚀速率[25], 但很难在热力学上避免发生腐蚀。Tuaev等[26]研究了碳载体孔结构对催化剂活性的影响, 发现中孔结构有利于阻止Pt失活。炭黑的抗电化学腐蚀性和孔结构均存在一定的缺陷, 较难满足理想电催化剂载体的条件。由图3可知炭黑在电化学腐蚀前后, 结构发生了明显的改变[27]。
近年来对碳载体的研究主要集中在新型碳载体的开发和利用上: 炭气凝胶具有可控的孔结构, 可根据应用需要, 设计孔性质; 碳纳米管和石墨烯具有低阻抗、高导电性和高电化学稳定性等优异性质。新型碳载体有望克服炭黑的缺点, 是很有潜力的PEMFC载体材料。本文主要综述了近年来研究者在炭气凝胶、碳纳米管和石墨烯等新型碳载体上的研究进展。

图2 Pt纳米粒子在炭黑载体上的失活示意图[21]
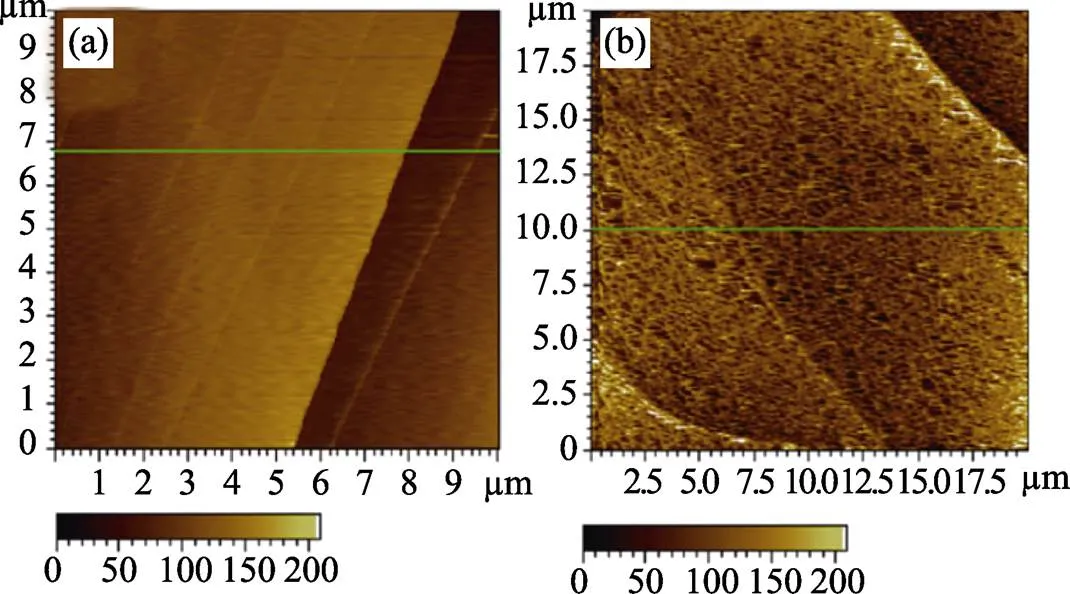
图3 炭黑电化学腐蚀前(a)后(b)的原子力显微镜照片[27]
1 炭气凝胶
炭气凝胶是一种非晶态碳材料,其纳米多孔三维网络结构可控(图4), 具有高比表面积(600~ 1100 m2∙g–1)、高孔隙率(80%~98%)和高稳定性[28]的特点。载体孔结构对PEMFC非常重要, 合适的孔结构有利于传质和阻止Pt失活。通过调控炭气凝胶的孔结构有望制备出满足PEMFC要求的载体。
为研究孔结构对PEMFC性能的影响, Ouattara等[29]制备出具有不同孔径分布的炭气凝胶。由图5可知, 载体孔径在25~30 nm时, PEMFC性能最佳; PEMFC的传质阻力主要依赖于炭气凝胶孔结构, 孔径大于40 nm时, 导电高聚物易堵塞孔结构, 增大传质阻力。Smirnova等[30]研究表明, 炭气凝胶孔径由16 nm增大到20 nm, 电池性能逐步增强; 孔径20 nm的炭气凝胶负载0.1 mg∙cm–2Pt时, 达到最大功率密度800 mW∙cm–2。合适的载体孔结构有利于减小传质过程导致的功率损失。Ouattara等[31]针对如何更好地调控膜电极的水, 设计了三维多孔炭气凝胶载体, 0.4 V电压下电池功率提高了40%。Wang等[32]发现N掺杂梯度孔炭气凝胶有利于传质, 掺杂微量Fe元素后, 催化氧还原活性非常好, 起始电压和半波电压分别为918和798 mV,比相同条件下Pt/C催化剂分别高出117和206 mV。
在PEMFC的使用过程中, 启动、急停都会加速碳载体的电化学腐蚀。Ouattara等[33]模拟PEMFC的启动和急停条件, 加速老化测试炭气凝胶抗电化学腐蚀性能, 相同条件老化14 h后, Pt/C和Pt/炭气凝胶活性比表面积分别减少了17.57%、56.27%。炭气凝胶由于石墨化程度较低, 抗电化学腐蚀性比Pt/C催化剂要差。为了提高炭气凝胶石墨化程度, Singh等[34]通过高温高压凝胶、高温惰性气氛石墨化, 制备出中孔比表面积490 m2∙g–1、平均孔径4.9 nm的炭气凝胶, PEMFC的起始电位为964 mV, 半波电位为814 mV, 优于相同条件下Pt/C催化剂。
