Risk factors for de novo hepatitis B during solid cancer treatment
Rie Sugimoto, Masayuki Furukawa, Takeshi Senju, Yoshihusa Aratake, Mototsugu Shimokawa, Yuki Tanaka,Hiroki Inada, Tatsuya Noguchi, Lingaku Lee, Masami Miki, Yuji Maruyama, Risa Hashimoto, Terumasa Hisano
Rie Sugimoto, Masayuki Furukawa, Takeshi Senju, Yoshihusa Aratake, Yuki Tanaka, Hiroki Inada,Tatsuya Noguchi, Lingaku Lee, Masami Miki, Yuji Maruyama, Risa Hashimoto, Terumasa Hisano,Department of Hepato-Biliary-Pancreatology, National Hospital Organization Kyushu Cancer Center, Fukuoka City 811-1395, Fukuoka Prefecture, Japan
Mototsugu Shimokawa, National Hospital Organization Kyushu Cancer Center, Clinical Research Institute, Fukuoka City 811-1395, Fukuoka Prefecture, Japan
Mototsugu Shimokawa, Department of Biostatistics, Yamaguchi University Graduate School of Medicine, Ube City 755-8505, Yamaguchi Prefecture, Japan
Abstract BACKGROUND Reactivation of hepatitis B virus (HBV) during anticancer treatment is a critical issue. When treating patients with solid tumors, it is unclear whether specific cancer types or treatments affect HBV reactivation in hepatitis B surface antigen(HBsAg)-negative and hepatitis B core antibody (HBcAb)-positive patients, socalled de novo hepatitis B patients. The risk of de novo hepatitis B may vary based on different background factors.AIM To determine the frequency and risk factors for de novo hepatitis B during solid tumor treatment.METHODS This retrospective cohort study comprised 1040 patients without HBsAgs and with HBcAbs and/or hepatitis B surface antibodies (HBsAbs). The patients were treated for solid cancer from 2008 to 2018 at the National Kyushu Cancer Center and underwent HBV DNA measurements. Patient characteristics and disease and treatment information were investigated. HBV DNA measurements were performed using TaqMan polymerase chain reaction (PCR). To identify the risk factors associated with HBV DNA expression, the age, sex, original disease,pathology, treatment method, presence or absence of hepatitis C virus (HCV), and HBsAb and/or HBcAb titers of all subjects were investigated. In patients with HBV DNA, the time of appearance, presence of HBsAgs and HBsAbs at the time of appearance, and course of the subsequent fluctuations in virus levels were also investigated.RESULTS Among the 1040 patients, 938 were HBcAb positive, and 102 were HBcAb negative and HBsAb positive. HBV DNA expression was observed before the onset of treatment in nine patients (0.9%) and after treatment in 35 patients (3.7%),all of whom were HBcAb positive. The HBV reactivation group showed significantly higher median HBcAb values [9.00 (8.12-9.89) vs 7.22 (7.02-7.43), P =0.0001] and significantly lower HBsAb values (14 vs 46, P = 0.0342) than the group without reactivation. Notably, the reactivated group showed a significantly higher proportion of cancers in organs related to digestion and absorption (79.0% vs 58.7%, P = 0.0051). A high HBcAb titer and cancers in organs involved in digestion and absorption were identified as independent factors for HBV reactivation (multivariate analysis, P = 0.0002 and P = 0.0095). The group without HBsAbs tended to have a shorter time to reactivation (day 43 vs day 193), and the frequency of reactivation within 6 mo was significantly higher in this group (P =0.0459) than in the other group.CONCLUSION A high HBcAb titer and cancers in organs involved in digestion and absorption are independent factors that contribute to HBV reactivation during solid tumor treatment.
Key Words: Hepatitis B; Reactivation; Solid cancer treatment; Digestion and absorption organ; Hepatitis B surface antibody; Hepatitis B core antibody titer
INTRODUCTION
Approximately 240 million people are positive for hepatitis B virus (HBV) infection worldwide[1], and more than 780000 people die every year of HBV complications. The mortality rates vary by region, with 2.6 cases per 100000 individuals in Asia and 0.5 cases per 100000 individuals in the United States. The reported hepatitis B surface antigen (HBsAg) prevalence rate in Japan is 0.31% according to Japan blood donation data[2].
