Role of gut microbiome in regulating the effectiveness of metformin in reducing colorectal cancer in type 2 diabetes
Qi-You Huang, Fei Yao, Chuan-Ren Zhou, Xiao-Ying Huang, Qiang Wang, Hui Long, Qing-Ming Wu
Qi-You Huang, Fei Yao, Chuan-Ren Zhou, Xiao-Ying Huang, Qiang Wang, Qing-Ming Wu, Institute of Infection, Immunology and Tumor Microenvironment, Medical College, Wuhan University of Science and Technology, Wuhan 430065, Hubei Province, China
Hui Long, Department of Gastroenterology, Tianyou Affiliated Hospital, Wuhan University of Science and Technology, Wuhan 430064, Hubei Province, China
Abstract The prevalence of colorectal cancer (CRC) and type 2 diabetes mellitus (T2DM) is increasing globally. It is rarely noticed that the incidence of CRC is higher in patients with T2DM. What needs to be mentioned is that metformin, a commonly used clinical drug for T2DM, attracts scholars’ attention because of its benefits in lowering the risk of developing CRC. Hence, we try to find the common grounds of initiation of T2DM and CRC and the reason why metformin reduces the risk of CRC in patients with T2DM. We noticed consistent changes of gut microbiota,such as elevated Bacteroides, Prevotella and Bifidobacterium and depressed Firmicutes and Lactobacillus. Furthermore, many studies in recent years have proved that the efficacy of metformin, such as improving blood glucose, depends on the gut microbiota. Coincidentally, the progression of CRC is inseparable from the contributions of gut microbiota. Therefore, we first proposed the concept of the metformin-gut microbiota-CRC (in T2DM) axis to explain the effect of metformin in reducing CRC in patients with T2DM. In this review, we elaborated the new concept and its potential clinical application value.
Key Words: Metformin; Colorectal cancer; Gut microbiota; Type 2 diabetes mellitus
lNTRODUCTlON
Several decades ago, colorectal cancer (CRC) was rarely diagnosed. To date, it has become the fourth deadliest cancer in the world, killing an average of 90000 people every year. The accumulation of environmental and genetic factors leading to genetic mutations and epigenetic changes are the factors in the carcinogenesis of intestinal epithelial cells[1]. Nevertheless, recent studies have shown that the occurrence of CRC is closely related to the gut microbiota, and the alterations of gut microbiota are inconsistent at different stages[2,3]. Table 1 summarizes the main alterations of gut microbiota in human studies related to CRC from 2014 to 2019.
There are more than 547 million patients with diabetes mellitus in the world, of which 373 million have been diagnosed. The number of young patients aged 20-39 exceeded 60 million in 2013[4]. Recent studies have shown that type 2 diabetes mellitus(T2DM) is also closely related to changes in gut microbiota. Table 2 summarizes the main alterations of gut microbiota in patients with T2DM. Interestingly, metformin(systematic name: 1-carbamimidamido-N,N-dimethylmethanimidamide, C4H11N5), as one of the most well-known drugs to treat T2DM, has been shown to have a positive pharmacological effect on restraining CRC in T2DM patients. Moreover, more and more studies have proved that its pharmacological function is mediated by gut microbiota.
Epidemiological evidence suggests that CRC is predisposed in patients with T2DM[5]. However, after comparing and analyzing the data of current research, the consistent changes of some gut microbiota were observed in patients with T2DM and patients with CRC. Table 2 summarizes the similarities in changing gut microbiota in patients with T2DM and patients with CRC. Therefore, based on the facts above, we set up a new concept called the metformin-gut microbiota-CRC axis (in T2DM) to explain that metformin reduces the incidence of CRC in patients with T2DM. More importantly, the new concept can be extended to be the drug-gut microbiota-diseases axis. If this theory is proven to be effective, it will provide new ideas for the use of clinical drugs and the application of gut microbiota for the treatment of clinical diseases.
METFORMlN-GUT MlCROBlOTA-CRC AXlS lN T2DM
Metformin suppresses CRC in T2DM
To date, epidemiological evidence suggests that T2DM is one of the risk factors for CRC. However, the latest meta-analysis of observational studies displayed insulin therapy significantly increased the risk of CRC [risk ratio (95% confidence interval(CI)): 1.69 (1.25, 2.27)][6]. Compared to this, the latest meta-analysis from observational studies demonstrated that metformin therapy was associated with a significantly lower overall survival [pooled risk ratio = 0.75, 95%CI: 0.66-0.86][7], lower CRC-specific survival [hazard ratio (95%CI): 0.66 (0.50-0.87)][8], and a lower risk of colorectal neoplasm [risk ratio (95%CI): 0.63 (0.50-0.79)] in patients with T2DM[9].In vivo,Tomimotoet al[10]suggested administration of metformin significantly reduced the number of tumors larger than 2 mm in diameter in Apc (Min/+) mice. Subsequent studies have shown that metformin suppressed colorectal aberrant crypt foci inhumans[11,12]and an azoxymethane-induced animal mode of CRC[13]. In patients with T2DM, it has been reported that metformin has a positive prophylactic effect on colorectal adenomas[14]and CRC[15]. In a multicenter double-blind, placebo-controlled,randomized phase 3 trial, Higurashiet al[16]proved the administration of low-dose metformin for 1 year to patients was safe and reduced the prevalence and number of metachronous adenomas or polyps after polypectomy.