炭气凝胶上存在许多活泼碳悬空键, 比较容易发生电化学腐蚀反应。炭气凝胶表面改性可提高抗电化学腐蚀性和催化活性。Wang等[35]制备了KOH活化掺N炭气凝胶催化剂, 掺杂N引入了大量的缺陷结构, KOH活化后进一步优化了炭气凝胶的孔结构, 无需负载Pt, 催化氧还原反应半波电压为790 mV。Fabien等[36]在炭气凝胶表面涂覆SnO2载体的抗电化学腐蚀性较好, 加速氧化测试后负载催化剂的活性比表面积和质量比活性不降反增; 由图6可知, 相比于炭气凝胶而言, SnO2涂覆炭气凝胶负载Pt催化剂加速氧化测试后, Pt粒子团聚现象减少。强氧化剂与炭气凝胶悬空键结合, 有利于在动力学上减缓电化学腐蚀。Berthon等[37]制备了氟化炭气凝胶, 5×103次循环测试后氟化载体催化剂活性比表面积仅减小10%, 远低于相同条件下Pt/炭气凝胶(25%)和Pt/C(15%)。

图4 炭气凝胶CA20(a)、CA30(b)和CA40(c)的SEM照片[28]
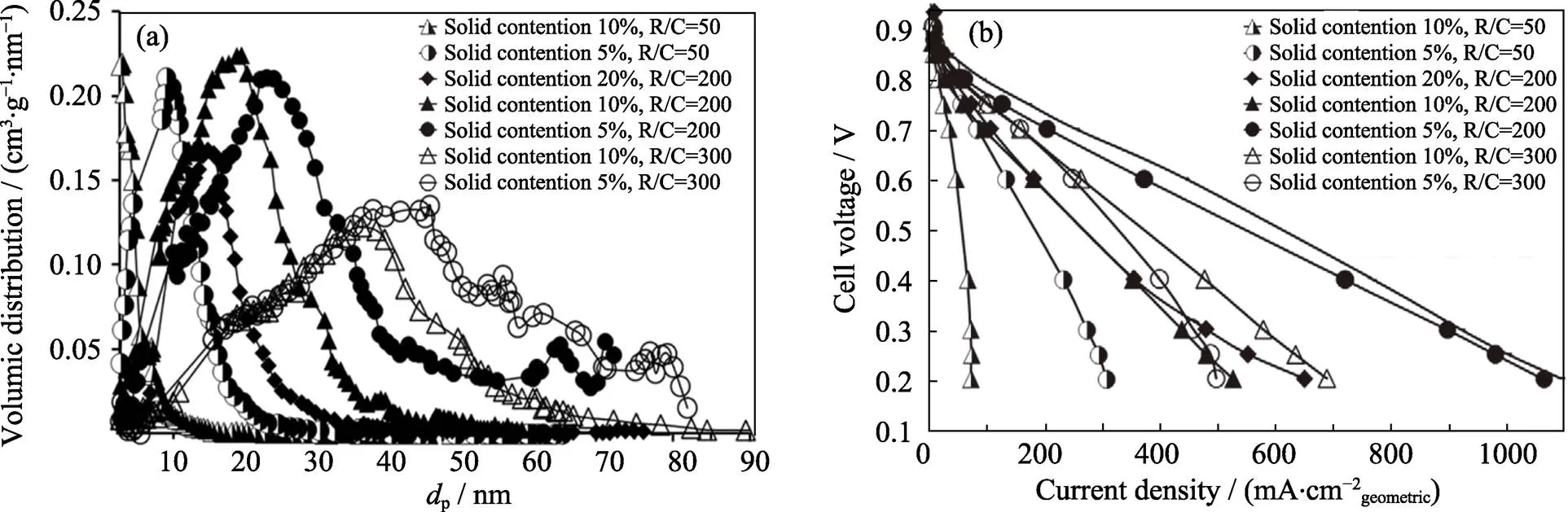
图5 压汞法测定的不同固含量、不同间苯二酚(R)和碳酸钠(C)摩尔比的炭气凝胶孔径分布曲线(a), 及其对应的单电池极化曲线(b)[29]

图6 炭气凝胶(a)及SnO2涂覆炭气凝胶(b)负载Pt催化剂加速氧化测试(AST P1)后的TEM照片和加速氧化测试前后的Pt粒子统计分布图[36]
炭气凝胶具有中孔比表面积高、活性位点多、传质阻力小等特点, 非常适合作为PEMFC载体。可控的纳米多孔三维网络结构是炭气凝胶作为载体最具有吸引力的特点。然而, 炭气凝胶目前存在抗电化学腐蚀性较差等缺点。表面改性、提高石墨化程度是增强炭气凝胶抗电化学腐蚀性的主要方法。目前, 以炭气凝胶为载体的PEMFC正处于应用前期。
2 碳纳米管
碳纳米管是呈六边形排列的碳原子层构成的无缝管, 是一种特殊的一维纳米结构, 具有阻抗低、导电性高、稳定性好的特点。根据管壁中碳原子层数目, 碳纳米管可分为单壁碳纳米管和多壁碳纳米管[38], 结构如图7所示。很多研究[39-42]表明, Pt/碳纳米管电催化活性、抗电化学腐蚀性和抗CO毒性均优于同条件下的Pt/C, 是很有潜力的载体材料。近年来, 研究者在增强碳纳米管负载催化剂的稳定性、增大碳纳米管比表面积等方面开展了深入的研究。
Pt与碳纳米管掺杂的杂原子之间的电子转移可增强催化活性。Zhao等[43]制备了含Fe的N掺杂碳纳米管, 催化氧还原反应半波电压为850 mV, 密度泛函理论模型研究表明结构中大量的吡啶N加快了电子传递速度。二氧化钛稳定性较好、具有一定催化活性, Mohammad等[44]合成了一种TiSi2O涂覆碳纳米管, 负载Pt后催化氧还原反应, 半波电压比Pt/碳纳米管高出30 mV。Gao等[45]研究表明, 利用高度分散晶体Ta2O5修饰的碳纳米管作为载体, 稳定性非常好、催化活性也较好, 104次循环伏安测试之后, 半波电压和活性比表面积基本不发生变化, Pt−Ta2O5/碳纳米管电化学活性比表面积为78.4 m2∙g−1, 0.9 V时的质量比活性为0.23 A∙mg−1Pt, 是相同条件下Pt/C和Pt/碳纳米管的2.2和3.4倍。Ta2O5和碳纳米管协同作用, 改变了Pt电子结构, 形成了Pt–O–Ta化学键, 使得Pt变得更加稳定。
为增大碳纳米管比表面积, Sahoo等[46]将多层碳纳米管上层沿轴向打开, 制备出了具有石墨烯“翅膀”的石墨烯–碳纳米管杂化材料。研究结果表明, 相比于Pt/C催化剂, Pt/石墨烯–碳纳米管杂化材料具有高催化活性, 阴极Pt负载量为0.3 mg∙cm–2时, 最大功率密度高达1000 mW∙cm–2。石墨烯–碳纳米管杂化材料兼具石墨烯片层和碳纳米管一维结构, 电导率高、反应活性位点多, 碳纳米管和石墨烯片层协同作用有利于Pt分散, 是一种新型载体材料。Priji等[47]研究了Pt-Sn/石墨烯–碳纳米管载体催化剂, 当Pt、Sn原子比为3 : 1时, 60 ℃功率密度为568 mW∙cm–2, 比同等条件下Pt/碳纳米管高出23%且阴极催化剂负载量远低于性能相当的Pt/C。Meenakshi等[48]报道了具有高活性和稳定性的Pt3Sc/石墨烯–碳纳米管催化剂, 60 ℃时的功率密度为760 mW∙cm–2, 加速氧化测试前后的质量比活性均高于Pt/C。