Chemotherapy has improved the prognosis of patients with various types of cancers. However, the management of complications accompanying anticancer drug therapy is becoming important. Studies have shown that reactivation of HBV occurs during anticancer drug treatment[3], and reactivation of HBV has become a critical issue because of its high fatality rate. Acute reactivation of HBV in HBsAg-positive cases (so-called persistently infected HBV patients) has been reported since 1982[4,5]and countermeasures have been established[6,7]. Furthermore, HBsAg-negative, hepatitis B core antibody (HBcAb)-positive and/or hepatitis B surface antibody (HBsAb)-positive(known as a past infection) status is known to cause reactivation, known asde novohepatitis. This issue was first observed in patients with hematologic malignancies.Multiple studies have evaluated and determined the types of anticancer drugs that are associated with HBV reactivation[8]. For example, one study of rituximab examined the frequency and timing of reactivation and mortality rate of patients with reactivation[9].R-CHOP therapy with rituximab for B-cell lymphoma shows very high rates of HBV reactivation of 8.3% in 1.5 years and 41.5% in 2 years[9,10]. In solid cancers, HBV reactivation may also occur, although less frequently[6,11]. For chemotherapy for solid cancers, guidelines for managing HBV infection were prepared in 2009[6]. However,whether the type of original cancer or the type and amount of the anticancer drug affects the reactivation frequency is unclear. Furthermore, the risk factors for and timing of reactivation are unknown.
Mochidaet al[11]reported that HBV DNA appeared in 3.2% of HBsAg-negative,HBsAb-positive and/or HBcAb-positive patients with autoimmune diseases who were treated with immunosuppressive treatments. We believe that it is necessary to consider solid cancers independently of autoimmune diseases because the treatment period, types of drugs, and prognosis differ greatly between autoimmune diseases and solid cancers. One analysis of 344 HBsAg-negative and HBc/HBsAb-positive patients reported that seven patients showed reactivation[12], but the relationship between the underlying disease, HBcAb titer, and HBsAb level has not been clarified.
In this study, we retrospectively reviewed the frequency of HBV DNA expression and the risk factors at the time of treatment for solid cancer in HBsAg-negative as well as HBcAb-positive and/or HBsAb-positive patients.and experimental
MATERIALS AND METHODS
Patients
This study included patients aged 20 years or older who received any treatment for solid cancer from 2008 to 2018 at the National Kyushu Cancer Center. Among patients without HBsAgs and with HBcAbs and/or HBsAbs, follow-up examinations of HBV DNA expression were conducted according to the guidelines of the Japan Society of Hepatology[4]. Those that had not been measured for HBVDNA before or during treatment were excluded from the analysis.
HBV DNA assessment
Blood HBV DNA measurements were performed using the COBAS TaqMan HBV Test, v 2.0 (Roche Diagnostics, K.K. Tokyo, Japan) (~2017/4/2) and COBAS 6800-8800 system HBV (Roche Diagnostics K.K.) (2017/4/3~), which is based on the polymerase reaction assay. The HBV DNA values were measured before the start of treatment,every month for 3 mo after the start of treatment, and every 3 mo thereafter until the completion of treatment. If HBV DNA expression was undetected after treatment, the measurements were continued for 1 year from the completion of treatment. The frequencies at which the HBV DNA value was higher than the detection sensitivity and at which the HBV DNA value was 1.3 Log IU/mL or higher were examined.
Clinical parameters
To identify the risk factors associated with HBV DNA expression, the age, sex, original disease, pathology (stage, existence of overlapping cancer, presence or absence of surgery, and presence or absence of myelosuppression), treatment method(chemotherapy alone, chemoradiotherapy, and postoperative adjuvant chemotherapy),presence or absence of hepatitis C virus (HCV), and HBsAb and/or HBcAb titers of all subjects were investigated.
