Exercise, microglia, and beyond - workout to communicate with microglia
Microglia are brain-resident immune cells that use their ramified processes to survey the brain parenchyma. They maintain brain homeostasis by mediating immune responses through cytokine release,phagocytosing pathogens and protein aggregation (Salter et al., 2017).Furthermore, in both healthy and disease states, microglia interacting with neurons regulate synaptic formation, elimination and plasticity(Salter et al., 2017). It is thought that these functions may be altered by environmental stimuli such as lifestyle, stress, infection and air pollution, the effects of which may result in brain dysfunction and the development of neurodegenerative diseases (Branchi et al., 2014; Hanamsagar et al., 2017).
It has been suggested that exercise, a seemingly beneficial environmental stimulus, is able to modulate brain function by inducing functional changes in microglia. As an example, we recently showed that exercise stimulated microglia to engulf excess synapses in a mouse model of autism spectrum disorder (ASD), which resulted in a reversal of behavioral abnormalities (Andoh et al., 2019). While it is well known that exercise plays a role in preventing lifestyle diseases and osteoporosis,it may also be a therapeutic strategy to treat brain diseases such as depression and dementia (Pedersen et al., 2015). Exercise is advantageous as a therapeutic strategy, because it is essentially noninvasive and free from side effects when compared to interventions such as brain surgery and medication. Further, exercise is thought to have positive effects on both the physiological and psychological condition of a patient; these synergistic effects may lead to improvements in quality of life.
In order to utilize exercise for clinical purposes, it is essential to determine the type, strength, frequency and duration of exercise that is needed to provide a benefit. In the case of schizophrenia, a variety of exercises such as strength training, aerobic exercise and yoga have been shown to improve symptoms (Pedersen et al., 2015). In ASDs,playing with full-body interactive video games has been reported to provide therapeutic effects (Mairena et al., 2019). Video game-related recreation is attractive as it can provide exercise therapy without mental stress. In general, when exercise has beneficial effects on disease, there is a positive correlation between exercise intensity and therapeutic effect (Pedersen et al., 2015). However, it is difficult to utilize vigorous exercise as therapy for patients with some neurodevelopmental (i.e.,ASDs and schizophrenia) and neurodegenerative disorders (i.e., Parkinson’s disease and multiple sclerosis) because they often suffer from a considerable decline in motor skills. Thus, it may be necessary to start with moderate exercise to first improve the patient’s motor abilities and gradually increase the intensity to achieve a reduction in pathological symptoms - including those associated with mental disorders.
Would it be too late to start exercise intervention after the onset of disease? Previous studies have reported that exercise contributes to both the prevention and attenuation of depression and dementia(Pedersen et al., 2015). For ASDs and schizophrenia, exercise that is initiated after the onset of disease can improve behavioral symptoms(Pedersen et al., 2015; Mairena et al., 2019). In Parkinson’s disease and multiple sclerosis, exercise is used as a rehabilitation therapy to prevent the further deterioration of motor abilities (Pedersen et al., 2015).
There is accumulating evidence that exercise exerts positive effects in a variety of central nervous system disorders, but the underlying cellular and molecular mechanisms behind these benefits have yet to be fully elucidated. Several studies have suggested that the hippocampus is the region of the brain most affected by exercise (Cotman et al.,2007). Exercise promotes neurogenesis, angiogenesis, synaptic plasticity and carbohydrate metabolism in the hippocampus, which leads to anti-depressant effects and improved cognitive function. In the cerebral cortex and cerebellum, exercise was reported to promote angiogenesis.Further, it has been shown that exercise elevates the expression levels of brain-derived neurotrophic factor, insulin-like growth factor 1 (IGF-1)and vascular endothelial growth factor in several brain regions. IGF-1 production is also increased in peripheral organs such as the muscle and liver, which results in an increase of IGF-1 in the brain.
Inflammation, both inside and outside of the brain, is another key process for understanding the cellular and molecular links between exercise and its effect on brain functions. It has been reported that exercise can decrease systemic inflammation (Cotman et al., 2007). The increase in growth factors and reduction of pro-inflammatory cytokines that can be seen after exercise are signs of neuroprotection; therefore, researchers are interested in investigating how these changes affect the function of neurons. However, some researchers - ourselves included - have placed their hopes on microglia as the primary cell type responsible for the changes in brain function that are observed after exercise. Microglia are a major cell type involved in orchestrating inflammatory responses in the brain. For example, they are the main source of IGF-1 in the brain;IGF-1 suppresses microglial activation, leading to a dampened immune response (Labandeira-Garcia et al., 2017). In the 2000s, multiple studies were conducted to determine whether exercise modulated the cell number, morphology and cytokine expression of microglia in animal models of several brain diseases. However, few reports have further investigated whether and how microglial functions, such as phagocytosis and environmental surveillance, are affected by exercise. Here, we introduce several studies that have determined that exercise modulates microglial function, resulting in the amelioration of neurodegenerative diseases.