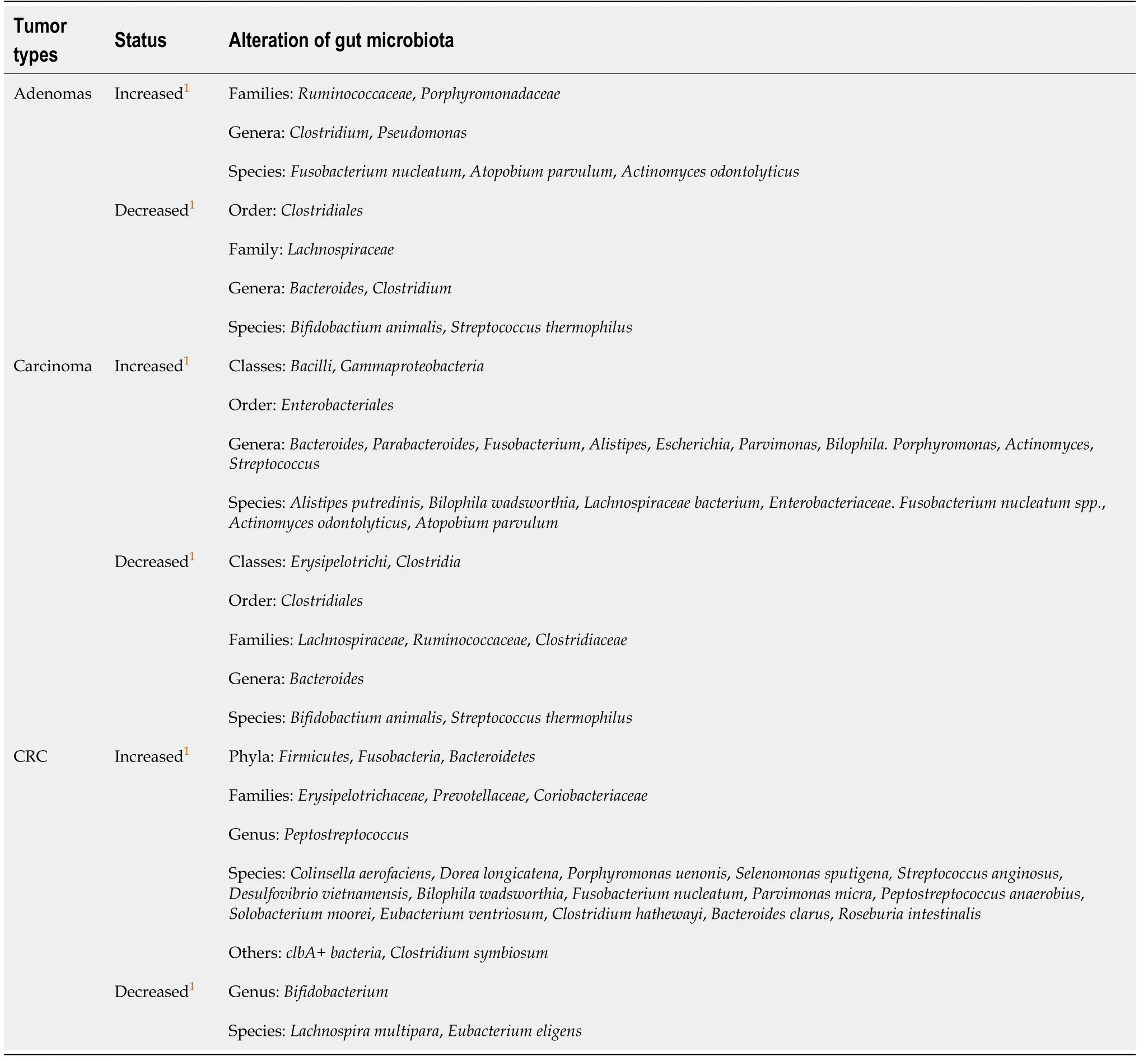
Table 1 Summary of the alteration of gut microbiota in patients with colorectal cancer from 2014 to 2019
At the cellular level, the effect of metformin is mainly manifested in inhibiting the proliferation of CRC cells as a result of AMPK activation and increased reactive oxygen species production[17,18]on the one hand and accelerating the apoptosis of CRC cells induced by immune cells and cytokines on the other hand[19]. Complementarily,metformin may go through a variety of pathways or mechanisms, such as by alteration of cellular responses to oxidative stress, protection of mitochondrial structuresviasuppressing reactive oxygen species production and NF-kB activity[20-22], and repression of epithelial-mesenchymal transition induced by IL-6 or TGF-β[19,23]. At the genetic level, it has been shown that the upregulation of adenosine A1 receptor[24],blocking of encoding DNA replication proteins and protooncogene protein synthesis[25,26], and regulation of the SNAIL/miR-34:ZEB/miR-200 system[27]are the targets of metformin. In addition, key sites for metformin action have been shown in multiple signaling pathways, such as attenuation of cell stemnessviainhibiting theWnt3a/β-catenin[28]and AMPK/PI3K/Akt pathways[29].
