Status epilepticus as an initial manifestation of hepatic encephalopathy: A case report
Bin Cui, Lin Wei, Li-Ying Sun, Wei Qu, Zhi-Gui Zeng, Ying Liu, Zhi-Jun Zhu
Bin Cui, Lin Wei, Wei Qu, Zhi-Gui Zeng, Ying Liu, Zhi-Jun Zhu, Liver Transplantation Center,National Clinical Research Center for Digestive Diseases, Beijing Friendship Hospital, Capital Medical University, Beijing 100050, China
Li-Ying Sun, Liver Transplantation Center, National Clinical Research Center for Digestive Diseases, Intensive Care Unit, Beijing Friendship Hospital, Capital Medical University, Beijing 100050, China
Abstract BACKGROUND Status epilepticus in patients with hepatic encephalopathy (HE) is a rare but serious condition that is refractory to antiepileptic drugs, and current treatment plans are vague. Diagnosis may be difficult without a clear history of cirrhosis.Liver transplantation (LT) is effective to alleviate symptoms, however, there are few reports about LT in the treatment of status epilepticus with HE. To our knowledge, this is the first report of status epilepticus present as initial manifestation of HE.CASE SUMMARY A 59-year-old woman with a 20-year history of heavy drinking was hospitalized for generalized tonic-clonic seizures. She reported no history of episodes of HE,stroke, spontaneous bacterial peritonitis, ascites or gastrointestinal bleeding.Neurological examination revealed a comatose patient, without papilledema.Laboratory examination suggested liver cirrhosis. Plasma ammonia levels upon admission were five times normal. Brain computed tomography (CT) was normal,while abdominal CT and ultrasound revealed mild ascites, liver cirrhosis and splenomegaly. Electroencephalography (EEG)showed diffuse slow waves rhythm,consistent with HE, and sharp waves during ictal EEG corresponding to clinical semiology of focal tonic seizures. The symptoms were reversed by continuous antiepileptic treatment and lactulose. She was given oral levetiracetam, and focal aware seizures occasionally affected her 10 mo after LT.CONCLUSION Status epilepticus could be an initial manifestation of HE. Antiepileptic drugs combined with lactulose are essential for treatment of status epilepticus with HE,and LT is effective to prevent the relapse.
Key Words: Status epilepticus; Hepatic encephalopathy; Decompensated alcoholic liver cirrhosis; Antiepileptic drugs; Liver transplantation; Case report
INTRODUCTION
Hepatic encephalopathy (HE) is a brain dysfunction syndrome caused by liver insufficiency and/or portosystemic shunting. It is characterized by a wide clinical spectrum ranging from mild neuropsychological symptoms to altered consciousness or coma[1,2]. It typically presents with alteration in behavior, impairment of consciousness and alterations in motor tone[2]. According to the report of Adams and Foley[3], seizures occur in one-third of cases of end-stage of liver disease, while Rudleret al[4]reported that only 0.7% of patients present with status epilepticus in cirrhosis.Status epilepticus can be a manifestation of HE[5,6]. The incidence and pathophysiology of status epilepticus in patients with HE are still not clear, and its presence suggests a poor prognosis[6-8]. It is difficult to identify status epilepticus from HE in the absence of a history of severe liver diseases. There are no reports of status epilepticus as the initial symptom of HE. We report an intriguing case of a 59-year-old woman with status epilepticus as an initial manifestation of HE.
CASE PRESENTATION
Chief complaints
A 59-year-old female patient was admitted to our hospital for generalized tonic-clonic seizures for several hours with loss of consciousness.
History of present illness
She had a history of heavy alcohol consumption, approximately 100 g/d for more than 20 years, as well as elevated transaminases for 5 years without any examinations and treatments.
History of past illness
She had a 4-year history of hypertension and was treated with antihypertensive drugs.History of HE, epilepsy, stroke, brain trauma or hepatitis B/C virus infection was denied.
Personal and family history
The patient had no significant personal and family history.