Exercise has been suggested to improve symptoms of multiple sclerosis and Parkinson’s disease. In the brains of these patients, microglia are often activated and immune responses are enhanced, which leads one to predict that exercise could reduce the severity of disease through the regulation of microglial activation. Using a mouse model of experimental autoimmune encephalomyelitis, which shares pathology with multiple sclerosis, Benson et al. (2015) conducted a study in which mice were exercised by voluntary wheel running. In the exercise group,there was a decrease in the number of Ionized calcium binding adaptor molecule 1-positive cells, presumed to be mostly microglia. These cells exhibited ramified morphology in the spinal cord. The level of reactive oxidative stress - which induces inflammation, demyelination and neuronal death - was also reduced, likely reflecting a decrease in microglial activation. Further, it was shown that exercise delayed disease onset and reduced pain hypersensitivity, indicating that exercise may prevent experimental autoimmune encephalomyelitis by blocking microglial activation.
Sung et al. (2012) investigated whether treadmill running could ameliorate the pathology of Parkinson’s disease. They used a mouse model of Parkinson’s disease that is induced by injecting mice with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid, which selectively penetrates and kills dopaminergic neurons. Treadmill running suppressed the loss of dopaminergic neurons in the substantia nigra pars compacta and striatum, improving motor performance in the Rotarod test. In the same brain regions, exercise reduced the number of CD11b positive cells, most of which are presumed to be microglia. The expression level of microglial inducible nitric oxide synthase, which promotes inflammation, was also reduced. Further, the authors showed that exercise enhanced CD200-CD200R signaling, a neuron-microglia interaction pathway that is known to suppress microglial activation, in the ventral midbrain. These results suggest that exercise decreases microglial activation, thus suppressing further loss of dopaminergic neurons. It should be noted that in this study, exercise was introduced in the disease development phase. Therefore, further studies are required to determine whether exercise can restore degenerated neural circuits in the late phase of disease.
Two recent studies reported that exercise reversed abnormalities in neural circuits. Giorgetti et al. (2019) showed that exercise improved the neurotransmission of α motor neurons, which is known to decrease during normal aging. In C57BL/6J mice, neurotransmission from α motor neurons to muscles begins to decrease at 12 months of age. The researchers introduced voluntary wheel running to mice starting at 16 months of age, when the decline in neurotransmission was complete.Two months of wheel running restored neurotransmission to the level observed in 12-month old mice. Aging increased the gene expression levels of microglial activation markers such as CD86, CD68, MHCII and CD11b. Conversely, neuronal genes related to synapses, axons and neurotransmitters were downregulated with aging. These transcriptomic changes were normalized by voluntary running. Together, these results suggest that the increased microglial activation that occurs with aging causes dysfunction of α motor neurons, which can be reversed by exercise. Furthermore, the depletion of microglia (via colony stimulating factor 1 receptor inhibition) in mice from 16 to 18 months of age resulted in effects similar to those obtained by voluntary running. Thus,exercise was able to directly change microglial state and function.
Andoh et al. (2019) showed that exercise reversed synaptic abnormalities in a mouse model of ASD that was induced by maternal immune activation using the synthetic double-stranded RNA, polyinosinic:polycytidylic acid, to mimic viral infection. The authors introduced voluntary wheel running in adolescent mice that were exhibiting ASDlike behaviors and had excess synapses in the hippocampal CA3 region.They found that one month of exercise normalized the synaptic density in the hippocampus to control levels and attenuated ASD-like behaviors. Next, they tested whether exercise was able to decrease synaptic density by promoting synaptic engulfment by microglia. They showed that the ability of microglia to engulf synapses was impaired in ASD mice during the postnatal period, but that exercise during adolescence reversed it. Further, it was shown that exercise activated a portion of the dentate granule cells, whose axons provide synaptic contacts with CA3 pyramidal cell dendrites. This may induce synaptic competition in the CA3 region, leading to neuronal activity-dependent synaptic engulfment by microglia. Finally, increased synaptic engulfment was blocked by administration of minocycline, a tetracycline antibiotic that is widely used to suppress microglial activation. This is the first study to show that exercise “activates” microglia via increased neuronal activity.