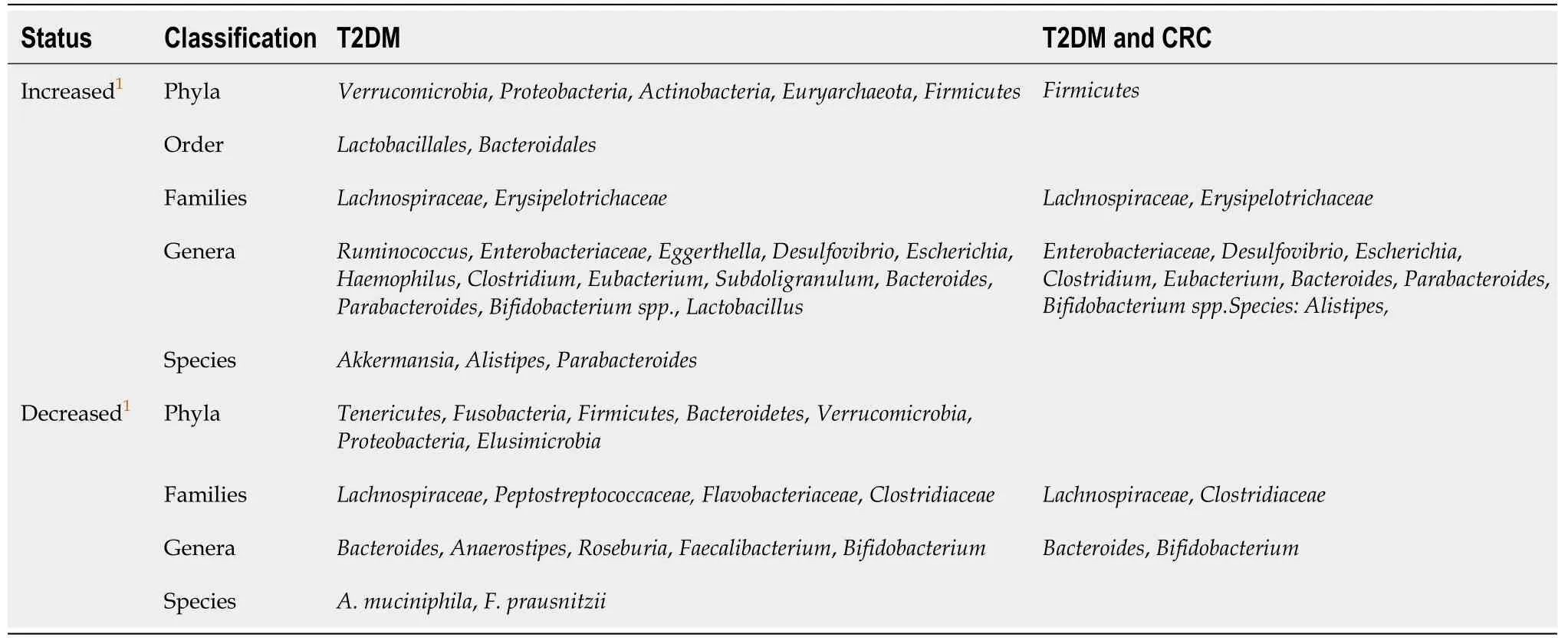
Table 2 Summary of the alteration of gut microbiota in patients with type 2 diabetes mellitus and the common alteration with both type 2 diabetes mellitus and colorectal cancer from 2012 to 2019
To sum up, in both humans and animals or at both the individual and cellular level,metformin has shown an effect of inhibiting the development of CRC. However, as a treatment for T2DM, the underlying mechanism has not been fully elucidated.
Metformin’s action depends on gut microbiota
The changes of gut microbiota have been investigated to be associated with metformin administration, contributing to the beneficial therapeutic effects[30,31]. Decreased profiles ofIntestinibacter spp.andClostridium spp.were observed upon administration of metformin to health young men with a corresponding increase ofEscherichia/Shigella spp.andBilophila wadsworthia[32]. To date, there are many studies focusing on the role of the gut microbiota that mediate the effect of metformin in regard to improving the metabolic phenotype.
In diet-induced obese mice, an increase in the population ofAkkermansia, a mucin degradation bacteria, induced the improved glucose homeostasis in the cases of metformin administration[33]. Subsequent work focused on the effects of improving metabolism and delaying T2DM progression in animal models[34,35]. The study addressed by Forslundet al[36]supported the opinion from the perspective of the human gut genome that metformin could reduce the consumption of short-chain fatty acid-producing groups in T2DM and improve the transfer of functional microbial groups. Through the implementation of 16S rRNA gene sequencing, it was observed that the participating microbiota were mainlyAkkermansia muciniphilaand short-chain fatty acid-producing bacteria, includingButyrivibrio,Bifidobacterium bifidum,Megasphaera, andPrevotellaafter the metformin intervention[37,38].
Overall, at the phylum level, the main shifts by metformin in patients with T2DM are reflected in the increase ofBacteroidetes, Actinobacteria, andProteobacteriawith a corresponding decrease ofFirmicutesandVerrucomicrobia. At the genus level, the increased bacteria were mainlyBacteroides, Streptococcus, Collinsella, Escherichia,Clostridium, andSubdoligranulum, while the decreased bacteria wereFaecalibacteriumandRuminococcus[39,40]. Finally, through a comparative analysis of currently available data, we found that the alteration of gut microbiota involved in the inhibition of CRC by metformin in T2DM may be mainly the increase ofFirmicutesand decrease ofBacteroidetes, Fusobacteria, andBacteroidetesat the phyla levels. At the genus level, it is mainly manifested as the increase ofBifidobacteriumand decrease ofFusobacterium (F.)nucleatum.
In addition, Baueret al[41], Sunet al[42], and Pryoret al[43]confirmed that metformin improved metabolic dysfunction, such as hyperglycemia,viathe glucose-SGLT1-sensing glucoregulatory pathway,B. fragilis-GUDCA-intestinal FXR axis, and the model of host-microbe-drug-nutrient interactions, respectively. These three new findings are profound to help understand how metformin and gut bacteria together affect disease progression. Table 3 lists their details and the involved types of gut microbiota. Not only that, with the participation of gut microbiota, other fields have gradually revealed the role of metformin, including anti-inflammatory effects,downregulation of interleukin expression, and ameliorating polycystic ovary syndrome in an animal model[44]. Moreover, metformin accelerated fatty acid oxidation by regulating host metabolism and longevity under the action of regulating a multipressure metabolic system[43].
Therefore, it can be confidently said that the gut microbiota, such asAkkermansia, B.fragilisandE. coli, mediate the pharmacological effects of metformin in different fields separately.
