RNAi在抗蚊媒病毒感染中的研究进展
魏勇,何玉兰,郑学礼
综 述
RNAi在抗蚊媒病毒感染中的研究进展
魏勇,何玉兰,郑学礼
南方医科大学公共卫生学院病原生物学系,广州 510515
蚊媒病因具有较高的发病率和传播率使其成为全球关注的重要公共卫生问题。蚊虫作为蚊媒病的传播媒介,研究其与蚊媒病毒两者之间的相互作用机制将有助于蚊媒病的防控。蚊虫抵御蚊媒病毒的先天免疫降低和病毒成功逃避蚊虫免疫屏障为病毒在蚊虫体内的持续感染和蚊媒病的暴发流行造成了潜在风险。RNA干扰(RNA interference, RNAi)途径作为蚊虫体内强大的抗病毒防御屏障,通过产生多种小RNA降解病毒RNA,从而达到抑制病毒复制和传播的目的。本文对小干扰RNA (small interfering RNA, siRNA)、微小RNA (microRNA, miRNA)、Piwi蛋白相作用RNA (Piwi-interacting RNA, piRNA)等3种小分子RNA在蚊虫体内发挥抗蚊媒病毒感染的先天免疫机制的相关研究进行了综述,以期为蚊媒病的防控提供理论参考。
蚊虫;蚊媒病毒;siRNA;miRNA;piRNA
蚊媒病是一类由病媒蚊虫传播的自然疫源性疾病,常见的有流行性乙型脑炎、登革热、黄热病等。随着全球气候变暖、交通运输便捷和旅游业发展,蚊虫在全球范围内快速扩张,这为蚊媒病的暴发流行和传播扩散构成了潜在风险。绝大多数蚊媒病毒以及在全球发病率和死亡率占较大比重的均是RNA病毒,如黄病毒科黄病毒属(正链RNA病毒)、披膜病毒科甲病毒属(正链RNA病毒)、布尼亚病毒科白蛉病毒属(负链RNA病毒)[1,2]。黄病毒科黄病毒属包括黄热病病毒(yellow fever virus, YFV)、登革病毒(Dengue virus, DENV)、乙型脑炎病毒(Japanese encephalitis virus, JEV)、西尼罗河病毒(West Nile virus, WNV)和寨卡病毒(Zika virus, ZIKV)等[3],披膜病毒科甲病毒属包括基孔肯雅病毒(Chikungunya virus, CHIKV)、辛德毕斯病毒(Sindbis virus, SINV)、塞姆利基森林病毒(Semliki forest virus, SFV)、罗斯河病毒(Ross river virus, RRV)等[4],布尼亚病毒科白蛉病毒属包括托斯卡纳病毒(Toscana virus, TOSV)、裂谷热病毒(Rift Valley fever virus, RVFV)等[5]。在过去的几十年间,登革热至少在128个国家或地区暴发流行,每年平均有3.9亿人口感染登革热,登革热一直是重点关注的全球公共卫生问题[6]。寨卡病毒病是近年来新兴的蚊媒传播性疾病,2016年2月世界卫生组织(WHO)宣布将寨卡疫情列为全球紧急公共卫生事件。自从2015年巴西发生大规模寨卡病毒感染疫情后,目前已有64个国家和地区报告了寨卡疫情[7]。寨卡病毒感染引起的成人格林-巴利综合征(Guillain-Barre syndrome, GBS)和新生儿出生缺陷,如产前感染所致的小头畸形,在近年来引发了全球广泛关注[8]。因目前缺乏蚊媒病相应的有效疫苗,抑制病毒在蚊虫体内复制和阻断病毒传播将是控制蚊媒病暴发流行的有效途径[9]。因此,研究蚊虫体内抗蚊媒病毒感染的先天免疫机制将有助于制定相应的蚊媒病控制策略。本文将对目前蚊虫体内3大主要抗病毒的RNAi途径的相关研究进行综述,以期为蚊媒病的防控提供理论参考。