图7 碳纳米管原子结构示意图(a~c), 隧道电子显微镜照片(d), TEM微观形貌照片(e)[38]
碳纳米管的导电性和稳定性优异, 抗电化学腐蚀性较好。然而, 碳纳米管的活性比表面积较小, 表面惰性导致负载Pt催化剂能力较弱。近年来, 研究者们通过制备碳纳米管杂化材料、碳纳米管–过渡金属复合材料等方法, 极大地增强了碳纳米管负载Pt催化剂的催化活性和稳定性, 增大了碳纳米管载体的活性比表面积, 增强了Pt负载能力。但是对于工业化生产, 碳纳米管作为PEMFC催化剂载体还面临着合成方法和价格的问题。
3 石墨烯
石墨烯具有二维平面结构, 其理论比表面积大(2630 m2∙g–1)、电导率高(106 S∙cm–1)、抗电化学腐蚀性好[49], 是很有应用前景的PEMFC载体材料。大量研究表明, 以石墨烯作为Pt、Pt合金和非贵金属催化剂载体的催化剂, 性能均优于商业Pt/C。
石墨烯具有诸多优异性能, 但其本征二维结构的片层间范德华力较强, 容易发生重组、团聚, 导致负载的Pt随之团聚、脱落和失活。通过二维片层石墨烯架构而得到的具有三维结构的石墨烯材料, 能避免片层间团聚。Liu等[50]制备了具有三维结构和缺陷的石墨烯泡沫, 比表面积高达1500 m2∙g–1, 载体催化剂的电化学活性比表面积为101 m2∙g–1(比Pt/C高50%), 质量比活性为176 A∙g–1Pt(比Pt/C高30%)。石墨烯气凝胶具有本征纳米多孔三维网络结构。Eylul等[51]使用超临界CO2溶剂将Pt负载在石墨烯气凝胶上, 载体催化剂电化学活性比表面积为102 m2∙g–1, 质量比活性为30.6 A∙g–1Pt。
以炭黑为阻隔和连接石墨烯片层的“空间桥梁”, 形成具有三维结构的石墨烯–炭黑杂化材料, 能够阻止石墨烯片层团聚[52-54]。Li等[55]使用聚苯并咪唑将炭黑和石墨烯片层结合, 制备炭黑–石墨烯杂化材料, 研究表明以这种材料为载体的催化剂, 质量比活性为183 A∙g–1Pt(Pt/C为149 A∙g–1Pt), 膜电极开始工作后, 最大电流密度由500 mA∙cm–2增大到2250 mA∙cm–2; 由图8可知, 1000次循环伏安测试之后, 电流密度保持在1500 mA∙cm–2, 峰电压为1.0~1.5 V, 而Pt/C峰电压值降低为0 V。聚苯并咪唑可锚定Pt, 且石墨烯抗电化学腐蚀性较好, 故而炭黑–石墨烯载体性能优异。除炭黑之外, 碳纳米管、碳纤维等也可以作为阻隔并连接石墨烯片层的“空间桥梁”[56-61]。
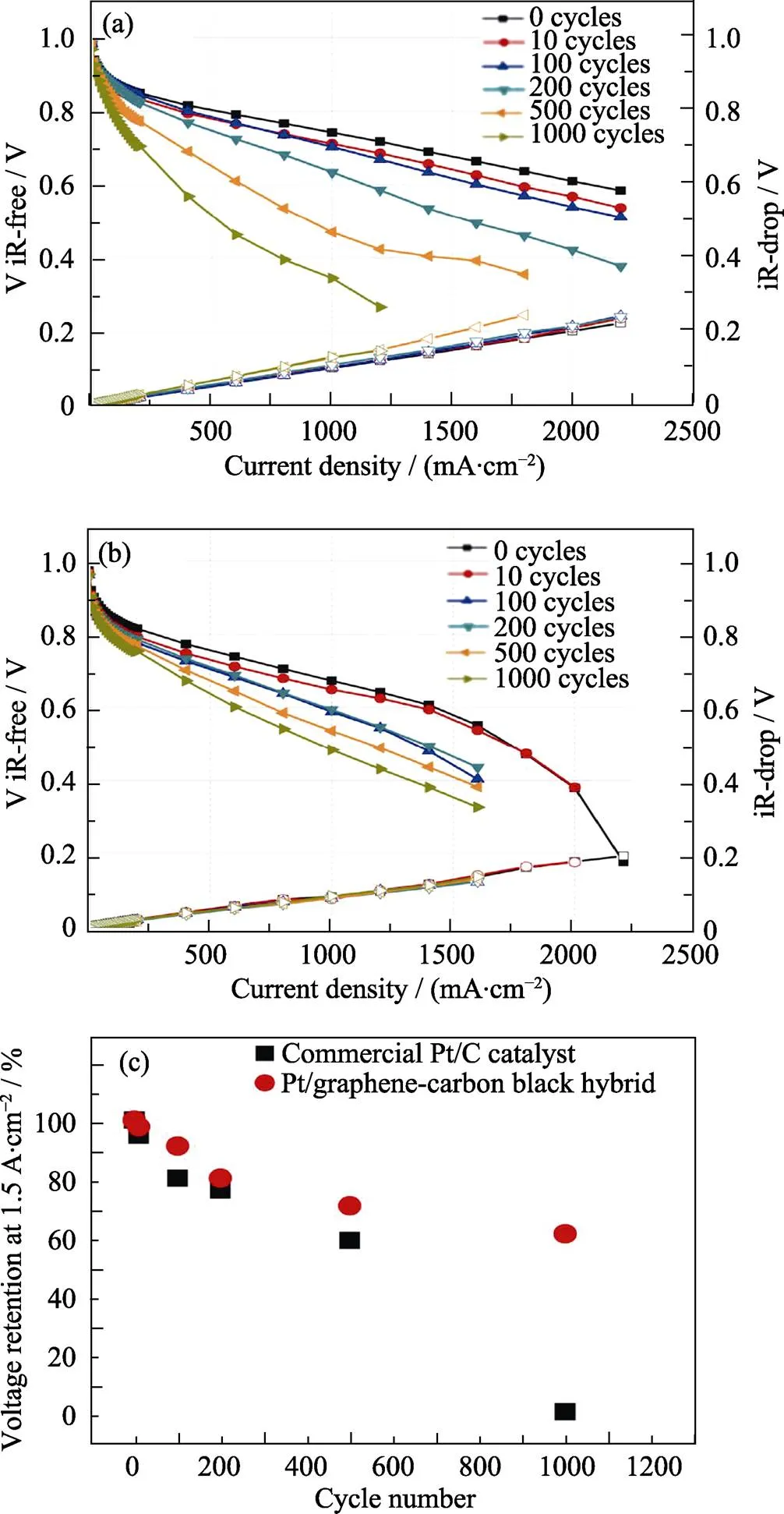
图8 商业Pt/C(a), Pt/炭黑–石墨烯杂化材料(b)为阴极催化剂的PEMFC经加速氧化测试后的极化曲线; 不同循环次数后的电压保留值(c)[55]