In patients with HBV DNA, the time of appearance, presence of HBsAgs and HBsAbs at the time of appearance, and course of the subsequent fluctuations in virus levels were also investigated. Patients who did not undergo HBcAb and HBsAb measurements before the start of treatment and those who did not undergo for HBV DNA measurements at the times stated in the guidelines (once every 1-3 mo) were excluded from the analysis.
Statistical analysis
All patients who met the eligibility criteria were included in the analysis, and these data were analyzed based on intention to treat. HBcAb titers are expressed as the mean and standard deviation (SD) or in contingency tables. Student’sttest was used when appropriate to examine significant differences in HBcAb titers. Comparisons between two groups in HBsAb titer were performed using Wilcoxon’s test. Categorical variables were compared using Fisher’s exact test, and continuous variables were compared using an unpaired 2-tailedt-test. Univariate and multivariate logistic regression analyses were used to examine the risk factors for reactivation. APvalue less than 0.05 was considered to indicate statistical significance. All statistical analyses were performed using JMP version 9.0.2 (SAS Institute Inc., Cary, NC, United States).
RESULTS
Among the patients who received treatment for solid cancer at our hospital from 2008 to 2018, we identified 1099 patients without HBsAgs and with HBcAbs and/or HBsAbs. HBV DNA was appropriately measured in 1040 patients. The median followup period was 387 d (31-2724 d). Among the 1040 patients, 938 had HBcAbs with/without HBsAbs, and 102 had HBsAbs alone. The primary diseases were colorectal cancer, lung cancer, head and neck cancer, stomach cancer, liver cancer,breast cancer, esophageal cancer, gynecological cancer, pancreatic cancer, biliary tract cancer, urological cancer, and others (Table 1).
Among the 938 patients with HBcAbs with/without HBsAbs, the appearance of HBV DNA was observed in 44 patients; all were HBcAb positive. No patients with HBsAbs alone showed HBV DNA expression. The rate of positive HBV DNA expression in patients with HBcAbs was 4.7% (44/938). HBV DNA expression was observed before the onset of treatment in nine patients (0.9%) and after treatment in 35(3.7%). Among these 35 patients, the HBV DNA expression in 15 (1.6%) patients was higher than 1.3 Log IU/mL.
The median duration until the appearance of HBV DNA expression was 221 d (3-5040 d). The expression rate varied among the original diseases; the incidence rates in patients with hepatoma, pancreatic cancer, biliary tract cancer, and esophageal cancer were higher than those in patients with head and neck cancer, gastric cancer,pulmonary cancer, colon cancer, breast cancer, and gynecologic cancer. HBV DNA expression was not observed in patients with urological cancer (Table 2). None of the patients in whom HBV DNA was detected had previously been treated for hepatitis B.
Of the 35 patients with viral reactivation, 31 received chemotherapy, 11 received surgical resection, 6 received chemoradiation, and 5 received transarterial chemoembolization. The type of chemotherapy regimen varied, and the number of regimens ranged from one to five (median, one regimen). However, the specific regimens and anticancer agents that are risk factors for reactivation are also unidentified. The differences in reactivation rates according to primary disease were not extracted as a result of the differences in treatment regimens or anticancer drugs.
To determine whether the combination of HBcAbs, HBsAbs, or hepatitis B e antibodies with HCV contributed to the reactivation of HBV, each factor was compared in the virus reactivation group (RG) and the nonreactivation group (NRG)(Table 3). The median value of HBcAbs was significantly higher in the RG than in the NRG (P= 0.0001), and the median value of HBsAbs was significantly lower in the RG (P= 0.0342). There were no significant differences in the positive rate of hepatitis B e antibodies or HCV complication rate between groups.