1 蚊虫体内三大主要的RNAi途径
蚊虫的先天免疫屏障和蚊媒病毒的免疫逃避机制是影响病毒在蚊虫体内成功复制和传播的关键因素。蚊虫体内抗病毒的效应分子(抗菌肽(antimicrobial peptide, AMP)、活性氧(reactive oxygen species, ROS)和酚氧化酶级联反应的组分等),以及效应分子所依赖的信号通路(JAK-STAT、Toll和Imd等信号通路)是蚊虫体内免疫防御的重要方面[10~12]。此外,RNA干扰(RNAi)途径是蚊虫体内最为强大的抗病毒防御体系[13]。RNAi是指由双链RNA(dsRNA)诱发的具有高度保守的小RNA片段可高效特异性降解同源mRNA的现象,是转录后水平的基因沉默(post- transcriptional gene silencing, PTGS)。RNAi途径主要包含3种类型的小RNA:小干扰RNA (siRNA)、微小RNA (miRNA)和Piwi蛋白相作用RNA (piRNA),其中siRNA为蚊虫体内抗病毒免疫的主要小RNA分子[13~15]。RNAi具有高度的序列特异性,严格按照碱基互补配对原则与同源基因的mRNA结合并进行降解,从而实现针对目的基因的精准沉默;RNAi具有高效抑制基因表达的特性,表型可达到缺失突变体表型的程度;RNAi还具有可遗传性和可传播性,RNA干扰效应能稳定遗传给下一代,也可穿过细胞界限,传播至扩散处细胞乃至整个机体[16,17]。利用RNAi特性发展的RNAi技术可广泛地应用于基因功能的探索、传染性疾病和恶性肿瘤的治疗领域[18~21]。
2 siRNA在蚊虫体内的抗病毒作用
siRNA途径可分为内源性通路和外源性通路。内源性siRNA一般由细胞内基因双向转录形成的部分互补配对或由RNA依赖性RNA聚合酶(RNA- dependent RNA polymerase, RDRP)进行的RNA链复制等形成的dsRNA经剪切修饰而产生,在生物体不同生长发育时期发挥调控作用。外源性siRNA一般是由转基因技术或病毒感染细胞后产生的复制中间体—dsRNA经过核糖核酸酶III(RNase III)家族中的Dicer-2核酸内切酶剪切成长度约为21~25 nt的双链小RNA分子[22,23]。细胞内siRNA通过与AGO、TRBP和PACT等蛋白分子结合形成RNA诱导的沉默复合物(RNA-induced silencing complex, RISC),siRNA的随从链被降解,引导链通过碱基互补配对的方式找到靶基因的mRNA或同源的病毒RNA,然后RISC中的Ago-2蛋白降解其mRNA或病毒RNA,阻止翻译表达或病毒复制(图1)[24,25]。
Li等[26]首次在冈比亚按蚊()细胞系中验证了siRNA的抗病毒作用,并表明其抑制效应依赖于Ago-2蛋白。Sánchez-Vargas等[27]研究发现沉默埃及伊蚊() siRNA通路会增加蚊虫体内登革2型病毒的复制,这表明siRNA通路在蚊虫体内病毒复制过程中发挥抑制作用。Khoo等[28]通过转基因技术破坏埃及伊蚊中肠的siRNA通路,发现这样会增加中肠内辛德毕斯病毒(SINV)的复制和播散率。Basu等[29]通过基因敲除抑制埃及伊蚊Dcr-2酶的表达,发现蚊虫体内抗辛德毕斯病毒的免疫反应下降;另外,Dcr-2基因突变型的埃及伊蚊较野生型的蚊虫体内具有显著增加的黄热病病毒复制水平[30]。