Gut microbiota affects the occurrence and development of CRC
At present, many studies have confirmed that gut microbiota affect the occurrence and development of CRC. In humans, Yachidaet al[2]used metagenomics analysis techniques to analyze the changes of gut microbiota in different stages of CRC on samples from a large cohort of 616 participants. They found the relative abundance ofFusobacterium nucleatum spp.was significantly elevated continuously from intramucosal carcinoma to more advanced stages. In addition,Atopobium parvulumandActinomyces odontolyticus,which co-occurred in intramucosal carcinomas, were significantly increased only in multiple polypoid adenomas and/or intramucosal carcinomas. Moreover, Yuet al[45]has shown thatF. nucleatumwas abundant in CRC tissues in patients with recurrence post chemotherapy.F. nucleatumpromoted CRC resistance to chemotherapy by targeting TLR4 and MYD88 innate immune signaling and specific microRNAs to activate the autophagy pathway. By 16S rRNA gene sequencing of stool samples in taxon-based analysis, stool of conventional adenoma patients was depleted in a network ofClostridiaoperational taxonomic units from familiesRuminococcaceae, Clostridiaceae,andLachnospiraceaeand enriched in the classesBacilliandGammaproteobacteria,orderEnterobacteriales,and generaActinomycesandStreptococcus[46].
In animal models through fecal bacteria transplantation, the intestinal microbiota of CRC patients promoted the progression of intestinal adenomas in Apcmin/+mice[47].Donohoeet al[48]proved that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner by a gnotobiotic mouse model. Zhuet al[49]found that editing of the gut microbiota reduced carcinogenesis in colitisassociated CRC. Qinet al[50]suggested that the gut microbiome, under specific dietary exposures, stimulates a reprogramming of the enhancer landscape in the colon with downstream effects on transcription factors, which may be associated with CRC development. In addition, the immune system plays an important role in the effect of gut microbiota on CRC. Cremonesiet al[51]demonstrated that gut microbiota modulated T cell trafficking in human CRC. Wanget al[52]has shown that a purified membrane protein fromAkkermansia muciniphilaor pasteurized bacterium blunts colitis-associated tumorigenesis by modulation of CD8+ T cells. Meanwhile, Longet al[53]has also shown thatPeptostreptococcus anaerobiuspromoted the occurrence of CRC and regulated tumor-related immunity. At the cellular level, Belchevaet al[54]demonstrated that gut microbial metabolism drives transformation of MSH2-deficient colon epithelial cells.
To sum up, in both humans and animals, gut microbiota is closely related to CRC,and this relationship refers to the immune system. In humans, studies have elaborated changes in the types of gut microbiota, and animal and cell experiments have further elaborated the possible mechanisms by which gut microbiota affects CRC. In Wonget al[55]’s review, the gut microbiota is thought to influence colorectal carcinogenesisviamicrobial-derived factors such as metabolites or genotoxins, promotion of cancer, or proliferating as opportunistic microorganisms in the tumor-associated microenvironment and the activation of procarcinogenic signaling pathways.
Changes in different gut microbiota by metformin at different stages of CRC
As we described before, the changes in the gut microbiota are inconsistent at different stages of development of CRC. According to the interaction between metformin and gut microbiota, does metformin mediate the changes of different gut microbiota at different stages of the development of CRC?
Based on the eighth Union for International Cancer Control Tumor Node Metastasis Classification of Malignant Tumors, at the phylum level in stage 0/pTis, the enriched bacteria are mainlyActinobacteriaaccompanied by a certain amount ofProteobacteria,Firmicutes, andFusobacteria.In stage SI/II, the bacteria that are enriched areProteobacteri,Firmicutes,Fusobacteria, andBacteroidetes, and they show roughly the same abundance. In the stage SIII/IV, the enrichment ofFirmicutesandBacteroidetesis moreobvious.Proteobacteri,Actinobacteria, andFusobacteriaare also present, but their abundance is not very high and is roughly equal[2].
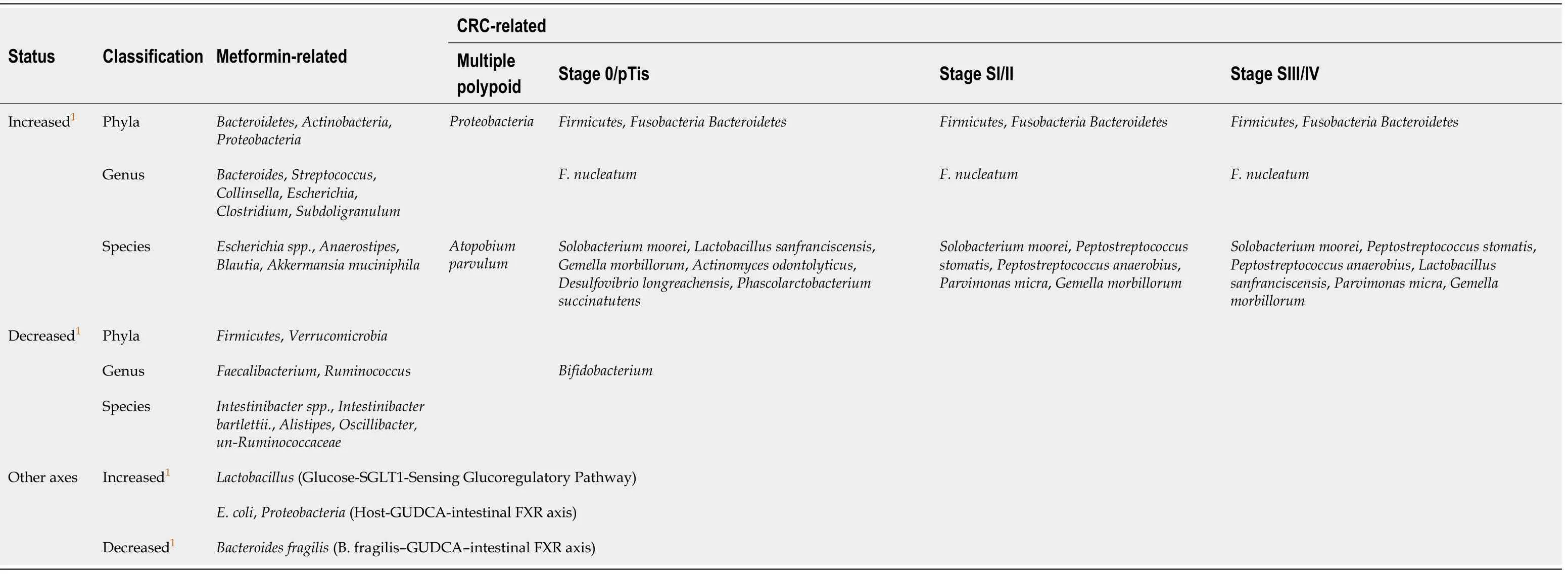
Table 3 Summary of alteration of gut microbiota by metformin in patients with type 2 diabetes mellitus that may affect colorectal cancer from 2014 to 2020
In a nonblinded, one-armed intervention study, the relative abundance of 11 bacterial genera significantly changed during metformin intervention but returned to baseline levels after treatment cessation[32], which demonstrated that the effects of metformin on the gut microbiota are transient. However, for patients with T2DM, this condition is nothing to worry about because they require long-term medication.Moreover, there was a significant decrease of the phylumFirmicutesand of the ratio ofFirmicutestoBacteroidetesafter taking metformin[39,56]. The other result of the microbiome analyses indicated a shift in the bacterial distribution in fish exposed to metformin, leading to an increase ofProteobacteriaand a reduction ofFirmicutesandActinobacteria[57]. Compared with the previous data, metformin use from intramucosal cancer to advanced stage is likely to mediate its anticancer effect by provoking different gut microbiota. In stage 0/pTis,Actinobacteriamay be the main target of the action of metformin, which is specifically manifested by attenuating its abundance. In stage SI/II and SIII/IV, the decrease of the phylumFirmicutesand of the ratio ofFirmicutestoBacteroidetesmay be the main function of metformin. Of course, these processes may also be accompanied by the regulation of other flora. Table 3 details the changes. Therefore, the use of metformin to interfere with different types of gut microbiota at different stages of the development of CRC may be a means to improve the survival rate and prognosis of CRC in the future.