The patient characteristics were also examined (Table 4). Age, presence/absence of double cancer, stage of cancer, type of cancer, and presence/absence of myelosuppression (fewer than 1000 Leukocytes, fewer than 500 neutrophils) were also examined (Table 4). There were no significant differences between the HBV RG and NRG in age, presence/absence of double cancer, cancer stage, and presence/absence of bone marrow suppression. However, the proportion of cancers related to digestion and absorption, such as cancers in the oral cavity, pharynx, esophagus, stomach,hepatobiliary pancreas, and colon, was significantly higher in the RG than in the NRG(P= 0.0051). The proportion of hepatoma was significantly higher in the RG (P=0.0150).
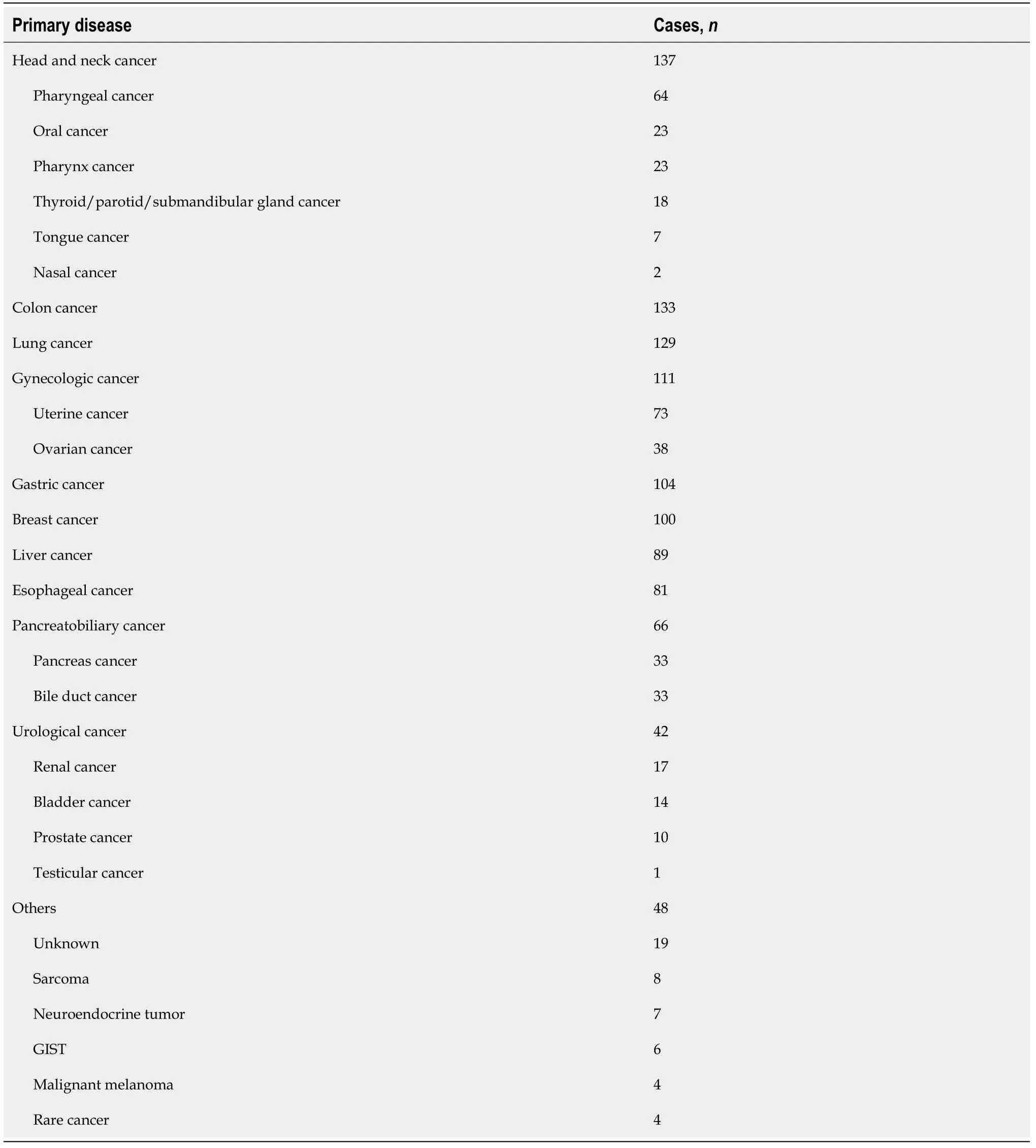
Table 1 Primary disease in all hepatitis B surface antigen-negative, hepatitis B core antibody- and/or hepatitis B surface antibodypositive patients
We also examined the types of treatment (Table 5). There were no significant differences between the HBV RG and NRG regarding treatment with steroids,chemotherapy, molecular targeted drugs, immune checkpoint inhibitors, radiotherapy,or surgery.