多项研究表明,在敲除siRNA通路的相关成分后,成蚊体内或培养的蚊虫细胞系中的病毒复制水平会显著升高[27,31]。成蚊或不同细胞系感染蚊媒病毒后会导致该病毒来源的siRNA (virus-derived small interfering RNA, vsiRNA)产生[32,33]。Myles等[34]研究表明vsiRNA对蚊虫体内抗甲病毒感染具有重要调控作用。能够表达dsRNA结合蛋白和病毒RNA沉默抑制因子(viral suppressors of RNA silencing, VSRs)的重组甲病毒感染埃及伊蚊和冈比亚按蚊后,蚊虫体内的vsiRNA显著下降,从而导致病毒复制量和蚊虫死亡率显著增加。

图1 siRNA的生物合成和作用机制
单链RNA逆转录形成双链RNA,经Dicer-2核酸内切酶剪切成双链小RNA分子,其引导链与Ago-2等相应蛋白组成RNA诱导沉默复合物,通过碱基互补配对的方式结合靶标RNA,并将该靶标RNA降解。
蚊媒病毒在受到蚊虫宿主先天免疫消除的同时,也在不断进化适应宿主的生理环境,并对抗媒介蚊虫的抗病毒免疫途径。通过鉴别蚊媒病毒基因组中编码的病毒RNA沉默抑制因子(VSRs),了解病毒蛋白对蚊虫免疫途径的拮抗作用,将有助于理解蚊媒病毒在蚊虫体内长期感染和传播的机制,并应用于蚊媒病的防控[35]。布尼亚维拉病毒(Bunyamwera virus, BUNV)S片段上的非结构蛋白(non-structure proteins, NSs)是VSRs,NSs缺陷性BUNV在Dcr-2缺陷性的蚊虫细胞系中的复制水平要高于在正常蚊虫细胞系中的复制水平,NSs缺陷性BUNV在埃及伊蚊体内的感染能力要低于野生型BUNV[36]。Kakumani等[37]通过体外实验证明登革热病毒(DENV) NS4B蛋白能够干扰Dicer对siRNA的加工处理过程。哺乳动物细胞中的基因沉默实验显示NS4B蛋白的跨膜结构域3 (transmembrane domain 3, TMD3)和TME5参与VSRs抑制病毒增殖的活性,其具体机制目前尚不明确。Samuel等[38]研究发现在Dcr-2缺陷性的蚊虫细胞系中表达黄病毒衣壳蛋白(yellow fever virus capsid, YFC)的SINV与不表达YFC的SINV的增殖能力和毒力相似,并且验证了YFC作为VSRs拮抗siRNA途径。YFC对siRNA途径拮抗作用可能是非特异性结合双链RNA(dsRNA),干扰Dicer产生vsiRNA。虽然VSRs有多种不同的蛋白,但它与dsRNA非特异性结合是探讨其拮抗作用的主要内容。
3 miRNA在蚊虫体内的抗病毒作用
miRNA是一类内源性的非编码小RNA (18~ 25 nt),通过降解mRNA或抑制mRNA翻译来调控靶基因转录后表达水平[39]。与siRNA途径相似,miRNA途径也始于dsRNA剪切成小的双链RNA,其中一条引导链加载到RISC中,然后RISC中Ago-2蛋白降解靶基因的mRNA或同源的病毒RNA[40]。miRNA与siRNA途径的主要区别在于所发生的亚细胞结构位置和所参与的效应蛋白分子[41]。siRNA的转录、剪切和加工过程主要发生在细胞质中,而编码miRNA的基因在宿主RNA聚合酶Ⅱ的作用下转录成miRNA初始转录物(primary miRNA, pri-miRNA),然后由Drosha剪切加工成miRNA前体物(precursor miRNA, pre-miRNA),这一过程均是在细胞核内完成。pre-miRNA输出到细胞质后,由Dicer-1进一步加工至成熟的miRNA,并加载到RISC中发挥RNA干扰作用(图2)[42]。
