Purification and Characterization of a Novel Lipase from Antarctic Krill
CHEN Xin, WANG Chunlan, XU Jiakun, 3), *, WANG Fang, JIANG Yihui,CHEN Yixuan, 5), and ZHAO Xianyong, 4), *
Purification and Characterization of a Novel Lipase from Antarctic Krill
CHEN Xin1), 2), WANG Chunlan2), XU Jiakun2), 3), *, WANG Fang2), JIANG Yihui1), 2),CHEN Yixuan2), 5), and ZHAO Xianyong2), 4), *
1),,201306,2),,,,266071,3),266237,4),,266071,5),,266100,
Lipase from Antarctic krill, with a molecular weight of 71.27kDa, was purified with ammonium sulfate precipitation and a series of chromatographic separations overion exchange (DEAE) and gel filtration columns (Sephacryl S-100), resulting in 5.2% re- covery with a 22.4-fold purification ratio. The optimal pH and temperature for enzyme activity were 8.0 and 45℃, respectively. Puri- fied lipase hadmandmaxvalues of 3.27mmolL−1and 2.4Umg−1, respectively, using-nitrophenyl laurate as the substrate. Lipase activity was enhanced by adding Ca2+and Mg2+ions in the concentration ranges of 0–0.5mmolL−1and 0–0.3mmolL−1, respectively, while the activity was inhibited by a further increase in these ion concentrations. Fe3+and Cu2+ions showed obvious inhibitory effects on enzyme activity, and the inhibition rates were 71.8% and 53.3% when the ion concentrations were 0.5mmolL−1.
Antarctic krill; lipase; isolation and purification; enzymology properties
1 Introduction
Antarctic krill () is a type of crus- tacean zooplankton that lives in the Southern Ocean and feeds on microscopic phytoplankton (Alonzo, 2005). It is a keystone species in the Antarctic food chain, play- ing a crucial role in the Antarctic food web and providing an important source of food for whales, seals, squid, ice fish, penguins, albatrosses, and many other species of birds (Friedrich and Reinhard, 1996). With a maximum length of about 6cm and weighing up to 2g, krill can live for about 6 years (Hellgren., 1986). Antarctic krills have been used mainly in aquaculture and aquarium feeds, as well as in the pharmaceutical and health food industries (Živković., 2015). This crustacean is a rich source of endogenous enzymes, such as protease (Hahn Berg., 2001), lipase, chitinase (Saborowski., 1999),and glucosidase (Saborowski, 1993). Due to the uni- que geographical environment and natural climate of the Antarctic continent, enzymes from Antarctic krill exhi- bit higher activity, which may offer additional possibili-ties as industrial biocatalysts (Cai., 2009). These enzymes may affect the quality of the krill and the cor- responding industrial products during processing and sto- rage. Thus it is of great importance to clarify the proper- ties of the enzymes in Antarctic krill.
In this study, a new lipase was isolated from Antarctic krill by a comprehensive extraction method including am-monium sulfate precipitation, dialysis, ion exchange chro- matography, and size exclusion chromatography. The basic enzymatic properties of the purified lipase were character- ized, laying the foundation for its further development and utilization.
2 Materials and Methods
2.1 Experimental Materials
Antarctic krill was provided by the Liao Fishery Group after harvest from Antarctic waters in October 2016 and stored at −80℃ until use.
2.2 Reagents and Instruments
2.2.1 Reagents
Disodium hydrogen phosphate, sodium dihydrogen phos-phate, isopropyl alcohol, Triton X-100, ascorbic acid, ZnCl2, MgCl2, CuCl2, FeCl3, AlCl3, MnCl2, CaCl2, and other metal salts were purchased from Sinopharm Che- mical Reagent Co., Ltd. (Beijing, China).-nitrophenyl laurate was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan).
2.2.2 Instruments
A Mettler Toledo S20 pH meter (Mettler Toledo, Grei- fensee, Switzerland), the HH-1 electric constant tempe- rature water bath (Changzhou Guohua, Changzhou, Chi- na), a CR21GII high-speed refrigerated centrifuge (Hi- tachi, Tokyo, Japan), the AKTA Explorer FPLC System (GE, Milwaukee, WI, USA), a gel electrophoresis system (Bio-Rad, Hercules, CA, USA), and an ultrapure water purification system (Millipore, Bedford, MA, USA) were purchased from commercial sources.
2.3 Experimental Methods
2.3.1 Preparation of the crude enzyme extract
An appropriate amount of frozen Antarctic krill stored at −80℃ was thawed on ice, and suspended in 50mmolL−1Phosphate-buffered saline (PBS, pH 7.0) pre-cooled to 4℃ (m:v=1:2). The mixture was homogenized and centrifuged at 42600×at 4℃ for 30min to remove the insoluble components. The supernatant was used as a crude enzyme extract solution to which ascorbic acid was added to a final volume ratio of 1% to prevent the reduction of enzyme activity caused by oxidation.