The factors contributing to the emergence of the virus were examined using logistic regression analysis (Table 6). A high HBcAb titer and cancers involved in digestion and absorption were identified as independent factors that contributed to the appearance of HBV DNA. Among the reactivated cases, the group without HBsAbs tended to have a shorter time to reactivation (day 38.5vsday 197), and the frequency of reactivation within 6 mo was significantly higher in this group than in the other group (P= 0.0459) (Table 7).

Table 2 Number of patients with hepatitis B virus DNA expression and frequency of hepatitis B virus DNA expression by primary disease in hepatitis B core antibody-positive patients
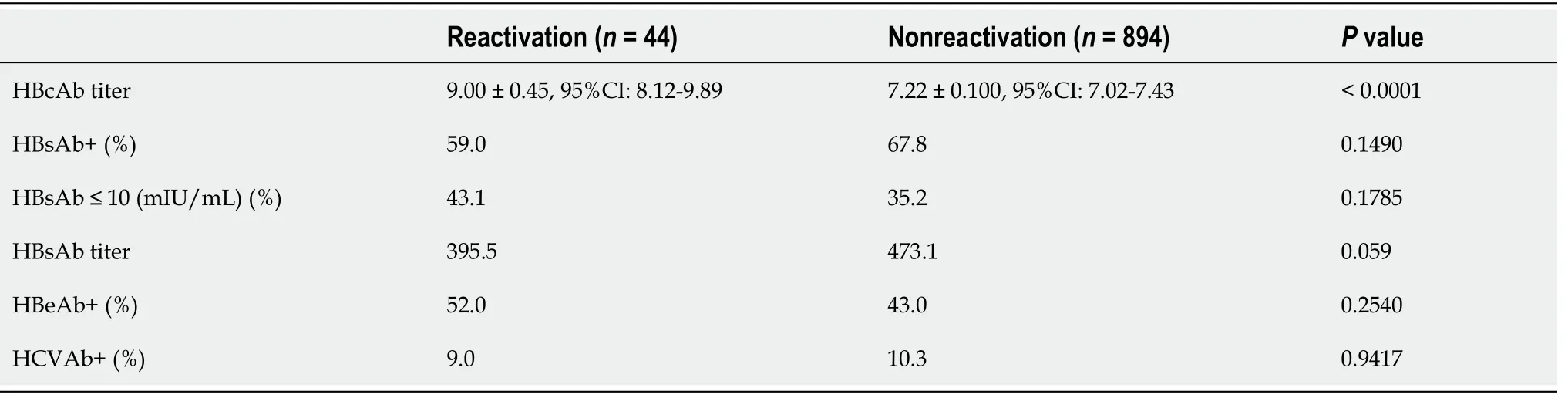
Table 3 Comparison of viral-related factors between the hepatitis B virus reactivation group and the nonreactivation group

Table 4 Comparison of background factors, including the type and status of underlying disease, between the hepatitis B virus reactivation group and the nonreactivation group
DISCUSSION
Here, we retrospectively reviewed the reactivation of HBV during chemotherapy for multiple types of solid carcinoma in our hospital.