石墨烯表面呈化学惰性, 活性位点少, 负载催化剂能力较弱, 在石墨烯表面加入杂原子、官能团或大分子, 能够在平面引入具有电化学活性的缺陷结构, 增大活性比表面积, 增强催化活性[62-64]。Sergey等[65]制备了掺氟、掺氧的氧化石墨烯, 掺氧氧化石墨烯负载的Pt颗粒粒径较小, 掺氟、掺氧石墨烯载体催化剂性能均高于Pt/C。
近年来, 研究者们在杂原子掺杂石墨烯作为非贵金属催化剂载体, 用于催化氧还原做了大量工作[66]。氮掺杂石墨烯增加了每摩尔氧气的电子转移数, 使氧气能在低电压下形成OH–。Xu等[67]研究了Co–B–N掺杂多孔石墨烯催化剂的电化学活性, 起始电位和半波电位分别为904和792 mV, 仅比商业Pt/C催化剂低0.06和0.04 V, 而Tafel斜率为58 mV∙dec−1, 低于Pt/C(71 mV∙dec−1)。Lei等[68]研究报道了N掺杂石墨烯气凝胶用于氧还原反应, 起始电压仅比相同条件下Pt/C低0.1 V, 稳定性和对甲醇的耐受性好于Pt/C。同时, Wang等[69]还通过研究表明, 石墨烯上吡啶N掺杂缺陷处催化氧还原活性较高, 产生的超电势最低为0.28 V, 大量吡啶N缺陷掺杂石墨烯在碱性环境中半波电位为850 mV, 催化活性较好。Yang等[70]设计掺Fe、掺N单层石墨烯为氧还原催化剂, 揭示了催化反应活性位点为Fe与吡啶N结合的缺陷位置。
石墨烯电导率高、抗电化学腐蚀性好且比表面积大。然而, 其在使用过程中易团聚、表面呈化学惰性。制备具有三维结构的石墨烯可以阻止片层团聚; 杂原子掺杂石墨烯, 可在表面引入具有催化活性的缺陷结构, 调节缺陷数目、尺寸和形状等, 改善石墨烯表面活性, 增强其负载能力和催化活性。单独的石墨烯片层较难在PEMFC中应用, 而三维结构的石墨烯材料和原子杂化石墨烯具有非常优异的性能, 有望成为PEMFC载体。
4 其他新型碳材料
碳纤维具有优异的导电性和独特的物理化学性质, 是一种很有潜力的电催化剂载体。Wang等[71-73]研究了氮掺杂多孔碳纤维、中空多孔碳纤维等不同形态的碳纤维, 多孔碳纤维负载Pt的抗电化学腐蚀性和催化活性均优于Pt/C, 电化学活性比表面积为52 m2∙g–1(Pt/C为41 m2∙g–1), 起始电压和半波电压分别为891和739 mV, 比Pt/C分别高出44和25 mV; 其最大功率密度为130 mW∙cm–2, 是同等条件下Pt/C的二倍。Song等[74]制备了一种直径100 nm、孔径5~30 nm的超细多孔碳纳米纤维负载的Pt催化剂, 电化学活性比表面积为71.9 m2∙g–1(Pt/C 为54.6 m2∙g–1), 起始电位和半波电位分别为969和763 mV均高于Pt/C, 功率密度为165 mW∙cm–2, 是Pt/C的1.25倍。
空心碳表面具有有序的介孔结构、内部中空, 电导率在0.003~1.4 S∙cm–1之间。Ying等[75]合成了Co–Pt二元合金催化剂, 并将其植入氮掺杂的空心碳中, 相同条件下质量比活性是Pt/C的13.5倍, 电化学活性比表面积为64.6 m2∙g–1(Pt/C为57.6 m2∙g–1), 半波电位为883 mV(Pt/C为864 mV), 加速氧化测试之后半波电位仅降低19 mV(Pt/C降低67 mV), 催化活性和耐久性都较好。Chen等[76]制备了氮掺杂石墨碳负载微量Co催化剂, 微孔、介孔结构和掺杂石墨N、吡啶N使得材料具有催化氧还原活性。
近年来研究表明, 碳纤维、空心碳等新型碳材料作为电催化剂载体性能优异, 但由于制备方法复杂, 难以工业化生产和大规模应用, 成本较高, 故而研究较少。