Physical examination
A comatose patient was observed on admission, and further clinical examination revealed no local neurological disorder or papilledema. Signs of decompensated cirrhosis showed mild jaundice, splenomegaly and lower extremity edema.
Laboratory examinations
Laboratory data showed anemia, thrombopenia, increased transaminases, ammonia and total bilirubin, decreased albumin and potassium and prolonged international normalized ratio (INR) and prothrombin time (PT). On admission, the patient’s plasma ammonium (NH4+) level was 210 μmol/L (reference range: 0-45 μmol/L); platelet count, 44 × 109/L (reference range: 100-300 × 109/L); aspartate transaminase, 58 U/L(reference range: 8-40 U/L); albumin, 24 g/L (reference range: 35-55 g/L); total bilirubin, 117.8 μmol/L (reference range: 3.4-20.5 μmol/L); blood potassium, 2.8 mmol/L (reference range: 3.5-5.5 mmol/L); INR, 2.01 (reference range: 0.8-1.2); and PT, 22.6 s (reference range: 10.2-14.3 s). Hepatitis B and C antigens were negative.
Imaging examinations
Brain computed tomography (CT) was normal. Abdominal CT and ultrasound revealed mild ascites, liver cirrhosis and splenomegaly. Magnetic resonance imaging(MRI) showed mild hyperintensities in the bilateral frontal and parietal lobes on T2-weighted sequences (Figure 1A) and hyperintensities on fluid low attenuation inversion recovery(FLAIR)-weighted sequences, similar to hypoxic/ischemic-like encephalopathy (Figure 1B). The hyperintensity area of MRI was significantly reduced after 2 mo (Figure 1C).
FINAL DIAGNOSIS
Interictal electroencephalography (EEG) showed diffuse slow waves in the parietooccipital lobe (Figure 2A), and ictal EEG revealed sharp waves in the left parietal regions (Figure 2B). Two months after symptom onset, background EEG activity was significantly improved (Figure 2C). According to the clinical symptoms, laboratory data, radiological examination and the patient’s responses to treatment, status epilepticus with HE and decompensated alcoholic liver cirrhosis were diagnosed.
TREATMENT
Intravenous midazolam was the initial therapy, but there was little improvement in seizures and consciousness level. Based on the laboratory results, we suspected status epilepticus related to HE. HE was resolved after 24 h therapy with lactulose, rifaximin and branched chain amino acids, and status epilepticus was resolved with continuous intravenous midazolam, which confirmed our diagnosis. Five days later, she presented with focal impaired awareness seizures, characterized by right upper extremity tonic.After 2 wk, she recovered from the episodes by increasing the dose of levetiracetam and lactulose. The level of consciousness returned to normal, seizures were terminated and blood ammonia decreased to 57 μmol/L. Levetiracetam and lactulose were continuously used. She underwent liver transplantation (LT) 3 mo after onset of disease. No complications were observed after LT. And the operation was completed in liver trasnsplantation center of Beijing Friendship Hospital, Capital Medical University.
OUTCOME AND FOLLOW-UP
During the first month after the operation, epileptic seizures were more frequent than before the surgery and returned to normal by adjusting the medication. It has been 10 mo since the operation, and she was followed up in the clinic. The patient was in stable condition with good consciousness level. She continued to receive levetiracetam for epilepsy and tacrolimus and mycophenolate mofetil for immunosuppressive treatment. Focal aware seizures lasting less than 5 sec affected her occasionally.
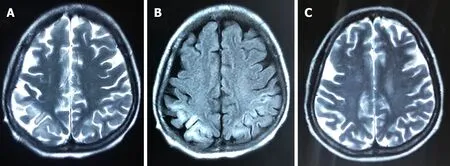
Figure 1 Images of magnetic resonance imaging. A: T2-weighted magnetic resonance imaging showed mild hyperintensities in bilateral frontoparietal lobe on day 5; B: The magnetic resonance imaging scans revealed hyperintensities in bilateral frontoparietal lobe on fluid low attenuation inversion recovery-weighted sequences 5 d after onset; C: The lesion of hyperintensity was shown to be significantly reduced after 2 mo.