Studies have shown that reactivation of HBV occurs during anticancer drug treatment[3]. HBsAg-positive patients require antiviral agents at the same time as the virus is detected[6,7].De novohepatitis that develops in HBsAg-negative and HBcAbpositive patients with malignant lymphoma has been reported in detail[8-10], and thereare some reports on reactivation during chemotherapy for solid cancer[11-13]. Several reports have demonstrated a relationship between HBV reactivation and the HBcAb titer in lymphoma patients[14,15]. Conversely, in one study examining the frequency of occult HBV infection, the HBcAb titer did not correlate with the frequency of viremia in tested blood samples from donors at the Japanese Red Cross[16]. Kotakeet al[12]reported no difference in the frequency of reactivation between patients with HBcAb values higher than 8.0 S/CO and those with lower values among solid tumor patients.In our study, however, a high HBcAb titer was an independent risk factor for HBV reactivation in solid tumor patients. Some HBV carriers enter an immune surveillance phase because of the predominance of host immunity that occurs with aging; such patients are called inactive carriers. HBsAg-negative, HBcAb-positive, and HBsAbpositive patients, so-called HBV-infected patients, are considered inactive carriers[17,18].Inactive carriers have high HBcAb titers, and HBV tends to appear when the immunity of these individuals is compromised. Because patients with a high HBcAb titer are considered inactive carriers, the result that reactivation tends to occur in these individuals is reasonable. Because reactivation occurred at a low frequency, even in patients with low HBcAb titers, it was not possible to determine the cutoff value for HBcAb titers to predict reactivation.
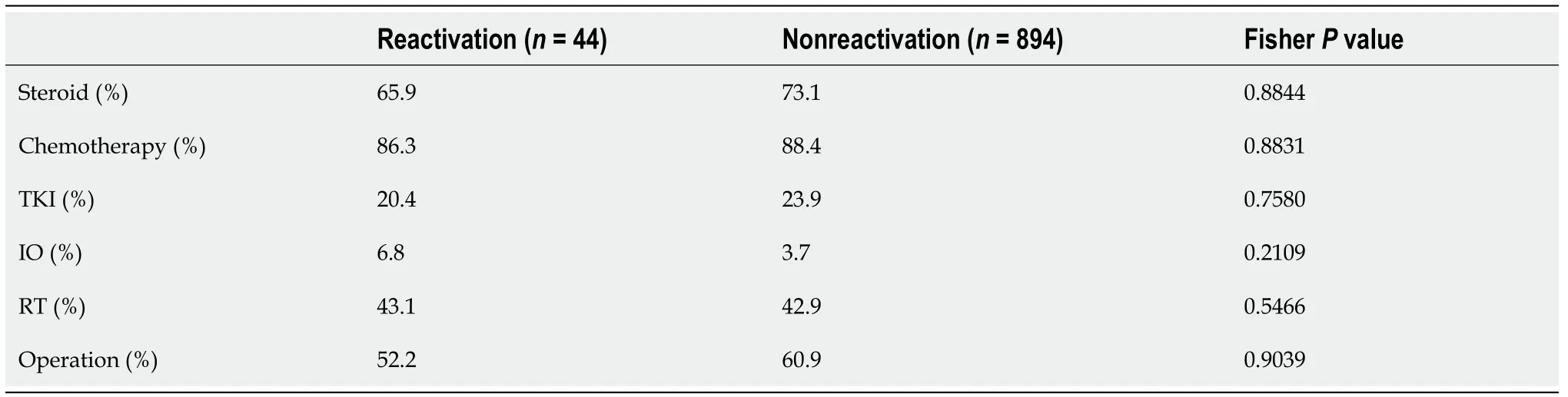
Table 5 Comparison of treatment regimens between the hepatitis B virus reactivation group and the nonreactivation group
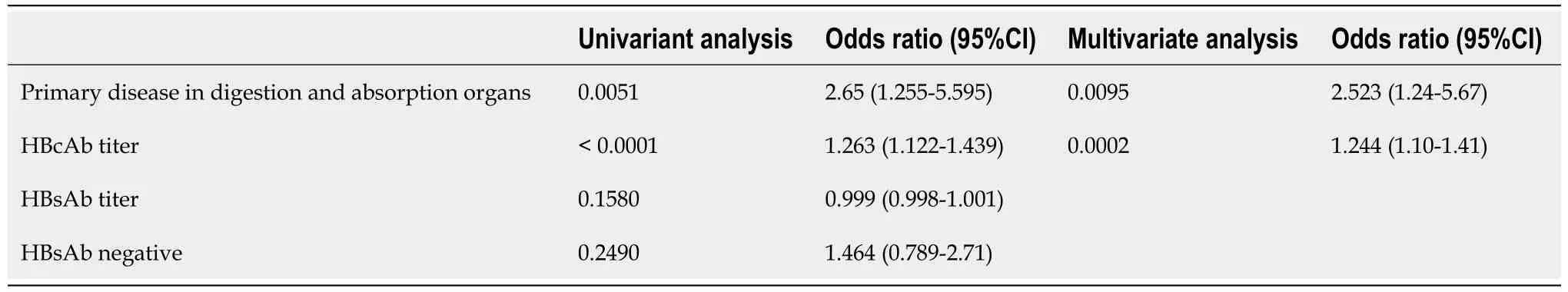
Table 6 Univariate and multivariate analysis of factors contributing to hepatitis B virus reactivation