多种蚊媒病毒均利用宿主miRNA通路来逃避宿主的抗病毒免疫反应,从而增加病毒复制和致病力[43]。Hussain等[44]在西尼罗河病毒(WNV)RNA序列的3¢端非编码区发现了类似miRNA的小RNA片段,并通过Northern印记杂交的方法检测到感染WNV的埃及伊蚊和白纹伊蚊()细胞系中存在一种病毒来源的成熟miRNA,命名为KUN-miR-1。KUN-miR-1通过上调转录因子GATA4的表达来促进病毒在蚊虫细胞内的增殖。Hussain 等[45]通过二代测序技术发现感染登革2型病毒(DENV-2)的埃及伊蚊体内含有6种病毒来源的miRNA样的小RNA,称为vsRNA 1~6;其中vsRNA5已证实与病毒增殖有关。蚊虫体内miRNA通路关键分子的功能丧失性突变将有助于鉴定其分子特性,并确定KUN-miR-1和DENV-2-vsRNA 1~6的生物发生机制和抗病毒免疫机制。
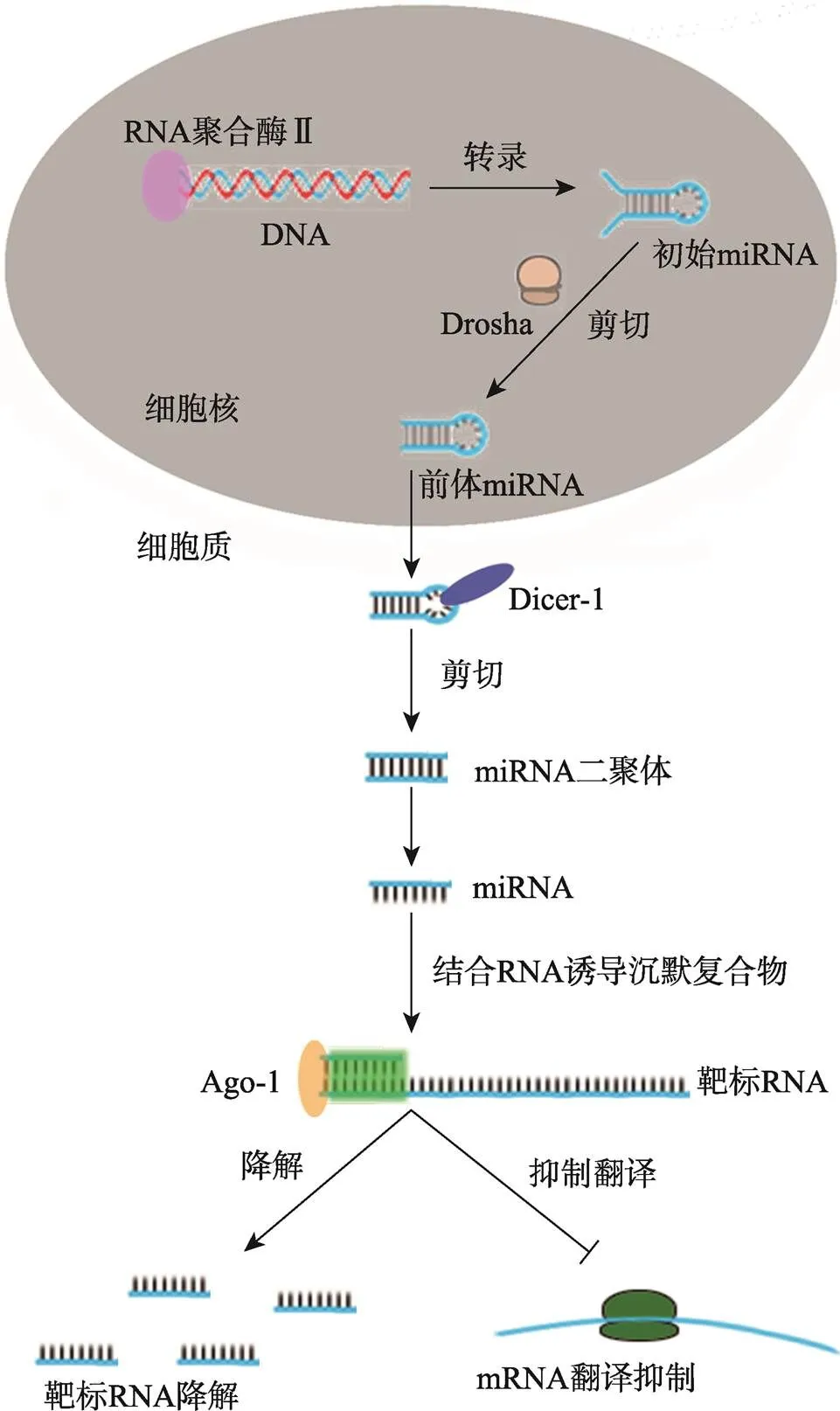
图2 miRNA的生物合成和作用机制
含miRNA序列的DNA经过转录后形成初始miRNA,然后经Drosha和Dicer-1等蛋白剪切加工修饰后形成成熟的miRNA,成熟的miRNA与Ago-1等相应蛋白组成RNA诱导沉默复合物,通过碱基互补配对的方式结合靶标RNA,并将该靶标RNA降解或抑制mRNA翻译。
当蚊虫感染蚊媒病毒后体内会出现多种miRNA差异性表达[46,47]。Su等[48]利用含DENV-2的血餐以及不含DENV-2的血餐分别喂食白纹伊蚊,鉴定蚊虫中肠内差异性表达的miRNA,相比于喂食不含DENV-2血餐的白纹伊蚊,一共有43个miRNA上调,4个miRNA下调;并且上调的miRNA中aal-miR- 4728-5p瞬时转入C6/36蚊虫细胞后能够增强DENV-2在细胞内的复制。Su等[49]再次利用含DENV-2的血餐喂食白纹伊蚊,将感染上DENV-2与未感染上DENV-2的白纹伊蚊中肠内miRNA进行差异性分析,发现感染上DENV-2的白纹伊蚊中肠内有15个miRNA上调,2个miRNA下调。其中miR-1767和miR-276-3p能够增强DENV-2在C6/36细胞内的复制,而miR-4448抑制DENV-2在C6/36细胞内的复制。
4 piRNA在蚊虫体内的抗病毒作用
piRNA途径可在siRNA途径缺陷的条件下进行抗病毒免疫,是RNAi介导的抗病毒免疫应答的相互补充[50]。与siRNA/miRNA相比,piRNA的生成不依赖Dicer,而依赖PIWI亚家族蛋白;piRNA的长度约为26~32 nt,piRNA基因簇主要分别在转座子和重复序列等区域;piRNA的3'端会出现甲基化修饰,可能与其稳定性或功能有关[51~53]。piRNA的生成涉及3种PIWI蛋白,包括Piwi、Aub和Ago-3,共同形成piRNA诱导的沉默复合物(piRNA-induced silencing complex, piRISC)[54]。