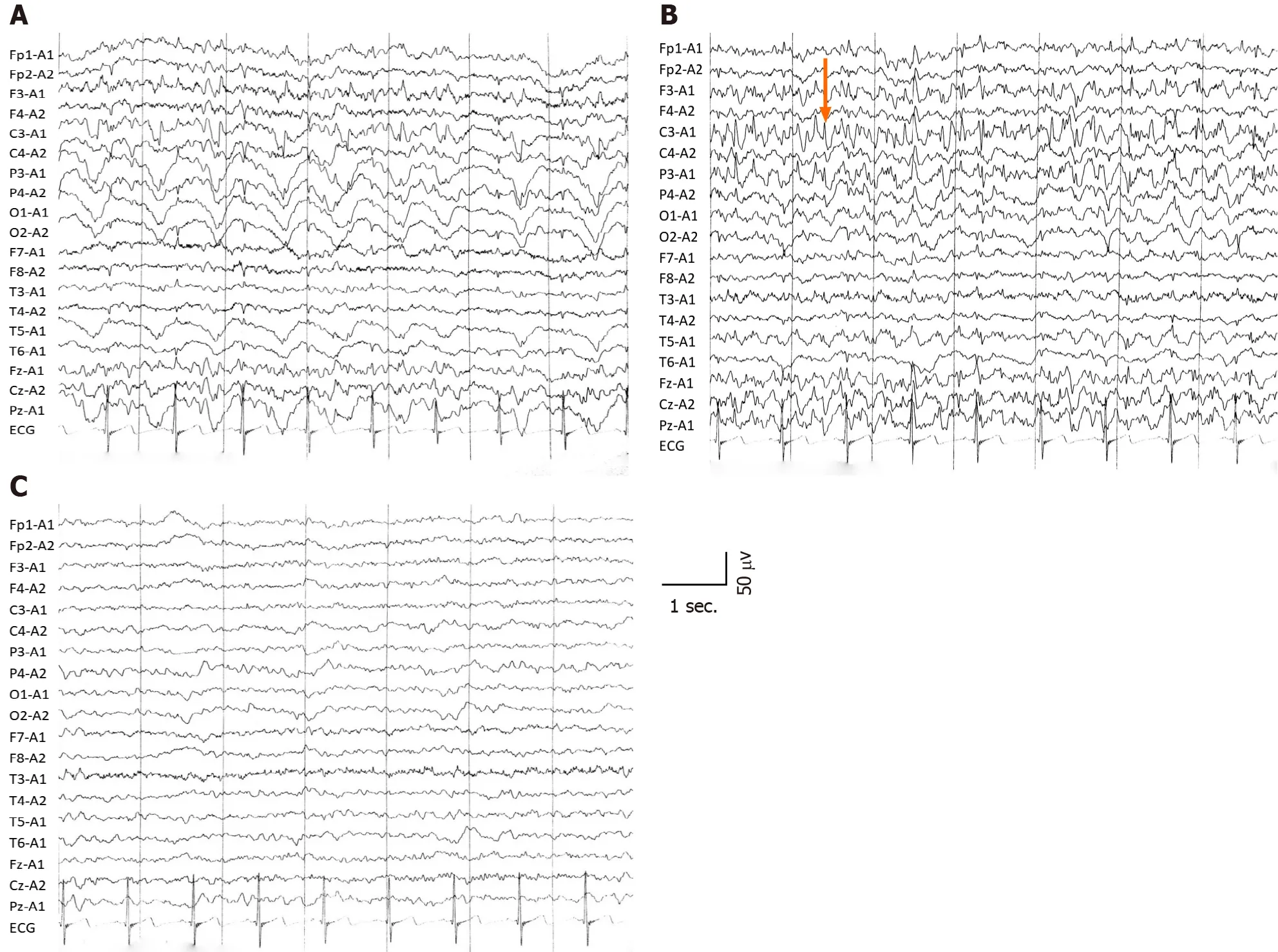
Figure 2 Electroencephalography showing different wave rhythms. A: Interictal electroencephalography showed slow wave rhythm activity; B:Epileptiform discharge (orange arrow) in left parietooccipital during ictal period, which corresponded well to the clinical symptoms; C: The slow waves rhythm and background activity improved after 2 mo.