Therapeutic effectiveness of metformin and changes in gut microbiome in recurrent CRC
Patients with stage III colon cancer have a risk of recurrence ranging between 15% and 50%[58]. Interestingly, the administration of metformin is associated with decreased recurrence of carcinoma[59], covering the colorectal adenoma (hazard ratio 0.572, 95%CI 0.385-0.852) in diabetic patients with previous colorectal adenoma[60].
So, how does the gut microbiota influence CRC recurrence? A recent study showed that higher fear of cancer recurrence was associated with lower relative abundance ofFirmicutesand higher relative abundance ofBacteroidetesat the phylum level and higher relative abundance ofBacteroidesand lower relative abundance ofLachnospiraceaeandRuminococcusat the genus level. The chemotherapy-induced changes in gut microbiota influence the fear of cancer recurrence[61]. In addition,colorectal adenoma resection gave rise to a significant increase ofParabacteroidespostoperatively. The microbiota signature ofParabacteroides,Streptococcus, andRuminococcusshowed an optimal discriminating performance of postoperative status[62]. These changes suggested that gut microbiota may be used in the future to prevent postoperative recurrence of CRC. Finally, it has been stressed that the intervention of metformin can directly affect the gut microbiota profile. In other words, metformin may inhibit the recurrence of CRC by changing the abundance structure of the corresponding gut microbiota.
The recurrence of CRC is resistant to routine chemotherapy and thought to be due to the enrichment of cancer stem cells. It has been reported that long term treatment of metformin impedes development of chemoresistance by regulating cancer stem cell differentiation in cancer cells[63]. Combination metformin with chemotherapeutics showed an overall modest but intriguing activity in patients with refractory CRC[64]and a synergistic inhibitory cytotoxicity in human CRC cells[65]. Moreover, metformin can act as an alternative radiosensitizing agent to 5-fluorouracil during neoadjuvant treatment for rectal cancer[66]. In addition, a large number of studies have confirmed that metformin, as a cancer stem cell-targeting agent in ovarian cancer, has a direct inhibitory effect on cancer stem cells[63,67]. The metformin intervention reduced expression of cancer stem cell markers and stemness-related genes in primary oral cancer cells[68].
Interestingly, microbiota also have a substantial role in the effectiveness of chemotherapy, chemoresistance, and related side effects[69]. For instance, the presence of specific bacterial species such asSlackiaandBlautia obeummay act as microbial markers associated with drug resistance monitoring[70]. The inhibition of the growth ofF. nucleatumsignificantly augments the efficiency of first-line chemotherapy treatments of CRC[71]. In addition, chemotherapy-induced changes in gut microbiota impact chemotherapyviainfluencing fear of cancer recurrence[61]. Moreover, in terms of cancer stem cells, gut microbiota regulate tumor metastasisviaimpacting circular RNA expression to regulate levels of corresponding miRNAs[72].
All in all, in the recurrence of CRC due to both the chemoresistance and the enrichment of cancer stem cells the gut microbiota plays a vital role, which may be one of the reasons why metformin can inhibit the recurrence of CRC in T2DM patients.
Metformin-gut microbiota-CRC (in T2DM) axis
Metformin, known as a “magic drug”, reduces the incidence of CRC in people with T2DM. Moreover, the gut microbiota has been shown to mediate the pharmacological effects of metformin and the progression of CRC. Finally, the shift of gut microbiota has similarity in the occurrence and development of T2DM and CRC. Therefore, a network of metformin-gut microbiota-CRC (in type 2 diabetes) axis could be assumed.In the network, the gut microbiota plays a key role as a bridge that affects not only the pharmacological effects of metformin but also the occurrence of CRC in T2DM patients.
We have hypothesized the potential mechanism of action of this axis around the gut microbiota. On the one hand, these mechanisms will pave the way for us to understand how gut microbiota can participate in drug therapy. On the other hand,they will provide ideas for the prospective clinical application and transformation of gut microbiota. More importantly, this axis can be expanded into a profound network of gut microbiota-drug-disease to explain the crosstalk relationship between gut microbiota, drugs, and diseases.