2.3.2 Lipase purification
The crude enzyme extracted above was used for further purification. The supernatant was fractionated by add- ing solid ammonium sulfate (35%–60% saturation).The resulting precipitate was collected by centrifugation at 42600×for 30min.Then it was redissolved in 50mmolL−1phosphate-buffered saline (PBS, pH 7.0) to a final ratio of 1:2 (m:v), and dialyzed against the same buffer. The dialyzed solution was filtered through a 0.45μm fil- ter and loaded onto a HiprepTMDEAE FF 16/10 column (2.0cm×20cm) (fast flow) equilibrated with buffer A (50mmolL−1phosphate-buffered saline (PBS, pH 7.0). The column was washed with buffer A to remove unbound proteins and the remaining bound proteins were eluted in a linear 0–1molL−1NaCl gradient of buffer B (50mmolL−1phosphate-buffered saline (PBS, pH 7.0) containing 1molL−1NaCl at a flow rate of 1mLmin−1. The eluted fractions were collected and analyzed for lipase activity. The fractions containing lipase activity were dialyzed a- gainst a 50mmolL−1phosphate-buffered saline (PBS, pH 7.0), prior to final purification using a HiPrep 26/60 Sep- hacryl S-100 gel filtration column where the protein was eluted using 50mmolL−1phosphate-buffered saline (PBS, pH 7.0) containing 0.15molL−1NaCl at a flow rate of 0.5mLmin−1with absorbance monitored at 280nm. The purity of the fractions at different purification stages was visualized by Coomassie Blue staining. Fractions showing a single band on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were pooled, concentrated, and dialyzed against buffer A prior to storage at −20℃ until use. The following marker proteins were used for co- lumn calibration to estimate the molecular mass: blue dextran (29kDa), bovine thyroglobulin (66kDa), apofer-ritin (150kDa), β-amylase (200kDa), alcohol dehydroge- nase (443kDa), albumin (669kDa), and phosphorylase (94kDa). The molecular mass of Antarctic krill lipase was estimated by gel filtration on a calibrated SuperdexTM200 column in 20mmolL−1phosphate-buffered saline (PBS, pH 7.0) containing 0.15molL−1NaCl at flow rate of 0.5mLmin−1.
2.3.3 Determination of lipase activity
Lipase activity was determined using a method de- scribed previously with minor modifications (Hatziniko- laou., 1999). Briefly, 0.1mL of solution A and 1.5mL of solution B were mixed and preheated at 40℃ for 4min. Lipase (0.1mL) was added to start the reaction, which was incubated in a thermostated water bath at 40℃ for 6min. Then 2mL ethanol was added to termi- nate the reaction and precipitate the protein. Absorbance was measured at 410nm using an ultraviolet and visible light spectrophotometer. In both cases, the enzyme sam- ple was boiled for 10min prior to receiving the same treatment and was used as a reference blank. One unit (U) of activity was defined as the amount of enzyme required to produce 1µmol of-nitrophenol per minute under the assay conditions. Buffer A is 3.33mLmin−1-nitrophenyl laurate solubilized in isopropanol. Buffer B is 0.1molL−1phosphate-buffered saline (PBS, pH 7.0) containing 0.4% Triton X-100. The protein concentration was determined using a BCA Protein Assay Kit (Chen., 2017).
2.3.4 Determination of optimum temperature and temperature stability
The enzyme was incubated at different temperatures (16, 25, 35, 40, 45, 50 and 55℃) for 6min, followed by an activity assay using-nitrophenyl laurate as the sub- strate to determine the optimum temperature.
To investigate the effect of temperature on enzyme sta- bility, the enzyme solution was placed at different tempe- ratures (0, 4, 10, 16, 25, 35, 45 and 55℃), and the activitieswere measured every 2h using the method described in Section 2.3.3 with-nitrophenyl laurate as the substrate.
2.3.5 Determination of the optimum pH
The enzyme solution was placed in buffer solutions of different pH values (5.0, 6.0, 7.0, 8.0, 9.0 and 10.0) to de- termine the optimum reaction pH. The buffers were 50mmolL−1Na2HPO4-citric acid (pH 5.0–8.0), 50mmolL−1gly- cine-NaOH (pH 9.0), and 50mmolL−1Na2HPO4-NaOH (pH 10.0).