DISCUSSION
HE is a clinical hallmark of patients with advanced liver disease and is always a sign of deterioration[2]. Status epilepticus is a rare but life-threatening manifestation of HE[5,6,9]. It is interesting that an excitatory neurotransmission condition can manifest with a condition characterized by neuroinhibition. There are few reports concerning the mechanisms of epilepsy in HE, so the pathophysiology remains unknown. Some events of nonconvulsive status epilepticus, which resemble grade 4 HE, were misdiagnosed as HE episodes[6,8], so it is important to identify HE and status epilepticus for patients.
Alcoholic cirrhosis is more likely to cause epilepsy[4,10]. There may be several reasons for this. First, the character of alcohol is lipophilic, which easily binds to lecithin in the brain, causing degeneration, dehydration and loss or necrosis of brain cellsvianeurotoxicity, leading to atrophy of brain cells and diffuse brain atrophy[11].Additionally, chronic alcohol addiction increases blood concentration of excitotoxic substances such as glutamate, aspartate and homocysteine, and alcohol withdrawal further increases the plasma level of homocysteine, lowers seizure threshold and increases the possibility of prolonged or sustained onset[12]. The “kindling” model is another underlying possible cause of seizures, which means that alcohol increases the excitability of the limbic system and subcortical structures[13]. Second, blood ammonia plays an important role in the pathogenesis by causing brain edema, energy failure and neurotransmitter alterations. Ammonia can diffuse freely across the blood-brain barrier, leading to excessive production of glutamine by glutamine synthetase in astrocytes and overactivation of the N-methyl-D-aspartate receptors, which can cause cell edema or even death. Moreover, hyperammonemia alters protein synthesis and exhausts the intermediate products of cell energy metabolism, leading to cell swelling,proteolysis, mitochondrial degradation and free-radical production. The effects of hyperammonemia depend on the sensitivity of the brain and are possibly reversible,so it may be a potential mechanism for treatment[14,15]. Third, with the increased permeability of the blood-brain barrier, other factors such as short-chain free fatty acids, phenols, thiols and pseudo-neurotransmitters have also been implicated[5,16].Other under-recognized factors such as undiagnosed neurodegenerative disorders or aging may contribute to seizures in those patients, and further research on the underlying mechanisms are needed.
MRI showed mild, high signal intensity in bilateral frontoparietal lobes on T2-weighted images, while high signal intensity in FLAIR-weighted sequences was the same as in hypoxic/ischemic-like encephalopathy. Timely and effective treatment could improve prognosis and reduce the hyperintensities in MRI. Some potential reasons are related to the alterations in neuroimaging.
As mentioned above, seizures are caused by many factors that affect brain metabolism or structure and can lead to brain damage. Seizures correspond to high energy consumption and blood flow increase; therefore, when the equilibrium of blood flow and energy demand in the focal region is disrupted, lactate is released by anaerobic metabolism, resulting in destruction of the blood-brain barrier. Animal models demonstrate that, if seizures are prolonged, they can cause failure of Na+/K+-ATPase pumps, leading to cell edema or even irreversible brain damage[17]. Hypoxia or neurotransmitter imbalance caused by HE is responsible for these changes. Acute hyperammonemia selectively affects the white matter and insular and perirolandic regions, causing hypomyelination, myelination delay and cystic changes in white matter, although these changes are reversible at the beginning. Ultimately, the severity of damage depends on the duration of hyperammonemia, sensitivity of brain and age of the patient[15,18-20].