UNDERLYlNG lNVOLVED MECHANlSMS
The above narrations provide an elaborate explanation about the metformin-gut microbiota-CRC axis (in T2DM), but we need to know more details about its underlying mechanisms. Figure 1 provides an exhaustive overview. There are two aspects: (1) Metformin may change the gut microbiota after entering the intestine. The changed gut microbiota has several cascade reactions to affect tumorigenesis,including reduction of inflammation, production of metabolites, and regulation of immunity; and (2) Metformin may play a pharmacological role to influence cancerrelated systems in colorectal epithelial cells in the presence of gut microbiota.
Metformin entering the intestine changes the composition of the gut microbiota leading to the cascade anticancer effects
Noticeably, before oral drugs are absorbed into our bloodstream, they must pass through our intestines for catabolism. In this process, the drugs are likely to first react with the intestinal microorganisms colonized in the intestinal epithelium, one of which is gut microbiota. Therefore, the drug may cause a cascade-like reaction related to gut microbiota by changing the composition and abundance of the gut microbiota. The drug may also be metabolized into other secondary products under the action of gut microbiota to exert subsequent efficacy. But the causation and deep interaction mechanism still remain exclusive. We have mentioned above that the gut microbiota involved in the inhibition of CRC by metformin in T2DM may be mainlyBacteroides,Ruminococcus,Clostridium,Firmicute,Lactobacillus, andE. coli. After these gut microbiota are changed, it may cause subsequent cancer suppressionviaregulation of inflammation and immunity and reduction of the production of genotoxic metabolites,etc.
Intestinal inflammation and related pathways are closely related to the initiation of CRC. In terms of regulating inflammation, after the mice were treated with metformin,the level of inflammatory markers TNF-α, IL-6, and IL-17α in plasma was reduced with a corresponding increase of the level of IL-10, which was accompanied by a reduction inHelicobacter pylori. Leeet al[44]suggested that fecal microbiota transplantation using metformin-treated mouse fecal material can upregulate the expression of GLP-1 and pattern recognition receptors TLR1 and TLR4. It has been shown that CRC-enriched genotoxic polyketide synthase (pks)+ E. coli,E. faecalis, andA. finegoldiiand TLR2 and/or TLR4 pathway-related bacteria, such asF. nucleatumandPeptostreptococcus anaerobius, are related closely to intestinal inflammation[55]. In our previous discussion, these bacteria are likely to be the targets of metformin, which provides a theoretical basis for our conjecture.
In terms of innate immune response, it has been proven that metformin specifically affects the accumulation of IFN-γ producing NK cells, IL-4, and IL-17 producing NKT cells and IL-12 producing macrophages/dendritic cells in the intestine in animal models[73]. In addition, the latest studies have shown that metformin could upregulate protein molecules involved in the classical immune pathway such as PMK-1/p38MAPK[74], RAGE ligands[75], and TLRs[76]. It should be mentioned that this process is likely to be mediated by gut microbiota.F. nucleatumhas been shown to activate autophagyviathe TLR4 and MYD88 pathways closely related to innate immunity[45].
In terms of adaptive immune response, it has been mentioned that metformin regulates adaptive immune cell infiltration[77,78], the expression of related immune factors, such as IFN-γ[79], IL-2[80], and IL-6[23,81,82], and T cell metabolic reprogramming in tumor tissues[83]. Nevertheless, it must be pointed out that these effects may also be manifested in the presence of gut microbiota. Cremonesiet al[51]proved that gut microbiota modulated T cell trafficking into human CRC including CCL5, CXCL9, and CXCL10 for cytotoxic T lymphocytes and T helper (Th) 1 cells, CCL17, CCL22, and CXCL12 for Th1 and regulatory T cells, CXCl3 for follicular Th cells, and CCL20 and CCL17 for IL-17-producing Th cells. Sethiet al[84]showed that gut microbiota depletion significantly reduced tumor burden in subcutaneous and liver metastasis models of pancreatic cancer, colon cancer, and melanoma. However, the effect was counteracted in Rag1-knockout mice that lacked mature T and B cells. In fact, gut microbiota is likely more complicated in the mechanisms of drug-mediated immunity affecting the development of CRC. A lot of work needs to be done to supplement this field.