2.3.6 Effect of metal ions on lipase activity
The effect of metal ions (Ca2+, Mg2+, Zn2+, Al3+, Cu2+, Mn2+and Fe3+) on lipase activity was determined at pH 8.0 and 40℃ after a 30min incubation. The salts used were CaCl2, MgCl2, ZnCl2, AlCl3, CuCl2, MnCl2andFeCl3with the concentration at 0–0.5mmolL−1. The percentage inhibition rate was calculated using the following equation:

2.3.7 Substrate specificity for various-nitrophenyl esters
Substrate specificity was defined using-nitrophenyl esters of various carbon chain lengths (C4–C16), such as-nitrophenyl butyrate (C4),-nitrophenyl hexanoate (C6),-nitrophenyl caprylate (C8),-nitrophenyl decanoate (C10),-nitrophenyl laurate (C12), and-nitrophenyl palmitate (C16). The concentration of the substrates was 4mmolL−1and lipase activity was determined as described in Section 2.3.3.
2.3.8 Kinetic parameters
The Michaelis-Menten kinetic parameters,mandmax, of the purified lipase were determined by measuring the increasing concentration of-nitrophenol produced from-nitrophenyl laurate (0.6–10mmolL−1). Themandmaxvalues were determined using Lineweaver-Burk plots.
3 Results and Discussion
3.1 Purification of Lipase from Antarctic Krill
Fractions (1mL) were analyzed for lipase activity us- ing-nitrophenyl laurate as the substrate. As shown in Fig.1A, seven peaks eluted after DEAE anion-exchange chromatography. The fractions showing lipase activity were used for further purification by size exclusion chro- matography. As shown in Fig.1B, two peaks were ob- served after size exclusion chromatography, and fractions from the first peak showed lipase activity.
The purification data reported in Table 1 indicate that lipase activity was purified 22.4-fold over that in the start- ing material, with a total recovery of 5.2%. After purifica- tion, the protein component of the lipase active fraction appeared homogeneous on SDS-PAGE (Fig.2), consist- ing of a single Coomassie Blue stained band.Determina- tion of the molecular mass by analytical gel filtration resulted in the lipase emerging from the column in a peak at 1.85 times of the void volume, with an estimated mo- lecular mass of 71.27kDa.
When compared with other enzyme purification meth- ods for Antarctic krill, this method yielded a superior li-pase purification ratio. Wu. (2008) isolated and purified trypsin from Antarctic krill, resulting in a purification ratio of 5.4 and a recovery rate of 26%. The purification ratio of a protease isolated from Antarctic krill, as described by Guo(2001), was 12.6, with a yield of 43%. Turkiewicz. (1985) isolated and purified endo- (1→3)-β-D-glucanase from Antarctic krill, resulting in an enzyme activity recovery rate of 2.6% with a purifica- tion ratio of 980. Si. (2014) isolated an arginine ki- nase from Antarctic krill with a purification ratio of 2.6 and a recovery rate of 86%. While our method yielded a final Antarctic krill lipase purification factor that was higher than those of other methods, the resulting enzyme activity recovery rate was a little lower.
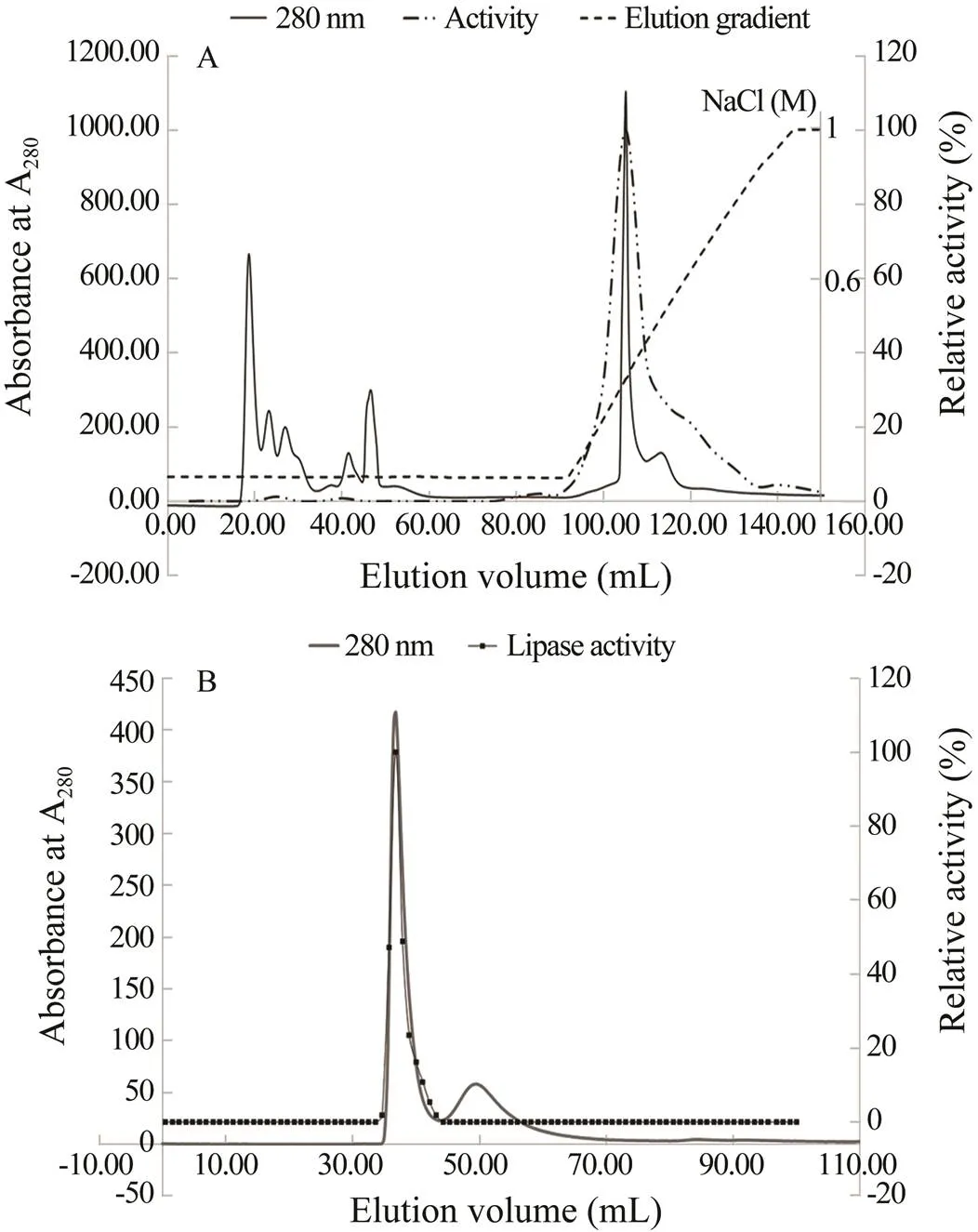