5 结语
炭黑是商业PEMFC中被广泛使用的Pt载体, 性能基本满足要求, 但微孔含量高、导电性较差、比表面积较小, 易发生电化学腐蚀、催化剂利用率低, 导致PEMFC性能持续性较差。通过研发具有特殊结构和优异性能的新型碳载体, 有望克服炭黑载体的缺点。
炭气凝胶具有可控的纳米多孔网络结构, 活性比表面积大, 能够均匀有效地负载Pt、缓解Pt失活、降低传质阻力, 然而抗电化学腐蚀性较差。表面改性和增强结构中石墨化程度, 是提高炭气凝胶抗电化学腐蚀性的有效措施。碳纳米管导电性和稳定性好, 抗电化学腐蚀性较好, 然而比表面积较低, 负载Pt能力较弱。碳纳米管杂化材料、碳纳米管–过渡金属复合材料极大地增大了碳纳米管载体的活性比表面积和负载Pt的能力。石墨烯电导率高、抗电化学腐蚀性好且比表面积大。然而, 其在使用过程中易团聚、表面呈化学惰性。制备三维结构石墨烯可以阻止片层团聚; 杂原子掺杂石墨烯, 可在表面引入具有催化活性的缺陷结构, 增强其负载能力和催化活性。本文综述的各类碳载体优缺点, 总结如表1所示。
PEMFC要求载体有大量的三维互通中孔结构、丰富的表面活性位点、具有高导电性和稳定性的特点, 同时能够大规模工业化生产。具有丰富中孔结构的高度石墨化炭气凝胶、改性炭气凝胶、三维结构石墨烯材料、高比表面积碳纳米管杂化材料等, 理论上满足大多数理想电催化剂载体的条件, 性能较好, 有望成为新一代PEMFC电催化剂载体。

表1 不同碳载体的性能比较
[1] DE L, ZHOU J R. Theoretical modeling of the PEMFC catalyst layer: a review of atomistic methods., 2015, 177(7): 4–20.
[2] SHARMA S, POLLET B G. Support materials for PEMFC and DMFC electrocatalysts-a review., 2012, 208(2): 96–119.
[3] CURTIN D E, LOUSENBERG R D, HENRY T J,. Advanced materials for improved PEMFC performance and life., 2004, 131(1): 41–48.
[4] BARBIR F. PEM electrolysis for production of hydrogen from renewable energy sources.y, 2005, 78(5): 661–669.
[5] KNIGHTS S, BASHYAM R, HE P,. PEMFC MEA and System Design Considerations. 220th ECS Meeting, Boston, Massachusetts, USA, 2011. 39–53.
[6] 马健新, 衣宝廉, 俞红梅,等. PEMFC膜电极组件(MEA)制备方法的评述. 化学进展, 2004, 16(5): 804–807.
[7] KONGKANAN A. Encyclopedia of sustainability science and technology encyclopedia of sustainability science and technology, 1. New York: Springer, 2017: 1–20.