对于piRNA的生成,目前提出了“乒乓”循环扩增模型,piRNA从转录前体物中循环扩增[55]。反义链piRNA与Aub和Piwi结合形成具有核酸酶活性的piRNA复合物(piRNA complex, piRC),piRC能结合正义链前体piRNA并将其剪切加工成具有成熟5¢端的前体piRNA,然后该正义链前体piRNA经Zuc等酶剪切3¢末端形成成熟的正义链piRNA;反之,正义链piRNA与Ago-3结合形成piRC,然后以同样的方式形成反义链piRNA (图3)[56]。piRNA途径在生殖遗传、配子形成、胚胎发育、基因转座、基因沉默和病毒增殖等方面均有调控作用。
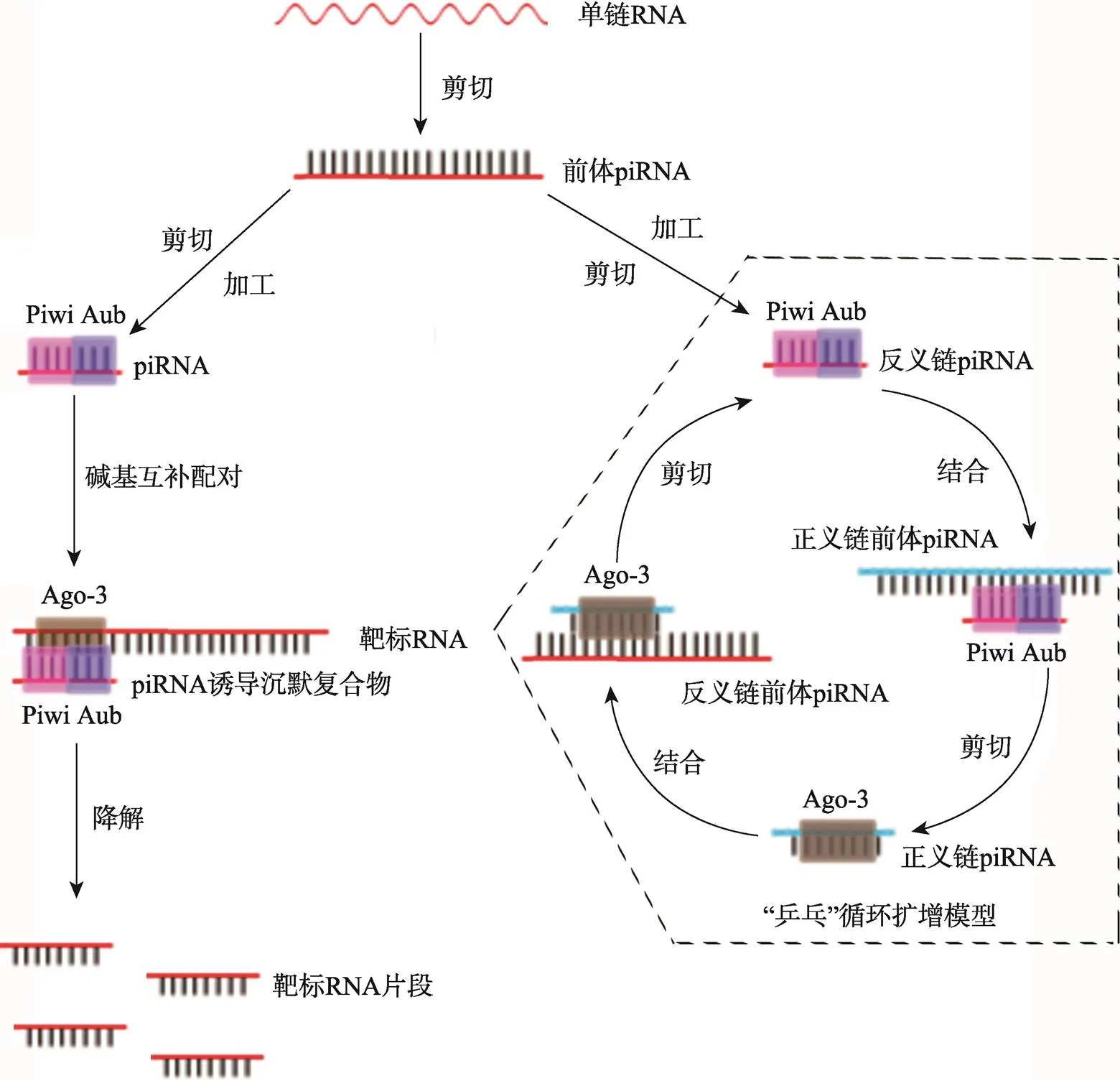
图3 piRNA的生物合成和作用机制
单链RNA经剪切形成前体piRNA,然后经Zuc等酶剪切加工后形成成熟的piRNA,成熟的piRNA与Piwi、Aub和Ago-3等蛋白组成piRNA诱导沉默复合物,通过碱基互补配对的方式结合靶标RNA,并将该靶标RNA降解。虚线框内为piRNA的“乒乓”循环扩增模型。
蚊虫或蚊虫细胞系感染蚊媒病毒后,能够产生一类依赖“乒乓”模型的病毒来源的piRNA (virus- derived piRNA, vpiRNA),这些vpiRNA不同于以往研究中来源于重复序列元件或piRNA簇的piRNA[57,58]。Morazzani等[57]在感染有基孔肯亚热病毒(CHIKV)的埃及伊蚊和白纹伊蚊体内检测到vpiRNA。Miesen等[59]用SINV感染埃及伊蚊细胞系后,通过免疫沉淀反应和小RNA的Northern免疫印迹检测到了Ago-3和Piwi-5蛋白特异性富集的vpiRNA。通过对这类vpiRNA测序分析,显示反义链vpiRNA倾向于与Piwi-5结合,而正义链vpiRNA倾向于与Ago-3结合,这也表明了这两种蛋白在“乒乓”模型中的作用机制。抑制vpiRNA在siRNA途径缺陷的蚊虫细胞系中表达,将会加重受病毒感染细胞的病变程度,这体现了piRNA途径在siRNA途径缺陷的蚊虫细胞系中的抗病毒作用[57]。Schnettler等[60]通过深度测序检测到塞姆利基森林病毒(SFV)来源的piRNA,敲除Piwi-4基因后会导致Aag2埃及伊蚊细胞内SFV复制增加,表明了piRNA途径在抗病毒免疫中的重要作用。
5 结语与展望
每年全球均有大量的蚊媒病暴发流行,并且目前大部分的蚊媒病缺乏有效的疫苗进行预防,所以阻止蚊媒病毒传播一直是蚊媒病预防控制的重要任务。目前有关化学杀虫剂和生物防治等方面的防控措施主要是通过控制蚊虫数量来控制蚊媒病的暴发流行。病毒感染和蚊虫防御机制以及两者动态平衡的演变过程一直是科研工作者关注和探索的问题,深入了解蚊虫先天免疫系统如何抵御病毒感染,病毒如何在蚊虫体内持续稳定增殖,以及不同蚊虫种类或病毒株如何影响疾病暴发流行等方面内容,将有助于提高现有策略的有效性,并提出新的蚊媒控制策略。RNAi途径作为蚊虫体内主要的抗病毒防御体系,目前我国科研工作者对蚊虫RNAi途径的研究主要集中在相关小RNA分子的作用机制,然而通过基因编辑加强蚊虫RNAi抗病毒免疫的相关研究较少,将转基因蚊广泛有效地应用于蚊媒病防控也是任重道远。
[1] Gubler DJ. Human arbovirus infections worldwide., 2001, 951: 13–24.