Fig.1 Chromatographic purification of Antarctic krill lipase. (A) Elution profile of the ammonium sulfate precipitated fraction from chromatography over a HiprepTM DEAE FF 16/10 column; (B) HiPrep 26/60 Sephacryl S- 100 gel filtration column. Elution was performed using a gradient (0–1molL−1 NaCl) at a flow rate of 1mLmin−1 for the HiprepTM DEAE FF 16/10 column and 50mmolL−1 phosphate-buffered saline (PBS, pH 7.0) containing 0.15molL−1 NaCl at a flow rate of 0.5mLmin−1 for the HiPrep 26/60 Sephacryl S-100 gel filtration column.

Table 1 Purification steps and fractions

Fig.2 Sodium dodecyl sulfate-polyacrylamide gel electro- phoresis analysis. M, Protein marker (5μL); C, crude lipase extract (1861μg); A, ammonium sulfate precipitation (167μg); D, DEAE FF fractions (63μg); S, HiPrep 26/60 Sephacryl S-100 fractions (S1, 6.25μg; S2, 12.50μg).
3.2 Effect of Temperature on Antarctic Krill Lipase Activity
Fig.3A shows that the lipase had no catalytic activity below 16℃. The enzyme activity increased significantly as the reaction temperature was raised from 16℃ to 45℃. Catalytic activity decreased with increasing temperature above 45℃, until it was completely inhibited at temperatures higher than 55℃.
According to Fig.3B, the lipase was highly stable at 16℃ and below, maintaining 95.0% of the original enzyme activity after 10h at 16℃. The lipase activity levels were 76.6%, 63.7% and 7.7% after 10h of storage at 25, 35, and 45℃, respectively. However, enzyme activity de- creased sharply with increasing incubation time at 55℃ and was totally inactivated after 2h. Antarctic crusta- ceans and South Baltic shrimp live in waters of different temperatures (Tsugawa., 1999). The apparent tem- perature optima for N-acetyl-β-D-glucosaminidase acti- vity fromis 40℃, whereas it is 50℃forThe corresponding val- ues for chitinase are 40℃ forica, and 45℃ for(Spindleret and Buchholz, 1988). In terms of lipase activity, the optimal reaction temperature is gener- ally 45–70℃. For example, the optimum reaction tem- perature for the lipase isolated from the liver tissue of seabass () (Sae-Leaw and Benjakul, 2018), the lipase isolated from smooth hound (), and the lipase isolated from red carp () (Kurtovic., 2010) are all 50℃. The lipase isolated from the gut of the shrimp,has optimal activity at 50℃ (Esakkiraj., 2010), while lipase recovered from the hepatopancreas of the Pacific white shrimp exhibits optimal activity at 55℃ (Kuepethkaew., 2017). The digestive lipase purified from crab () (CDL) shows maximal activity at 60℃ (Cherif., 2007), while the enzyme from the viscera of the gray mullet has an optimal temperature of 60℃ (Smichi., 2017); and that of the marine hydro- carbon degrading bacteria(Ka- dri., 2018) is 70℃. Compared with the marine organism-derived lipase, the lipase isolated from Antarctic krill showed a lower optimum temperature (45℃). More- over, this lipase displayed significantly lower tempera- ture stability, indicating its potential application value in the food processing, pharmaceutical, and chemical in- dustries that require low temperature.