[8] 孙世刚, 陈胜利. 电催化. 北京: 化学工业出版社, 2008, 242–253.
[9] GASTEIGER H A, KOCHAS S, SOMPALLI B,. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs., 2005, 56(1): 9–35.
[10] SHAO Y, YIN G, GAO Y. Understanding and approaches for the durability issues of Pt-based catalysts for PEM fuel cell., 2007, 171(2): 558–566.
[11] SHAO Y, LIU J, YONG W,. Novel catalyst support materials for PEM fuel cells: current status and future prospect., 2008, 19(1): 46–59.
[12] DICKS A L. The role of carbon in fuel cells., 2006, 156(2): 128–141.
[13] SHARMA S, POLLET B G. Support materials for PEMFC and DMFC electrocatalysts-a review., 2012, 208(2): 96–119.
[14] SHAHGALDI S, HAMELIN J. Improved carbon nanostructures as a novel catalyst support in the cathode side of PEMFC: a critical review., 2015, 94(1): 705–728.
[15] SHARMA S, POLLET B G. Support materials for PEMFC and DMFC electrocatalysts-a review., 2012, 208(2): 96–119.
[16] KONGKANAND A, MATHIAS M F. The priority and challenge of high-power performance of low-platinum proton-exchange membrane fuel cells., 2016, 7(7): 1127–1137.
[17] SHINOZAKI K, MORIMOTO Y, PIVOVAR B S,. Suppression of oxygen reduction reaction activity on Pt-based electrocatalysts from ionomer incorporation., 2016, 325(1): 745–751.
[18] BRUJIN F A D, DAM V A T, JANSSEN G J M. Review: durability and degradation issues of PEM fuel cell components., 2010, 8(1): 3–22.
[19] SHAO Y, YIN G, GAO Y. Understanding and approaches for the durability issues of Pt-based catalysts for PEM fuel cell., 2007, 171(2): 558–566.
[20] XIN W, LI W, CHEN Z,. Durability investigation of carbon nanotube as catalyst support for proton exchange membrane fuel cell., 2006, 158(1): 154–159.
[21] DUBAU L, CASTANHEIRA L, BERTHOME G,. An identical- location transmission electron microscopy study on the degradation of Pt/C nanoparticles under oxidizing, reducing and neutral atmosphere., 2013, 110(1): 273–281.
[22] THOMMES M, MORLAY C, AHMAD R,. Assessing surface chemistry and pore structure of active carbons by a combination of physisorption (H2O, Ar, N2, CO2), XPS and TPD-MS., 2011, 17(3): 653–661.
[23] MAILLAR F, BONNEFONT A, MICOUD F. An EC-FTIR study on the catalytic role of Pt in carbon corrosion., 2011, 13(10): 1109–1111.
[24] ZHAO Z, CASTANHEIRA L, DUBAU L,. Carbon corrosion and platinum nanoparticles ripening under open circuit potential conditions., 2013, 230(20): 236–243.
[25] MITTERMEIER T, WEI A, HASCHE,. PEM fuel cell start-up/shut-down lossestemperature for non-graphitized and graphitized cathode carbon supports., 2017, 164(2): 127–137.
[26] TUAEV X, RUDI S, STRASSER P. The impact of the morphology of a carbon support on the activity and stability of nanoparticle fuel cell catalysts., 2016, 6(23): 1670–1679.
[27] CHOO H S, KINUMOTO T, NOSE M,. Electrochemical oxidation of highly oriented pyrolytic graphite during potential cycling in sulfuric acid solution., 2008, 185(2): 740–746.
[28] AZADEH S, AHMAD R B, ALIREZA S. Correlation between structure and oxidation behavior of carbon aerogels., 2016, 7(1): 195–203.
[29] OUATTARA B M, BERTHON F S, BEAYGER C,. Influence of the carbon texture of platinum/carbon aerogel electrocatalysts on their behavior in a proton exchange membrane fuel cell cathode., 2012, 37(12): 10–20.
[30] SMIRNOVA A, WENDER T, GOBERMAN D,. Modification of carbon aerogel supports for PEMFC catalysts., 2009, 34(21): 8992–8997.
[31] OUATTARA B M, BERTHON F S, BEAYGER C,. Correlations between the catalytic layer composition, the relative humidity and the performance for PEMFC carbon aerogel-based membrane electrode assemblies., 2014, 39(3): 1420–1429.
[32] WANG Q C, CHEN Z Y, WU N,. N-doped 3D carbon aerogel with trace Fe as efficient catalyst for oxygen reduction reaction., 2017, 4(3): 2373–2377.
[33] OUATTARA B M, BEAYGER C, BERTHON F S,. Carbon aerogels as catalyst supports and first insights on their durability in proton exchange membrane fuel cells., 2015, 11(6): 726–734.
[34] SINGH R, SINGH M K, BHARTIYA S,. Facile synthesis of highly conducting and mesoporous carbon aerogel as platinum support for PEM fuel cells., 2017, 42(16):11110–11117.
[35] WANG Q C, LEI Y P, ZHU Y G,. Edge defects engineering of nitrogen-doped carbon for oxygen electrocatalysts in Zn-air batteries., 2018, 10(1): 29448–29456.
[36] FABIEN L, ASSET T, CHATENET M,. Activity and durability of platinum-based electrocatalysts with tin oxide–coated carbon aerogel materials as catalyst supports.,2019(1): 1–17.
[37] BERTHON F S, DUBAU L, AHMAD Y,. First insight into fluorinated Pt/carbon aerogels as more corrosion-resistant electrocatalysts for proton exchange membrane fuel cell cathodes., 2015, 6(6): 521–533.