[2] Beckham JD, Tyler KL. Arbovirus infections., 2015, 21: 1599–1611.
[3] Laureti M, Narayanan D, Rodriguez-Andres J, Fazakerley JK, Kedzierski L. Flavivirus receptors: diversity, identity, and cell entry., 2018, 9: 2180.
[4] Lim EXY, Lee WS, Madzokere ET, Herrero LJ. Mosquitoes as suitable vectors for alphaviruses., 2018, 10(2): E84.
[5] Wuerth JD, Weber F. Phleboviruses and the type I interferon response., 2016, 8(6): 174.
[6] Dash AP, Bhatia R, Sunyoto T, Mourya DT. Emerging and re-emerging arboviral diseases in Southeast Asia., 2013, 50(2): 77–84.
[7] Lowe R, Barcellos C, Brasil P, Cruz OG, Honório NA, Kuper H, Carvalho MS. The Zika virus epidemic in Brazil: From discovery to future implications., 2018, 15(1): 96.
[8] Weaver SC, Costa F, Garcia-Blanco MA, Ko AI, Ribeiro GS, Saade G, Shi PY, Vasilakis N. Zika virus: History, emergence, biology, and prospects for control., 2016, 130: 69–80.
[9] Pang T, Mak TK, Gubler DJ. Prevention and control of dengue-the light at the end of the tunnel., 2017, 17(3): e79–e87.
[10] Xi ZY, Ramirez JL, Dimopoulos G. Thetoll pathway controls dengue virus infection., 2008, 4(7): e1000098.
[11] Souza-Neto JA, Sim S, Dimopoulos G. An evolutionary conserved function of the JAK-STAT pathway in anti- dengue defense., 2009, 106(42): 17841–17846.
[12] Liu XM, Yuan ML. Progress in innate immunity-related genes in insects., 2018, 40(6): 451–466.刘小民, 袁明龙. 昆虫天然免疫相关基因研究进展. 遗传, 2018, 40(6): 451–466.
[13] Ding SW, Voinnet O. Antiviral immunity directed by small RNAs., 2007, 130(3): 413–426.
[14] Nandety RS, Kuo YW, Nouri S, Falk BW. Emerging strategies for RNA interference (RNAi) applications in insects., 2015, 6(1): 8–19.
[15] Lee WS, Webster JA, Madzokere ET, Stephenson EB, Herrero LJ. Mosquito antiviral defense mechanisms: a delicate balance between innate immunity and persistent viral infection., 2019, 12(1): 165.
[16] Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in., 1998, 391(6669): 806–811.
[17] Saleh MC, Tassetto M, van Rij RP, Goic B, Gausson V, Berry B, Jacquier C, Antoniewski C, Andino R. Antiviral immunity inrequires systemic RNA interference spread., 2009, 458(7236): 346–350.
[18] Puglise JM, Estep AS, Becnel JJ. Expression profiles and RNAi silencing of inhibitor of apoptosis transcripts in,, andMosquitoes (Diptera: Culicidae)., 2016, 53(2): 304–314.
[19] Kang S, Hong YS. RNA interference in infectious tropical diseases., 2008, 46(1): 1–15.
[20] Gandhi NS, Tekade RK, Chougule MB. Nanocarrier mediated delivery of siRNA/miRNA in combination with chemotherapeutic agents for cancer therapy: current progress and advances., 2014, 194: 238–256.
[21] Zheng WH, Lin ZQ, Zhuo M, Du HL, Wang XN. Research progress on influenza antiviral small RNAs., 2012, 34(5): 526–532.郑维豪, 林志强, 卓敏, 杜红丽, 王小宁. 抗流感病毒小RNAs研究进展. 遗传, 2012, 34(5): 526–532.
[22] Galiana-Arnoux D, Dostert C, Schneemann A, Hoffmann JA, Imler JL. Essential function in vivo for Dicer-2 in host defense against RNA viruses in drosophila., 2006, 7(6): 590–597.
[23] Wang XH, Aliyari R, Li WX, Li HW, Kim K, Carthew R, Atkinson P, Ding SW. RNA interference directs innate immunity against viruses in adult., 2006, 312(5772): 452–454.
[24] Matranga C, Tomari Y, Shin C, Bartel DP, Zamore PD. Passenger-strand cleavage facilitates assembly of siRNA into Ago2-containing RNAi enzyme complexes., 2005, 123(4): 607–620.
[25] Miyoshi K, Tsukumo H, Nagami T, Siomi H, Siomi MC. Slicer function ofargonautes and its involvement in RISC formation., 2005, 19(23): 2837–2848.