Fig.3 Lipase enzyme properties. A, Effect of temperature on enzyme activity. B, Effect of temperature on enzyme stability. C, Effect of pH on enzyme activity. D, Effect of metal ions on enzyme activity. Enzyme activity was determined as described in Section 2.3.3. The highest level of activity was taken as 100%, and the error bars represent standard deviations (n=3).
3.3 Effect of pH on Lipase Activity of Antarctic Krill
As shown in Fig.3C, the catalytic activity of the lipase increased with increasing pH values between 5.0 and 8.0, reaching a maximum at pH 8.0. The catalytic activity of the lipase decreased as pH was increased further between 8.0 and 10.0. Thus the optimum reaction pH of the Antarctic krill lipase was 8.0.
The pH of the reaction buffer is an important factor affecting enzyme reactivity. Under different pH conditions, an enzyme may have altered conformations that change binding or dissociation of the substrate with the active site of the enzyme molecule, which can affect the activity of the enzyme (Salamanca., 2002). The optimal pH value of lipase enzymes isolated from aquatic organisms is generally between 7 and 9. These enzymes include the lipases extracted from seabass liver () (Kuepethkaew., 2017); the hepatopancreas of red seabream () (Cherif., 2007); the powdered delipidated pyloric ceca of two New Zealand-sourced fish, Chinook salmon () and hoki () (Smichi., 2017); the pyloric caeca of sardines () (Smichi., 2010); the marine strain(Ra- mani, 2013); cod () (Gjellesvik, 1992); the common smooth-hound () (Achouri., 2017); the hepatopancreas of red sea bream,(Iijima., 1998); the strainPSU-AH13 (Chaiyaso, 2012); the marine strainsp. W007 (Yuan., 2016); the gray mullet (Gor-gun and Akpinar, 2012); and carp liver (Smichi., 2013). The reaction buffer pH for optimal activity of the Antarctic krill lipase was similar with these previously isolated marine lipases.
3.4 Effect of Metal ions on Antarctic Krill Lipase Activity
The effect of seven different metal ions on activity of the lipase purified from Antarctic krill was studied over a concentration range of 0–0.5mmolL−1. Based on the data displayed in Fig.3D, Ca2+enhanced lipase activity over all concentrations tested (0–0.5mmolL−1), while Mg2+ions activated the lipase in the concentration range of 0– 0.3mmolL−1, with increasing concentrations above 0.3mmolL−1showing an inhibitory effect. Other metal ions, including Zn2+, Al3+, Cu2+, Mn2+and Fe3+had inhibitory effects on lipase activity, with Fe3+and Cu2+showing sig- nificant inhibition of 71.8% and 53.3%, respectively, at the concentration of 0.5mmolL−1.
Metal ions assist in maintaining the structure of an enzyme by binding to the active site, as well as to the enzyme surface to increase solubility of the enzyme (Si- varamakrishnan., 2016). Sangeetha. (2011) showed that Ca2+and Mg2+activate lipase by interacting with amino acids on the surface of the enzyme, changing the solubility of free fatty acid molecules in solution, or by binding to the enzyme active site to promote activity (Xie., 2016). Adding some metal ions (Fe3+, Cu2+, Mn2+, Al3+) can denature the protein; thus, decreasing catalytic ability.
3.5 Substrate Specificity to Various p-Nitrophenyl Esters
Lipase activity was higher when-nitrophenyl palmitate (C8, 100%) was used as the substrate, followed in order of decreasing activity of-nitrophenyl hexanoate (C6, 68.43%,-nitrophenyl decanoate (C10, 48.31%),- nitrophenyl butyrate (C4, 37.35%),-nitrophenyl laurate (C12, 20.73%), and-nitrophenyl palmitate (C16, 0%). The data show that the lipase has a better hydrolysis effect on substrates with a length of 4–8 carbon atoms, but decreases with increasing carbon chain length from C10 to C16 (Fig.4), while to the substrate with a length of C16 the enzyme does not exhibit any hydrolysis activity.