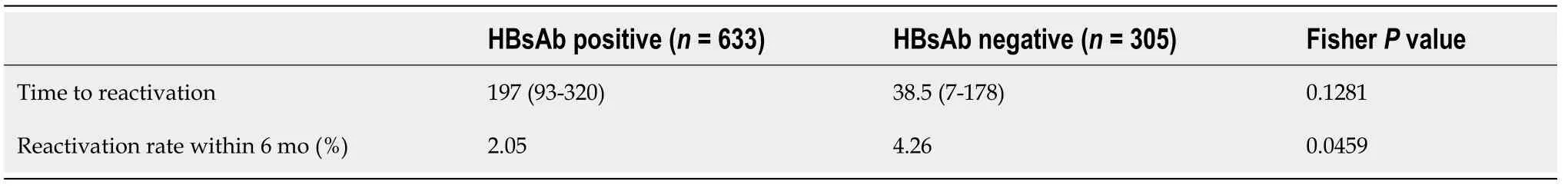
Table 7 Comparison of time to reactivation in the reactivation cases with or without hepatitis B surface antibody
Several reports have also examined the relationship between HBV reactivation and the presence of HBsAbs. A low HBsAb titer has been reported to be a risk factor for reactivating HBV during treatment for hematopoietic malignancies[10,19]. Conversely,one study showed that chemotherapy with rituximab tended to change the reactivation rate depending on the presence or absence of HBsAbs, but the difference was not significant[9]. No definitive conclusion was reached. Kotakeet al[12]reported that a HBsAb value ≤ 10.0 mIU/mL was a risk factor for reactivation in patients with solid tumors. In our study, the HBsAb titers were lower in the RG than in the NRG,but the presence of HBsAb and titer values were not identified as risk factors for reactivation in univariate analysis. However, we found a correlation between the presence or absence of HBsAbs and the time to reactivation. The frequency of reactivation within 6 mo was significantly higher in the HBsAb-negative group, and the HBsAb-positive group showed a long duration before reactivation. This suggests that the presence of HBsAbs might have a certain effect in suppressing reactivation during chemotherapy in the short term, but the suppression might be insufficient when chemotherapy is administered over the long term.
Some studies have reported the HBV reactivation rate during the treatment of hepatocellular carcinoma (HCC). The reactivation rate during sorafenib treatment was 8.7%[20], and the reactivation rate was 11% after transarterial chemoembolization[21]. In this study, the HBV reactivation rate after treatment for HCC was 10.5%, which was significantly higher than that in patients with other cancers. The high rate of reactivation during HCC treatment could be due to the inclusion of inactive carriers among the HCC patients[22]. However, none of the patients with liver cancer in whom HBVDNA was detected had previously been diagnosed with or treated for hepatitis B.