[38] BAUGHMAN R H. Carbon nanotubes-the route toward applications., 2002, 297(5582): 787–792.
[39] GIRISHKUMAR G, VINODGOPAL K, KAMAT P V. Carbon nanostructures in portable fuel cells: single-walled carbon nanotube electrodes for methanol oxidation and oxygen reduction., 2004, 108(52): 19960–19966.
[40] GIGAEK L, HYEONJN C, YONG T.durability of various carbon supports against carbon corrosion during fuel starvation in a PEM fuel cell cathode., 2019, 30(8): 1–12.
[41] YILSER D, ELIF D. Multi-walled carbon nanotubes decorated by platinum catalyst for high temperature PEM fuel cell., 2019, doi: 10.1016/j.ijhydene.2019. 01.051.
[42] YILSER D, ELIF D. Investigation of the effect of graphitized carbon nanotube catalyst support for high temperature PEM fuel cells., 2019, doi: 10.1016/j. ijhydene. 2019.01.111.
[43] ZHAO L, WANG Q C, ZHANG X Q,. Combined electron and structure manipulation on Fe containing N-doped CNTs to boost bifunctional oxygen electrocatalysis., 2018, 10(42): 35888–35895.
[44] MOHAMMAD N B, SUN S H, MENG X B,. TiSi2Ocoated N-doped carbon nanotubes as Pt catalyst support for the oxygen reduction reaction in PEMFCs., 2013, 117(30): 15457–15467.
[45] GAO W B, ZHANG Z P, DOU M L,. Highly dispersed and crystalline Ta2O5anchored Pt electrocatalyst with improved activity and durability towards oxygen reduction: promotion by atomic- scale Pt–Ta2O5interactions., 2019, doi: 10.1021/ acscatal.8b04505.
[46] SAHOO M, SCOTT K, RAMAPRABHU S. Platinum decorated on partially exfoliated multiwalled carbon nanotubes as high- performance cathode catalyst for PEMFC., 2015, 40(30): 9435–9443.
[47] PRIJI C, PUTHUSSERI D, RAMAPRABHU S. 1D-2D integrated hybrid carbon nanostructure supported bimetallic alloy catalyst for ethanol oxidation and oxygen reduction reactions., 2019, 44(10): 4951–4961.
[48] MEENAKSHI S G, RAMAPRABHU S. Highly efficient and ORR active platinum-scandium alloy-partially exfoliated carbon nanotubes electrocatalyst for proton exchange membrane fuel cell., 2019, doi: 10.1016/j. ijhydene.2019.02.161.
[49] SHENG Z H, SHAO L, CHEN J J,. Catalyst-free synthesis of nitrogen-doped graphenethermal annealing graphite oxide with melamine and its excellent electrocatalysis., 2011, 5(6): 4350–4358.
[50] LIU J F, DAIO T, KAZUNARI S,. Defective graphene foam: a platinum catalyst support for PEMFCs., 2014, 161(9): 838–844.
[51] EYLUL S Ö, ŞANSIM B B, SELMI E B,. Graphene aerogel supported Pt electrocatalysts for oxygen reduction reaction by supercritical deposition., 2017, 250(1): 174–184.
[52] ECE A, BEGUM Y K, AHMET M M,. An effective electrocatalyst based on platinum nanoparticles supported with graphene nanoplatelets and carbon black hybrid for PEM fuel cells., 2019, doi: 10.1016/j.ijhydene.2018.11.210.
[53] MELIKE S Y, BEGÜM Y K, SELMIYE A G,. Binary CuPt alloy nanoparticles assembled on reduced graphene oxide-carbon black hybrid as efficient and cost-effective electrocatalyst for PEMFC., 2018, 44(27): 14184–14192.
[54] SEVIM Y M, KAPLAN B Y, METIN,. A facile synthesis and assembly of ultrasmall Pt nanoparticles on reduced graphene oxide- carbon black hybrid for enhanced performance in PEMFC., 2018, 151(1): 29–36.
[55] LI Z F, XIN L, YANG F,. Hierarchical polybenzimidazole- grafted graphene hybrids as supports for Pt nanoparticle catalysts with excellent PEMFC performance., 2015, 16(1): 281–292.
[56] EMELINE R, YOHANN R J, LAURE G,. Optimization and tunability of 2D graphene and 1D carbon nanotube electrocatalysts structure for PEM Fuel Cells., 2018, 8(9): 377–387.
[57] YANG H N, KO Y D, KIM W J. 3D structured Pt/rGO-polyethyleneimine-functionalized MWCNTs prepared with different mass ratio of rGO and MWCNT for proton exchange membrane fuel cell., 2018, 43(9): 4439–4447.
[58] OH E J, HEMPELMANN R, NICA V,. New catalyst supports prepared by surface modification of graphene and carbon nanotube structures with nitrogen containing carbon coatings.,2017(1): 240–249.
[59] FU K, WANG Y, MAO L,. Facile one-pot synthesis of graphene-porous carbon nanofibers hybrid support for Pt nanoparticles with high activity towards oxygen reduction., 2016(1): 427–434.
[60] FU K, WANG Y, QIAN Y,. Synergistic effect of nitrogen doping and MWCNT intercalation for the graphene hybrid support for Pt nanoparticles with exemplary oxygen reduction reaction performance.,2018, 11(4): 1–13.
[61] CATIA A, SARA R, FRANCESCA S,. Graphene and carbon nanotube structures supported on mesoporous xerogel carbon as catalysts for oxygen reduction reaction in proton-exchange- membrane fuel cells., 2011, 36(8): 5038–5046.