Fig.4 Substrate specificity of lipase activity. Lipase activity toward p-nitrophenyl ester substrates of different carbon chain lengths was assayed as described in Section 2.3.3. The highest level of activity for each substrate was taken as 100%. Error bars represent the standard deviations (n=3).
3.6 Kinetic Studies
A kinetic study of lipase purified from Antarctic krill was carried out using-nitrophenyl laurate as the substrate over a concentration range of 0.6 to 10mmolL−1. The kinetic parameters, including the maximal velocity (max) and Michaelis-Menten constant (m) were derived from fitting the Lineweaver–Burk equation to the data points where the reciprocal of the initial velocity was plotted against the reciprocal of the substrate concentration (Lineweaver and Burk, 1934) at standard reaction conditions of pH 8.0 and 40℃. Themandmaxvalues of the purified lipase were 3.72mmolL−1and 2.4Umg−1, respectively.
4 Conclusions
In this study, a new kind of lipase was purified from the Antarctic krill and its properties were characterized. The Antarctic krill lipase had a molecular weight of 71.27kDa with an optimal pH value of 8.0. The optimum reaction temperature was 45℃, and the enzyme displayed significantly low activity when the temperature was below 16℃. Both Ca2+and Mg2+ions stimulated enzyme activity over a concentration range of 0–0.5mmolL−1and 0–0.3mmolL−1, respectively. Zn2+, Al3+, Cu2+, Mn2+and Fe3+had significant inhibitory effects on the enzyme. Theactivity was higher when-nitrophenyl palmitate was used as the substrate. Lipase perform essential roles in digestion, transport, and processing of dietary lipids (., tri- glycerides, fats, and oils). After long-term evolutionary adaptation, endogenous enzymes from Antarctic krill mayhave special structures, physiological and biochemical me- chanisms suited for low temperature environments, which is consistent with the observed low optimum reaction temperature. This study not only enriches the information of enzymes derived from Antarctic organisms, but also offers a novel biocatalyst for using in industrial processes with low temperature.
Acknowledgements
This work was supported by the Marine S&T Fund of Shandong Province for the Pilot National Laboratory for Marine Science and Technology (Qingdao) (Nos. 2018 SDKJ0304-4-2, 2018SDKJ0303-1), the Central Public-In- terest Scientific Institution Basal Research Fund, Chinese Academy of Fishery Sciences (Nos. 2017HY-XKQ01-01, 2016ZD0902, 2018GH10), the Central Public-Interest Sci- entific Institution Basal Research Fund, YSFRI, CAFS (No. 20603022018025), the Aoshan S&T Innovation Project from Qingdao National Laboratory for Marine Science and Technology (No. 2015ASKJ02-02-04), the Antarctic Marine Biological Resources Development and Utilization Project from the Ministry of Agriculture and Rural Affairs,People’s Republic of China (2017), and the Financial Fund of the Ministry of Agriculture and Rural Affairs, People’s Republic of China (Nos. NFZX2018, FSTICE2019).
Achouri, N., Smichi, N., Gargouri, Y., Miled, N., and Fendri, A., 2017. The smooth-hound lipolytic system: Biochemical cha- racterization of a purified digestive lipase, lipid profile andoil digestibility., 102: 1120-1129, DOI: 10.1016/j.ijbiomac.2017. 05.002.
Alonzo, F., Virtue, P., Nicol, S., and Nichols, P. D., 2005. Lipids as trophic markers in Antarctic krill. II. Lipid composition of the body and digestive gland ofin controlled conditions., 296 (1): 65-79, DOI: 10.1093/plankt/18.6.895.
Cai, Y. J., Wang, L., Liao, X. R., Ding, Y. R., and Sun, J., 2009. Purification and partial characterization of two new cold- adapted lipases from mesophilicsp. SYBC WU- 3., 44 (7): 786-790, DOI: 10.1016/j.proc- bio.2009.03.011.
Chaiyaso, T., Seesuriyachan, P., Zimmermann, W., and H-Kit- tikun, A., 2012. Purification and characterization of lipase from newly isolatedPSU-AH130 and its application for biodiesel production., 62 (4): 1615-1624, DOI: 10.1007/s13213-011-0418-z.
Chen, Y. J., Wang, C. J., Hou, W. Q., Wang, X. S., Gali, B. G., Huasai, S. M. J. D., Yang, S. Q., Wu, A. Q. M., Zhao, Y. F., Wu, Y. F., and Chen, A. R. G. L., 2017. Effects of antibacterial compounds produced byin Kou-miss on pathogenicOs and its cell surface characteristics., 16 (3): 742- 748, DOI: 10.1016/S2095-3119(16)61516-2.