In this study, the most important finding to highlight is that the reactivation rate significantly differed depending on the primary cancer, regardless of the type of chemotherapy. Cancers in organs involved in digestion and absorption, such as the tongue, pharynx, esophagus, stomach, hepatobiliary pancreas, and colon, showed significantly higher reactivation rates than cancers in other organs, such as the lung,thyroid, urinary gland, gynecological organs, and mammary gland. Why the reactivation rate was high in the treatment of carcinomas involved in digestion and absorption is still unclear, but we speculate the potential involvement of intestinal immunity and gut flora.
The immune responses of B cells, T cells, macrophages and other factors are strongly involved in controlling HBV. Rituximab, an anti-CD20 antibody used against lymphoma, and infliximab, an anti-TNFα antibody, are known to be the strongest factors for HBV reactivation[23]. The intestinal tract has recently been established as an immune organ, and one report showed that intestinal bacteria are involved in the elimination of HBV in childhood, which is mediated by toll-like receptor 4 (TLR4)[24].However, treatments for cancers in organs other than those involved in digestion and absorption also cause adverse events in the gastrointestinal system, so we may not be able to explain the difference in morbidity solely based on the involvement of gutrelated immunity. Many of the digestive and absorptive organs are closely connected to the liver through the portal vein. We speculate that substances produced by cancer treatments might directly affect hepatocytes.
In this study, among patients with detected HBV DNA, 62% showed reduced HBV DNA levels below the sensitivity limit without HBV treatment. Only 38% of patients showed an increase to 1.3 Log IU/mL or higher and needed treatment. The factors that predict whether HBV will reactivate or spontaneously disappear have not been identified. The detection rate of HBV DNA before cancer treatment was 0.9%, which was similar to that previously reported in HBsAg-negative blood donors[18].
The present study has some limitations, including its retrospective nature. In some cases, HBcAbs were not measured; we therefore did not investigate all cases. A multiinstitutional prospective study is required to address these questions.
CONCLUSION
The rate of HBV DNA expression in the HBcAb-positive group during solid tumor treatment was 4.8%. A high HBcAb titer and cancer in organs involved in digestion and absorption were identified as independent risk factors for HBV reactivation. The presence of HBsAbs correlated with the duration until HBV reactivation.
ARTICLE HIGHLIGHTS
Research background
Hepatitis B virus (HBV) reactivation occurs in both hepatitis B surface antigennegative and hepatitis B core antibody (HBcAb)-positive patients during chemotherapy for malignant solid tumors. The risk factors for such reactivation are unclear.
Research motivation
Identification of risk factors for HBV reactivation could result in more careful followup of patients with HBV DNA who are at high risk, whereas those at low risk could be followed up at less frequent intervals or stop follow-up altogether.
Research objectives
We aimed to identify the factors that contribute to hepatitis B reactivation during treatment of solid tumors. By analyzing many factors in a large cohort, we hoped to identify the most important ones and thus facilitate elucidation of the mechanism of hepatitis B reactivation.
Research methods
This was a retrospective cohort study of patients with solid tumors attending a single cancer center. Of particular note are the large cohort (1040 cases) and long follow-up period (10-years).
Research results
High HBcAb titer and cancers in organs involved in digestion and absorption were identified as independent factors for HBV reactivation.
Research conclusions
The site of the primary cancer was found to be a risk factor for hepatitis B reactivation during solid cancer treatment.
Research perspectives
Future prospective studies with a large cohort research are required to further investigate the relationship between sites of primary cancers and hepatitis B reactivation.
 World Journal of Clinical Cases2020年24期
World Journal of Clinical Cases2020年24期
- World Journal of Clinical Cases的其它文章
- Role of gut microbiome in regulating the effectiveness of metformin in reducing colorectal cancer in type 2 diabetes
- lmpact factors of lymph node retrieval on survival in locally advanced rectal cancer with neoadjuvant therapy
- Three-year follow-up of Coats disease treated with conbercept and 532-nm laser photocoagulation
- Virus load and virus shedding of SARS-CoV-2 and their impact on patient outcomes
- Cause analysis and reoperation effect of failure and recurrence after epiblepharon correction in children
- Effects of different acupuncture methods combined with routine rehabilitation on gait of stroke patients