[26] Li WX, Li H, Lu R, Li F, Dus M, Atkinson P, Brydon EW, Johnson KL, García-Sastre A, Ball LA, Palese P, Ding SW. Interferon antagonist proteins of influenza and vaccinia viruses are suppressors of RNA silencing., 2004, 101(5): 1350–1355.
[27] Sánchez-Vargas I, Scott JC, Poole-Smith BK, Franz AW, Barbosa-Solomieu V, Wilusz J, Olson KE, Blair CD. Dengue virus type 2 infections ofare modulated by the mosquito's RNA interference pathway., 2009, 5(2): e1000299.
[28] Khoo CC, Piper J, Sanchez-Vargas I, Olson KE, Franz AW. The RNA interference pathway affects midgut infection- and escape barriers for Sindbis virus in., 2010, 10: 130.
[29] Basu S, Aryan A, Overcash JM, Samuel GH, Anderson MA, Dahlem TJ, Myles KM, Adelman ZN. Silencing of end-joining repair for efficient site-specific gene insertion after TALEN/CRISPR mutagenesis in., 2015, 112(13): 4038–4043.
[30] Samuel GH, Wiley MR, Badawi A, Adelman ZN, Myles KM. Yellow fever virus capsid protein is a potent suppressor of RNA silencing that binds double-stranded RNA., 2016, 113(48): 13863–13868.
[31] Keene KM, Foy BD, Sanchez-Vargas I, Beaty BJ, Blair CD, Olson KE. RNA interference acts as a natural antiviral response to O'nyong-nyong virus (Alphavirus; Togaviridae) infection of., 2004, 101(49): 17240–17245.
[32] Brackney DE, Beane JE, Ebel GD. RNAi targeting of West Nile virus in mosquito midguts promotes virus diversification., 2009, 5(7): e1000502.
[33] Myles KM, Morazzani EM, Adelman ZN. Origins of alphavirus-derived small RNAs in mosquitoes., 2009, 6(4): 387–391.
[34] Myles KM, Wiley MR, Morazzani EM, Adelman ZN. Alphavirus-derived small RNAs modulate pathogenesis in disease vector mosquitoes., 2008, 105(50): 19938–19943.
[35] Schuster S, Zirkel F, Kurth A, van Cleef KWR, Drosten C, van Rij RP, Junglen S. A unique nodavirus with novel features: mosinovirus expresses two subgenomic RNAs, a capsid gene of unknown origin, and a suppressor of the antiviral RNA interference pathway., 2014, 88(22): 13447–13459.
[36] Szemiel AM, Failloux AB, Elliott RM. Role of Bunyamwera Orthobunyavirus NSs protein in infection of mosquito cells., 2012, 6(9): e1823.
[37] Kakumani PK, Ponia SS, S RK, Sood V, Chinnappan M, Banerjea AC, Medigeshi GR, Malhotra P, Mukherjee SK, Bhatnagar RK. Role of RNA interference (RNAi) in dengue virus replication and identification of NS4B as an RNAi suppressor., 2013, 87(16): 8870–8883.
[38] Samuel GH, Wiley MR, Badawi A, Adelman ZN, Myles KM. Yellow fever virus capsid protein is a potent suppressor of RNA silencing that binds double-stranded RNA., 2016, 113(48): 13863– 13868.
[39] Jonas S, Izaurralde E. Towards a molecular understanding of microRNA-mediated gene silencing., 2015, 16(7): 421–433.
[40] Pedersen IM, Cheng GF, Wieland S, Volinia S, Croce CM, Chisari FV, David M. Interferon modulation of cellular microRNAs as an antiviral mechanism., 2007, 449(7164): 919–922.
[41] Lee YS, Nakahara K, Pham JW, Kim K, He Z, Sontheimer EJ, Carthew RW. Distinct roles forDicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways., 2004, 117(1): 69–81.
[42] Xia MM, Shen XY, Niu CM, Xia J, Sun HY, Zheng Y. MicroRNA regulates Sertoli cell proliferation and adhesion., 2018, 40(9): 724–732.夏蒙蒙, 申雪沂, 牛长敏, 夏静, 孙红亚, 郑英. MicroRNA参与调控睾丸支持细胞的增殖与粘附功能. 遗传, 2018, 40(9): 724–732.
[43] Trobaugh DW, Klimstra WB. MicroRNA regulation of RNA virus replication and pathogenesis., 2017, 23(1): 80–93.
[44] Hussain M, Torres S, Schnettler E, Funk A, Grundhoff A, Pijlman GP, Khromykh AA, Asgari S. West Nile virus encodes a microRNA-like small RNA in the 3' untranslated region which up-regulates GATA4 mRNA and facilitates virus replication in mosquito cells., 2012, 40(5): 2210–2223.
[45] Hussain M, Asgari S. MicroRNA-like viral small RNA from Dengue virus 2 autoregulates its replication in mosquito cells., 2014, 111(7): 2746–2751.
[46] Campbell CL, Harrison T, Hess AM, Ebel GD. MicroRNA levels are modulated inafter exposure to Dengue-2., 2014, 23(1): 132–139.
[47] Slonchak A, Hussain M, Torres S, Asgari S, Khromykh AA. Expression of mosquito microRNA Aae-miR-2940-5p is downregulated in response to West Nile virus infection to restrict viral replication., 2014, 88(15): 8457– 8467.