Cherif, S., Fendri, A., Miled, N., Trabelsi, H., Mejdoub, H., and Gargouri, Y., 2007. Crab digestive lipase acting at high temperature: Purification and biochemical characterization., 89 (8): 1012-1018, DOI: 10.1016/j.biochi.2007.02.005.
Esakkiraj, P., Rajkumarbharathi, M., and Grasian Immanuel, A. P., 2010. Lipase production byCMST-Pi 1 isolated from the gut of shrimp.,60 (1): 37-42, DOI: 10.1007/s13213- 009-0003-x.
Friedrich, B., and Reinhard, S., 1996. A field study on the phy- siology of digestion in the Antarctic krill,with special regard to chitinolytic enzymes., 18 (6): 895-906, DOI: 10.1093/plankt/18.6.895.
Gjellesvik, D. R., Lombardo, D., and Walthe, B. T., 1992. Pancreatic bile salt dependent lipase from cod (): Purification and properties.–, 1124 (2): 123-134, DOI: 10.1016/0005-2760(92)90088-D.
Gorgun, S., and Akpinar, M. A., 2012. Purification and characterization of lipase from the liver of carp,L. (1758), living in Lake Todurge (Sivas, Turkiye)., 12 (2): 207-215, DOI: 10.4194/1303-2712-v12_2_03.
Guo, Q. Y., Chi, H., Yang, X. S., Li, X. Y., and Hang, Y. J., 2001. Partial purification and characterization of protease from An- tarctic krill ()., 37 (10): 92-95, DOI: 10.1002/clc.20818.
Hahn Berg, I. C., Kalfas, S., Malmsten, M., and Arnebrant, T., 2001. Proteolytic degradation of oral biofilmsand: Potential of proteases originating fromfor plaque control., 109 (5): 316-324, DOI: 10.1034/j.1600-0722.2001.00099.x.
Hatzinikolaou, D. G., Kourentzi, E., Stamatis, H., Christako- poulos, P., Kolisis, F. N., Kekos, D., and Macris, B. J., 1999. A novel lipolytic activity ofcells: Production, partial characterization and application in the synthesis of esters., 88 (1): 53-56, DOI: 10.1016/S1389-1723(99)80175-3.
Hellgren, L., Mohr, V., and Vincent, J., 1986. Proteases of Antarctic krill–A new system for effective enzymatic debridement of necrotic ulcerations., 42 (4): 403-404, DOI: 10.1016/j.bej.2014.09.012.
Iijima, N., Tanaka, S., and Ota, Y., 1998. Purification and characterization of bile salt-activated lipase from the hepatopancreas of red sea bream,., 18 (1): 59-69, DOI: 10.1023/A:1007725513389.
Kadri, T., Rouissi, T., Magdouli, S., Brar, S. K., Hegde, K., Khiari, Z., Daghrir, R., and Lauzon, J. M., 2018. Production and characterization of novel hydrocarbon degrading enzy- mes from., 112: 230-240, DOI: 10.1016/j.ij biomac.2018.01.177.
Kuepethkaew, S., Sangkharak, K., Benjakul, S., and Klomklao, S., 2017. Use of TPP and ATPS for partitioning and recovery of lipase from Pacific white shrimp () hepatopancreas.,54 (12): 3880-3891, DOI: 10.1007/s13197-017-2844-9.
Kurtovic, I., Marshall, S. N., Zhao, X., and Simpson, B. K., 2010. Purification and properties of digestive lipases from Chinook salmon () and New Zealand hoki ()., 36 (4): 1041-1060, DOI: 10.1007/s10695-010-9382-y.
Lineweaver, H., and Burk, D., 1934. The determination of enzyme dissociation constants., 56 (3): 658-666, DOI: 10.1021/ja01318a036.
Ramani, K., Saranya, P., Jain, S. C., and Sekaran, G., 2013. Li- pase from marine strain using cooked sunflower oil waste: Production optimization and application for hydrolysis and thermodynamic studies., 36 (3): 301-315, DOI: 10.1007/s00449-012-0785-2.
Saborowski, R., and Buchholz, F., 1999. A laboratory study on digestive processes in the Antarctic krill,, with special regard to chitinolytic enzymes., 21 (5): 295-304, DOI: 10.1007/s003000050365.
Saborowski, R., Buchholz,F., Vetter, R. H., Wirth, S. J., and Wolf,G. A., 1993. Soluble, dye-labelled chitin derivative adapted for the assay of krill chitinase., 105 (3-4): 673-678, DOI: 10.1016/0305-0491(93)90104-D.
Sae-Leaw, T., and Benjakul, S., 2018. Lipase from liver of seabass (): Characteristics and the use for defatting of fish skin., 204: 9-15, DOI: 10.1016/j. foodchem.2017.07.089.