Maternal stress induced by infection (bacterial or viral), toxins and/or exposure to air pollution affect the development and function of microglia in fetuses (Hanamsagar et al., 2017). These environmental factors are suggested to increase the risk of ASD development in children,implying that ASDs may be microglia-oriented diseases. An increasing number of studies have shown that microglial dysfunction causes the pathogenesis of not only ASDs but other neurodegenerative diseases as well, highlighting the potential usefulness of therapeutic strategies targeting microglia. The functional state of microglia is very sensitive to environmental stimuli, which raises the possibility that symptoms of brain disorders can be improved by regulating microglial function using environmental stimuli. The work by Andoh et al. (2019) provided an example of a therapeutic intervention for improving neurological disease through environmental manipulation of microglial function.
In the current perspective, we introduced studies that suggest that exercise can normalize the function of microglia when they are either highly activated or highly inactivated in brain diseases (Figure 1).Further studies focusing on the cellular and molecular mechanisms by which exercise attenuates symptoms of brain disease are needed to fully elucidate the effects of exercise on microglial functions.
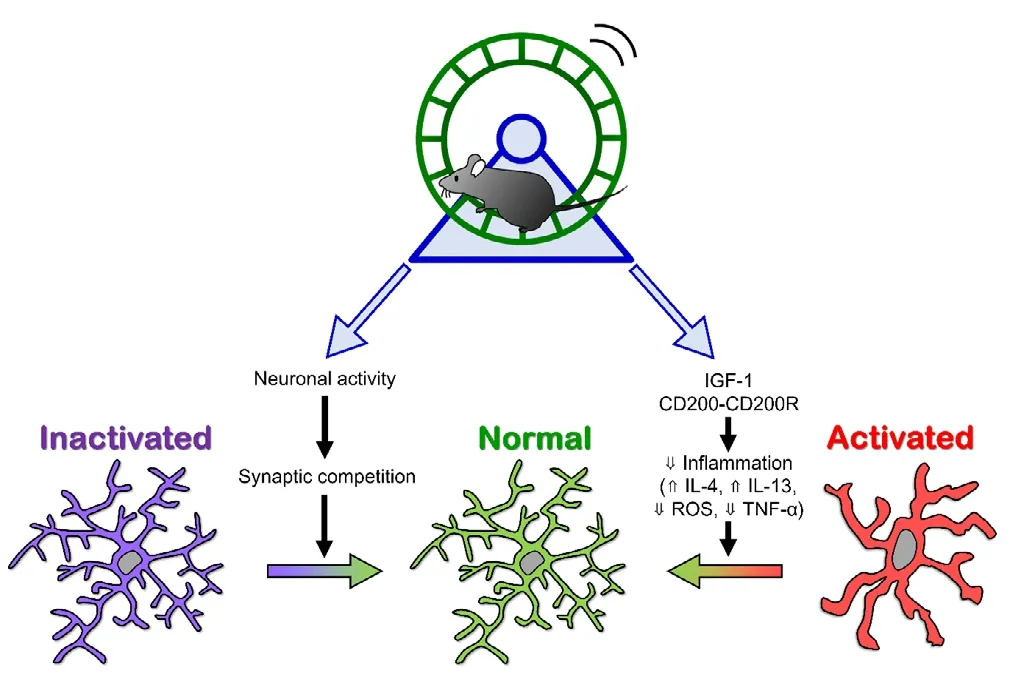
Figure 1 Possible mechanisms by which exercise modulates microglial states.
This work was supported in part by a Grant-in-Aid for Scientific Research(B) (17H03988, to RK) from JSPS and JST PRESTO (JPMJPR18H4, to RK).
Megumi Andoh, Ryuta Koyama*
Laboratory of Chemical Pharmacology, Graduate School of Pharmaceutical Sciences, The University of Tokyo, Bunkyo-ku,Tokyo, Japan
*Correspondence to:Ryuta Koyama, PhD,rkoyama@mol.f.u-tokyo.ac.jp.
orcid:0000-0001-7108-174X (Ryuta Koyama)
Received:December 29, 2019
Peer review started:January 10, 2020
Accepted:February 27, 2020
Published online:May 11, 2020
doi:10.4103/1673-5374.282241
Copyright license agreement:The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Anna Maria Colangelo, University of Milano-Bicocca,Italy; Heling Chu, Huashan Hospital, Fudan University, China.
- 中国神经再生研究(英文版)的其它文章
- The role of the TrkB-T1 receptor in the neurotrophin-4/5 antagonism of brain-derived neurotrophic factor on corticostriatal synaptic transmission
- Could non-invasive brain-stimulation prevent neuronal degeneration upon ion channel re-distribution and ion accumulation after demyelination?
- The role of exercise in brain DNA damage
- Combined effect of repetitive transcranial magnetic stimulation and physical exercise on cortical plasticity
- Should mast cells be considered therapeutic targets in multiple sclerosis?
- Neuroprotection mediated by natural products and their chemical derivatives