[48] Su JX, Li CX, Zhang YM, Yan T, Zhu XJ, Zhao MH, Xing D, Dong YD, Guo XX, Zhao TY. Identification of microRNAs expressed in the midgut ofduring dengue infection., 2017, 10(1): 63.
[49] Su JX, Wang G, Li CX, Xing D, Yan T, Zhu XJ, Liu QM, Wu Q, Guo XX, Zhao TY. Screening for differentially expressed miRNAs in(Diptera: Culicidae) exposed to DENV-2 and their effect on replication of DENV-2 in C6/36 cells., 2019, 12(1): 44.
[50] Varjak M, Maringer K, Watson M, Sreenu VB, Fredericks AC, Pondeville E, Donald CL, Sterk J, Kean J, Vazeille M, Failloux AB, Kohl A, Schnettler E.Piwi4 is a noncanonical PIWI protein involved in antiviral responses., 2017, 2(3): e00144–17.
[51] Yin H, Lin HF. An epigenetic activation role of Piwi and a Piwi-associated piRNA in., 2007, 450(7167): 304–308.
[52] Kirino Y, Mourelatos Z. Mouse Piwi-interacting RNAs are 2'-O-methylated at their 3' termini., 2007, 14(4): 347–348.
[53] Liu QP, An N, Cen S, Li XY. Molecular mechanisms of genetic transposition inhibition by piRNA., 2018, 40(6): 445–450.刘启鹏, 安妮, 岑山, 李晓宇. piRNA抑制基因转座的分子机制. 遗传, 2018, 40(6): 445–450.
[54] Siomi MC, Sato K, Pezic D, Aravin AA. PIWI-interacting small RNAs: the vanguard of genome defence., 2011, 12(4): 246–258.
[55] Brennecke J, Aravin AA, Stark A, Dus M, Kellis M, Sachidanandam R, Hannon GJ. Discrete small RNA- generating loci as master regulators of transposon activity in., 2007, 128(6): 1089–1103.
[56] Hayashi R, Schnabl J, Handler D, Mohn F, Ameres SL, Brennecke J. Genetic and mechanistic diversity of piRNA 3'-end formation., 2016, 539(7630): 588–592.
[57] Morazzani EM, Wiley MR, Murreddu MG, Adelman ZN, Myles KM. Production of virus-derived ping-pong-dependent piRNA-like small RNAs in the mosquito soma., 2012, 8(1): e1002470.
[58] Miesen P, Joosten J, van Rij RP. PIWIs go viral: arbovirus-derived piRNAs in vector mosquitoes., 2016, 12(12): e1006017.
[59] Miesen P, Girardi E, van Rij RP. Distinct sets of PIWI proteins produce arbovirus and transposon-derived piRNAs inmosquito cells., 2015, 43(13): 6545–6556.
[60] Schnettler E, Donald CL, Human S, Watson M, Siu RW, McFarlane M, Fazakerley JK, Kohl A, Fragkoudis R. Knockdown of piRNA pathway proteins results in enhanced Semliki Forest virus production in mosquito cells., 2013, 94(Pt 7): 1680–1689.
Research progress in RNA interference against the infection of mosquito-borne viruses
Yong Wei, Yulan He, Xueli Zheng
Mosquito-borne diseases have become an important public health issue of global concern because of their high incidence and transmission rate. As a vector for mosquito-borne diseases, studying the interaction mechanism between mosquitoes and mosquito-borne viruses will help control mosquito-borne diseases. The impaired innate immunity and immune barriers evasion caused by mosquito-borne viruses in mosquitoes pose a potential risk for the persistent infection of the virus in mosquitoes and the outbreak of mosquito-borne diseases. The RNA interference (RNAi) pathway, as a powerful antiviral defense barrier in mosquitoes, can inhibit viral replication and transmission by producing a variety of small RNAs to degrade viral RNA. In this review, we summarize the related studies on the innate immune mechanism against mosquito- borne virus infection in mosquitoes about small interfering RNA (siRNA), microRNA (miRNA), and Piwi-interacting RNA (piRNA), aiming to provide a theoretical reference for the prevention and control of mosquito-borne diseases.
Mosquito; Mosquito-borne viruses; siRNA; miRNA; piRNA
2019-10-15;
2019-12-14
国家自然科学基金项目(编号:31630011),广东省自然科学基金项目(编号:2017A030313625)和广州市科技计划项目(编号:201804020084)资助[Supported by National Natural Science Foundation of China (No. 31630011), Natural Science Foundation of Guangdong Province of China (No. 2017A030313625), Science and Technology Planning Project of Guangzhou City (No. 201804020084)]
魏勇,在读博士研究生,研究方向:传染病预防与控制。E-mail: smuweiyong@163.com
郑学礼,博士,教授,研究方向:传染病预防与控制。E-mail: zhengxueli2001@126.com
10.16288/j.yczz.19-262
2020/1/2 18:34:26
URI: http://kns.cnki.net/kcms/detail/11.1913.R.20191231.1147.003.html
(责任编委: 岑山)