Salamanca, M. H., Barría, C., Asenjo, J. A., and Andrews, B. A., 2002. Isolation, purification and preliminary characterization of cryophilic proteases of marine origin., 10 (4-5): 237-241, DOI: 10.1023/A:1016383212244.
Sangeetha, R., Arulpandi, I., and Geetha, A., 2011. Bacterial li- pases as potential industrial biocatalysts: An overview., 6 (1): 1-24, DOI: 10.3923/jm. 2011.1.24.
Si, Y. X., Song, J. J., Fang, N. Y., Wang, W., Wang, Z. J., Yang, J. M., Qian, G. Y., Shang, S. J., and Park, Y. D., 2014. Purification, characterization, and unfolding studies of arginine kinase from Antarctic krill., 67: 426-432, DOI: 10.1016/j.ijbiomac. 2014.03.044.
Sivaramakrishnan, R., and Incharoensakdi, A., 2016. Purification and characterization of solvent tolerant lipase fromsp. for methyl ester production from algal oil., 121 (5): 517-522, DOI: 10.1016/j.jbiosc.2015.09.005.
Smichi, N., Fendri, A., Chaâbouni, R., Rebah, F. B., Gargouri, Y., and Miled, N., 2010. Purification and biochemical characterization of an acid-stable lipase from the pyloric caeca of sardine ()., 162 (5): 1483-1496, DOI: 10.1007/s12010-010- 8920-5.
Smichi, N., Fendri, A., Triki, S., Arondel, V., Rebai, A., Gargouri, Y., and Miled, N., 2017. Biochemical characterization, cloning and molecular modeling of a digestive lipase from red seabream (): Structural explanation of the interaction deficiency with colipase and lipidic interface., 17 (6): 664-667, DOI: 10.1002/elsc. 201600246.
Smichi, N., Gargouri, Y., Miled, N., and Fendri, A., 2013. A grey mullet enzyme displaying both lipase and phospholipase activities: Purification and characterization., 58: 87-94, DOI: 10.1016/j. ijbiomac.2013.03.056.
Spindler, K. D., and Buchholz, F., 1988. Partial characterization of chitin degrading enzymes from two euphausiids,and., 9 (2): 115-122, DOI: 10.1007/BF00442038.
Tsugawa, K., Ishizaki, Y., and Takahashi, K. P., 1999. Increase in cold-stability of microtubules by cold adaptation ofcellsand., 124 (1): S126, DOI: 10.1016/S1095-6433(99)90498-1.
Turkiewicz, M., Galas, E., and Zielińska, M., 1985. Purification and partial characterization of an endo-(1→3)-β-D-glucanase fromDana (Antarctic Krill)., 4 (4): 203-211, DOI: 10.1007/BF00999765.
Wu, Z. Q., Jiang, G. L., Xiang, P., Yang, D., and Wang, N., 2008. Purification and characterization of trypsin-like enzymes from North Pacific krill ()., 30 (1): 67-72, DOI: 10.1007/s10529-007-9511-6.
Xie, C. X., Wu, B., Qin, S., and He, B. F., 2016. A lipase with b A. road solvent stability fromRQ3: Isolation, characteristics and application for chiral resolution of 1-phenylethanol., 39 (1): 59-66, DOI: 10.1007/s00449-015-1489-1.
Yuan, D. J., Lan, D. M., Xin, R. P., Yang, B., and Wang, Y. H., 2016. Screening and characterization of a thermostable lipase from marinesp. strain W007.,63 (1): 41-50, DOI: 10.1002/bab. 1338.
Živković, L. T. I., Živković, L. S., Babić, B. M., Kokunešoski, M. J.,Jokić, B. M., and Karadžića, I. M., 2015. Immobilization oflipase by adsorption onto biosafe meso/ macroporous silica and zirconia., 93: 78-83, DOI: 10.1016/j.bej.2014.09.012.
March 18, 2019;
April 28, 2019;
September 18, 2019
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2019
E-mail: xujk@ysfri.ac.cn
E-mail: zhaoxy@ysfri.ac.cn
(Edited by Qiu Yantao)
 Journal of Ocean University of China2020年1期
Journal of Ocean University of China2020年1期
- Journal of Ocean University of China的其它文章
- Circulation and Heat Flux along the Western Boundary of the North Pacific
- System Reliability Analysis of an Offshore Jacket Platform
- The Mineral Composition and Sources of the Fine-Grained Sediments from the 49.6˚E Hydrothermal Field at the SWIR
- Research Progress of Seafloor Pockmarks in Spatio-Temporal Distribution and Classification
- Application of the Static Headland-Bay Beach Concept to a Sandy Beach: A New Elliptical Model
- Climatology of Wind-Seas and Swells in the China Seas from Wave Hindcast