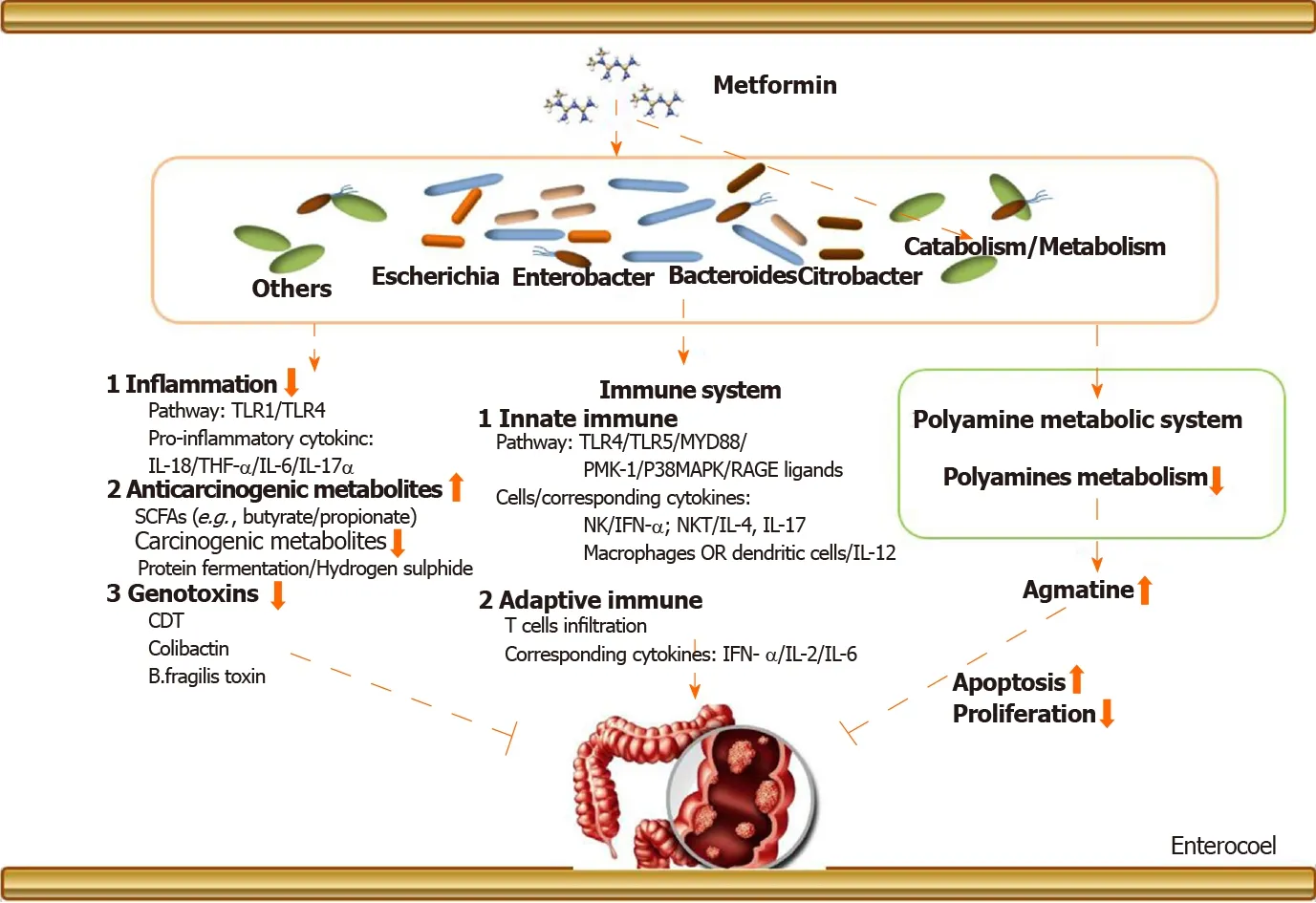
Figure 1 The potential metformin-gut microbiota-colorectal cancer axis in type 2 diabetes mellitus. (1) Metformin may change the gut microbiota after entering the intestine. The changed gut microbiota has cascade reactions to affect tumorigenesis, including reducing inflammation, regulating immunity, and producing metabolites, such as bile acid and genetic toxins. (2) Metformin may play a pharmacological role to influence cancer-related systems in colorectal epithelial cells in the presence of gut microbiota. CDT: Cytolethal distending toxin; SCFAs: Short-chain fatty acids.
In addition, it should be mentioned that after metformin changes the abundance of gut microbiota, the metabolites of the gut microbiota such as carcinogenic-related metabolites and genotoxicity will also change accordingly. Some of these metabolites are cancer-promoting, such as products of protein fermentation, hydrogen sulfide, bile acid metabolism, and ethanol, but some show protective effects. For example, shortchain fatty acids (butyrate and propionate) are anti-inflammatory molecules. Butyrate can inhibit histone deacetylase in colon epithelial cells and immune cells to downregulate proinflammatory cytokines and induce apoptosis in CRC cell lines.Therefore, the reduction of gut microbiota that produces procarcinogenic metabolites and the increase of gut microbiota that produces anti-inflammatory metabolites are likely to be the reason of metformin’s action. Similarly, metformin can directly slow down the abundance ofE. coli, restraining to the intestinal bacteria’s carcinogenic effects. On the other hand, metformin may also promote some floras that are beneficial for anticancer effects to become a dominant flora. This is consistent with the changes in gut microbiota caused by the metformin intervention mentioned in Table 3.
Role of polyamine metabolism system in the inhibition of CRC by metformin in the presence of gut microbiota
Polyamines are a class of compounds containing two or more amino groups. The raw materials for their synthesis are ornithine and arginine. The key enzymes are ornithine decarboxylase and arginine decarboxylase. It has been proven that they can regulate cell proliferation and apoptosis, and their biosynthesis is closely related to the formation and metastasis of cancer[85]. There is a correlation between the expression of ornithine decarboxylase and the prediction of cancer risk and treatment response in certain epithelial cancers. However, it must be pointed out that the function of the polyamine system is affected by metformin in the presence of several pathogens,includingShigella flexneri,Streptococcus pneumoniae,Salmonella enterica subsp.,enterica serovar Typhimurium, andHelicobacter pylori[86]. Therefore, metformin may inhibit the development of CRC by regulating the polyamine system under the action of certain gut microbiota.
Agmatine, a biogenic amine, is a polyamine that is converted from arginine by the action of arginine decarboxylase on the mitochondrial membrane of cells, and it has vasomotor implication and regulation of anti-inflammation[87]. Interestingly, the ability of bacteria to produce agmatine was enhanced under the administration of metformin in T2DM in a nutrient-dependent manner[43]. In addition, it must be emphasized that agmatine has a directly inhibitory effect on the proliferation of tumor cells with the corresponding acceleration of apoptosis[88]. As for CRC, agmatine has been confirmed to inhibit the proliferation of six types of human intestinal tumor cell lines in a concentration-dependent manner[89]. It is able to arrest proliferation in cell lines by depleting intracellular polyamine levels and enter mammalian cellsviathe polyamine transport system.