[62] GHOSHL A, BASU S, VERMAL A. Graphene and functionalized graphene supported platinum catalyst for PEMFC., 2013, 13(3): 355–363.
[63] GRIGORIEV S A, FATEEV V N, PUSHKAREV A S,. Reduced graphene oxide and its modifications as catalyst supports and catalyst layer modifiers for PEMFC., 2018, 11(8): 1–15.
[64] XIN L, YANG F, RASOULI S,. Understanding Pt nanoparticle anchoring on graphene supports through surface functionalization., 2016, 6(4): 2642–2653.
[65] SERGRY A G, VLADIMIR N F, ARTEM S. Reduced graphene oxide and its modifications as catalyst supports and catalyst layer modifiers for PEMFC., 2018, 11(10): 1045–1056.
[66] WANG Q C, LEI Y P, WANG D S,. Defect engineering in earth-abundant electrocatalysts for CO2and N2reduction., 2019, doi: 10.1039/ c8ee03781g.
[67] XU X, YAN X M, ZHONG Z,The construction of porous graphene tri-doped with B, N and Co for enhanced oxygen reduction reaction., 2019, doi: 10.1016/j.carbon.2019.01.039.
[68] LEI Y P, SHI Q, HAN C,. N-doped graphene grown on silk cocoon-derived interconnected carbon fibers for oxygen reduction reaction and photocatalytic hydrogen production., 2016, 9(8): 2498–2509.
[69] WANG Q C, JI Y J, LEI Y P,. Pyridinic-N-dominated doped graphene with abundant defects as superior oxygen electrocatalyst for ultrahigh-energy-density Zn-air batteries., 2018, 3(1): 1183–1191.
[70] YANG X D, ZHENG Y P, YANG J,Modeling Fe/N/C catalysts in monolayer graphene., 2017, 7(1): 139–145.
[71] WANG Y, JIN J, YANG,. Highly active and stable platinum catalyst supported on porous carbon nanofibers for improved performance of PEMFC., 2015, 177(1): 181–189.
[72] WANG Y, LI G, JIN J H,. Hollow porous carbon nanofibers as novel support for platinum-based oxygen reduction reaction electrocatalysts.,2017, 42(9): 5938–5947.
[73] WANG Y, JIN J H, YANG S L,. Nitrogen-doped porous carbon nanofiber based oxygen reduction reaction electrocatalysts with high activity and durability.,2016, 41(26): 11174–11189
[74] SONG J, LI G, QIAO J. Ultrafine porous carbon fiber and its supported platinum catalyst for enhancing performance of proton exchange membrane fuel cells., 2015, 177(13): 46861–46878.
[75] YING J, LI J, JIANG G P,. Metal-organic frameworks derived platinum-cobalt bimetallic nanoparticles in nitrogen-doped hollow porous carbon capsules as a highly active and durable catalyst for oxygen reduction reaction., 2018, 225(1): 496–503.
[76] CHEN Z Y, WANG Q C, ZHANG X B,N-doped defective carbon with trace Co for efficient rechargeable liquid electrolyte-/all-solid-state Zn-air batteries.,2018, 60(9): 548–555.
Research Progress on Advanced Carbon Materials as Pt Support for Proton Exchange Membrane Fuel Cells
LUO Yi, FENG Junzong, FENG Jian, JIANG Yonggang, LI Liangjun
(Science and Technology on Advanced Ceramic Fibers and Composites Laboratory, College of Aerospace and Engineering, National University of Defense Technology, Changsha 410073, China)
Proton Exchange Membrane Fuel Cell (PEMFC) has the characteristics of high energy conversion efficiency, high power density, fast start-up at room temperature, low noise and zero pollution, which is expected to alleviate the energy crisis and reduce carbon dioxide emissions. It has broad application prospects in rail transit, aerospace and other fields. Catalyst is one of the key materials of PEMFC. Moreover, Pt catalysts are widely used and considered difficult to be replaced because of their good activity and stability in oxygen reduction reaction. Pt is expensive because of its limited storage. However, Pt loading could be significantly lessened by Pt support to improve PEMFC utilization. Carbon materials are widely used as Pt supports because of their low cost, high specific surface area, pore structure, adjustable conductivity and surface properties, but commercial carbon black supports have low utilization efficiency and poor electrochemical corrosion resistance for Pt. For realizing the large-scale application of PEMFC, it is necessary to develop new carbon supports which can uniformly disperse Pt, efficiently utilize Pt, be resistant to electrochemical corrosion, and have good conductivity, thus the performance and sustainability of PEMFC are improved. Carbon aerogels, carbon nanotubes, graphene and other new carbon supports with unique structures and properties, which are expected to improve PEMFC performance and life, have attracted the attention of many researchers. In this paper, the research progress on new carbon material as Pt support for PEMFC in recent years is reviewed systematically, and the development trend is also commented appropriately.
Proton Exchange Membrane Fuel Cell; carbon aerogel; carbon nanotube; graphene; review
TQ15
A
1000-324X(2020)04-0407-09
10.15541/jim20190169
2019-04-22;
2019-08-03
国家自然科学基金(51172279, 51302317, 51702360) National Natural Science Foundation of China (51172279, 51302317, 51702360)
罗燚(1994–), 男, 博士研究生. E-mail: nudtluoyi@163.com
LUO Yi(1994–), male, PhD candidate. E-mail: nudtluoyi@163.com
冯坚, 研究员. E-mail: fengj@nudt.edu.cn
FENG Jian, professor. E-mail: fengj@nudt.edu.cn