Chronic liver disease with HE is often reversible and treatable, while acute fulminant HE can lead to diffuse swelling of the brain and structural damage of the brainstem, which are difficult to treat[2]. The mortality rate reaches 50% in patients with severe HE and cirrhosis within 1 year[21,22]. A retrospective study found that the prognosis of cirrhosis patients with epileptiform discharge is poor[7]. In-hospital mortality in patients with cirrhosis and status epilepticus can reach 75%[4]. We suppose that the increase in frequency of seizures after LT is due to the rapid correction of metabolism function. The purpose of therapy is to terminate seizures quickly and reverse HE actively to prevent serious neuronal damage.
The American Association for the Study of Liver Diseases and the European Association for the Study of the Liver guidelines recommend lactulose combined with the nonsystemic antibiotic rifaximin to prevent recurrence of HE, and the combination can reduce mortality[1]. At present, there are no standard treatments for status epilepticus with cirrhosis. Guidelines from the American Epilepsy Society suggest that intramuscular midazolam and intravenous lorazepam, diazepam and phenobarbital are established and effective initial treatments in adults with convulsive status epilepticus (Level A), while in children, intravenous lorazepam and diazepam are recommended (Level A). If the seizure lasts for more than 20 min, secondary therapy with intravenous fosphenytoin, valproic acid, levetiracetam or phenobarbital is recommended. If all the above measures fail, and seizure exceeds 40 min, repeating second-line therapy or anesthetic doses of thiopental, midazolam, pentobarbital or propofol is necessary[23]. EEG is recommended to monitor the discharge activity during treatment. Diazepam is risky in patients with liver disease because it may aggravate or induce HE[1,24], so intravenous midazolam was our first choice. Antiepileptic drugs need to be taken continuously to prevent relapse. Levetiracetam, lacosamide,topiramate, gabapentin and pregabalin are recommended first, and drugs undergoing extensive hepatic metabolism such as phenytoin, valproate and carbamazepine are the last resort[24]. Plans for drug withdrawal should be developed and executed in consultation with neurologists according to EEG and clinical symptoms[25].
Slow wave rhythm was a marked EEG change in patients with HE[7], and sharp waves were located in the left parietal region in the ictal stage, consistent with the clinical symptoms of the present patient. The background activities of EEG were improved with continuous treatment, and combined with laboratory data, the diagnosis of status epilepticus as the initial manifestation of HE based on decompensated alcoholic cirrhosis was supported.
Although not all manifestations are reversible by LT[1], it remains the final option for HE. Some reports suggest that damage to the central nervous system is reversible when elevated blood ammonia concentration does not exceed 200-400 mg/dL[26], and continuous improvement in cognition has been observed during the first year post-LT[10]. It has been also reported that 10% of patients present with seizures post-LT[9].Seizure is not an indication for LT, and more attention should be paid to seizures prior to LT to prevent fatal status epilepticus post-LT. However, there are lack of efficient neurological evaluations that can be undertaken to assess the degree of damage and the possibility of recovery for those patients. Effective measures are urgently needed to evaluate neurological dysfunction and to identify patients with a potential benefit from LT. The data of this study support the idea that LT is effective to treat status epilepticus in patients with HE.
CONCLUSION
In view of the high mortality of status epilepticus with HE, early diagnosis and treatment play a crucial role in prognosis. To prevent progressive deterioration of liver function, it is important to select the proper antiepileptic drugs. Although not all neurological symptoms can be improved, LT remains the final option for status epilepticus with HE in alcoholic liver cirrhosis.
 World Journal of Clinical Cases2020年24期
World Journal of Clinical Cases2020年24期
- World Journal of Clinical Cases的其它文章
- Role of gut microbiome in regulating the effectiveness of metformin in reducing colorectal cancer in type 2 diabetes
- lmpact factors of lymph node retrieval on survival in locally advanced rectal cancer with neoadjuvant therapy
- Three-year follow-up of Coats disease treated with conbercept and 532-nm laser photocoagulation
- Virus load and virus shedding of SARS-CoV-2 and their impact on patient outcomes
- Risk factors for de novo hepatitis B during solid cancer treatment
- Cause analysis and reoperation effect of failure and recurrence after epiblepharon correction in children