To date, there are few reports on the use of agmatine metabolism as a direct therapeutic target in clinical research, but there are studies affirming that the agmatine metabolism system has the potential to treat tumors. The role of metformin and gut microbiota is like a fuse in the antitumor effect mediated by the agmatine metabolic system. Moreover, Pryoret al[43]suggested that theEscherichia, Bacteroides, Enterobacter,andCitrobactergenera produce the most agmatine in the intestine, and these bacteria were abundant in the gut of patients after metformin intervention. Furthermore, this inhibitory effect of agmatine on tumors was probably attributable to an interaction between agmatine and the intracellular polyamine metabolism system[90,91]. For example, agmatine regulates the functional gene delivery system of spermidine/spermine acetyltransferase in cancer cell[90]. Therefore, the exploration of a metformingut microbiota-cell polyamine metabolism system-cancer chain helps us to understand how metformin inhibits CRC by regulating the agmatine metabolism system in the presence of gut microbiota. From the perspective of drugs-gut microbiotapharmacological effects-diseases, this will be an interesting and profound finding.
To sum up, metformin may indirectly restrain clinical colorectal tumors by regulating the agmatine metabolism system in the presence of gut microbiota. In future studies, more attention should be attached to the drug’s interaction with gut microbiota to the intracellular polyamine system because the intervention of the polyamine system may be one of the important methods for clinical cancerous treatment.
FUTURE EXPECTATlONS
One clear goal moving forward is to explore how to better apply the gut microbiota in clinical treatment. Gut microbiota may directly induce metformin’s anti-CRC effectviathe structure of its own composition, the production of secondary metabolites, and the regulation of the immune system after interplay with metformin. As a consequence,accumulated information has proven that metformin influences colorectal tissue carcinogenesis, and this effect is dependent on gut microbiota. Then we need to figure it out that what types of gut microbiota play key roles in this process.
Herein, we advocate to build a complete network of relationships of drugs-gut microbiota-diseases. This concept will provide a new treatment strategy for the diagnosis and treatment of clinical diseases by using gut microbiota. However, in order to achieve this goal, the relationship between changes in the structure and function of the gut microbiota and the pharmacological effects of drugs needs to be thoroughly explained. For example, composition of gut microbiota is a determinant for development of gastrointestinal adverse effects following metformin intake[32]. Wuet al[92]investigated the pharmacodynamic and pharmacokinetic effects of metformin mediated by the gut microbiotain vivo. The pharmacodynamic indexes were evaluated, and metformin concentrations were measured with a validated liquid chromatography-tandem mass spectrometry method after oral administration. They described the pharmacodynamic differences between metformin in sterile diabetic rats and conventional diabetic rats. Compared to conventional diabetic rats, fasting blood glucose and cmax in pseudosterile diabetic rats were significantly increased with a corresponding reduction in oral glucose and t1/2α. All of these elaborated that these differences in pharmacodynamics and pharmacokinetics may be due to the decrease in the expression ofOct1in the liver, contributing to the changes in liver uptake of metformin[92]. Napolitanoet al[93]suggested that metformin has complex effects that alter bile acid recycling and gut microbiota (positive correlation between the microbiota abundance of the phylumFirmicutesand changes in cholic acid and conjugates, negative correlation inBacteroidetes) due to gut-based pharmacology,which might provide insights into novel therapeutic approaches to treat T2DM and associated metabolic diseases. But the explanation of these pharmacodynamics and pharmacokinetic mechanisms is not enough for the determination of clinical drugs. In addition, the current majority of research focuses on the relationship between the changes in the profiles of gut microbiota and the effects of drugs on diseases (e.g.,metformin on CRC).
To date, the research in respect to the role of metformin to promote the formation of CRC in T2DM were mainly carried out in the observational studies of populations. In future work, more experiments need to be conducted to explore deeper mechanisms in individuals and in animal models, which can also verify the effect of an antihyperglycemia pharmaceutical for anticancer treatment. Furthermore, metformin, a biguanide derivative, has pleiotropic effects beyond glucose reduction, including antitumor, antihaze-induced pneumonia, and anti-aging. Other pharmacological effects and corresponding mechanisms of metformin need to be further discovered.For instance, we elaborated that metformin elicits its role in tumorsviaregulating the function of the immune system and how the effect is performed in the presence of gut microbiota based on the fact that the occurrence of tumors is closely related to the body’s immunity.
Overall, CRC is an urgent research topic undertaken by many researchers all over the world. Nevertheless, the diagnosis and treatment of CRC still has many unresolved issues, but the research on the gut microbiota has opened a new window.Through this window, we could see the emergence of gut microbiota as a novel way to fight tumors. However, the current research is limited to the changes in its quantity and distribution due to the limitations of current scientific research technologies and methods. When metformin is regarded as an anticancer drug in the intestine, the intestinal microbiota will change or affect the production of certain anticancer substances. Hence, it must be stressed again that establishing a complete drug-gut microbiota-disease relationship network is necessary for the potential medical application of gut microbiota. In addition, novel methods for microbiological research should be systematically carried out in order to explore the function and structure of gut microbiota in the future: structure functions, genomics, transcriptomics,proteomics, and metabolomics. These efforts will benefit the prevention, diagnosis,and treatment of clinical CRC. Advances in this field will also promote the progress of other microbial-related diseases and provide new ideas for the use of clinical drugs.
CONCLUSlON
In summary, metformin can inhibit the initiation of CRC in T2DM patients by changing the abundance of gut microbiota or under the participation of gut microbiota. Further research is needed to confirm this hypothesis and explore the mechanisms behind it. Moreover, in order to understand thoroughly the function and structure of gut microbiota, more cutting-edge scientific research methods and technologies are needed to implement.
 World Journal of Clinical Cases2020年24期
World Journal of Clinical Cases2020年24期
- World Journal of Clinical Cases的其它文章
- lmpact factors of lymph node retrieval on survival in locally advanced rectal cancer with neoadjuvant therapy
- Three-year follow-up of Coats disease treated with conbercept and 532-nm laser photocoagulation
- Virus load and virus shedding of SARS-CoV-2 and their impact on patient outcomes
- Risk factors for de novo hepatitis B during solid cancer treatment
- Cause analysis and reoperation effect of failure and recurrence after epiblepharon correction in children
- Effects of different acupuncture methods combined with routine rehabilitation on gait of stroke patients
