Multiplex PCR Sets of Novel Microsatellite Loci for Iwagaki Oyster Crassostrea nippona and Their Application in Parentage Assignment
LIU Kaikai, LI Qi, 2), , and LI Qiongzhen3),
Multiplex PCR Sets of Novel Microsatellite Loci for Iwagaki Oysterand Their Application in Parentage Assignment
LIU Kaikai1), LI Qi1), 2),*, and LI Qiongzhen3), *
1),,,266003,2),,266237,3),530021,
Iwagaki oyster,, widely distributes along the seashore of Eastern Asia, and has been considered to be a potential breeding species due to its delicious taste and high commercial value. In order to study its genetic background and population structure, we developed 46 novel polymorphic microsatellite markersusing next-generation sequencing technique and characterized them in 30 individuals. The number of alleles ranged from 3 to 22, while the observed and expected heterozygosities varied from 0.133 to 1.000 and 0.455 to 0.949, respectively. Fifteen microsatellite markers were selected and grouped into five highly informative multiplex PCRs for. We evaluated and validated these multiplex PCRs in a cultured population including 173 candidate parents and 486 offspring. In actual parentage analysis, 80% of the offspring were correctly assigned to their parental pairs using three multiplex PCRs. Furthermore, the success rate of parentage assignment reached 96% when the other two multiplex PCRs were added. These 46 microsatellite loci with high variability and the five multiplex PCRs described here provide a powerful tool for pedigree reconstruction, resource conservation and selective breeding program of.
; microsatellites; multiplex PCR; parentage assignment
1 Introduction
Iwagaki oyster,,is a marine bivalve belonging to Bivalvia, Ostreidae. It inhabits naturally along the coastal areas of Eastern Asia including Korea and Japan (Itoh., 2004; Yoon., 2008). The commercial value ofis high and its price is about five times of the price of Pacific oyster, because of its edibility in the summer when the other oyster species are not available in Japan (Itoh., 2004). Therefore, this species has broad market prospects and enjoys a great potential of aquiculture. Several hatcheries in China have gradually begun to introduce this species from Japan for artificial seedling and breeding in the recent years (Li, 2007; Yuan., 2008). Thus reasonable stock management and genetic improvement are becoming more and more important for sustainable development ofaquaculture industry. Molecular genetic markers are useful tools for genetic analysis and breeding in aquaculture. They play an essential role in stock identification, pedigree analysis, as well as genomic mapping (Liu and Cordes, 2004). Unfortunately, until now little information is available about the genetic background of.
Microsatellites, also known as simple sequence repeats (SSRs), have been widely used in the study of population genetic structure and parentage determination due to their high polymorphism, co-dominance and abundance in the genome. However, microsatellite genotyping is costly and time-consuming. Multiplex polymerase chain reaction (PCR) is a technique that can amplify multiple sites simultaneously in the same reaction, thus saving a lot of time and money associated with microsatellite genetic studies. In addition, this technique decreases the risk of handling errors by reducing the repeated operation of a large number of experimental samples (Porta., 2006). Therefore, the development of multiplex PCR of microsatellite can provide powerful tools for large-scale family analysis, population genetic research and gene mapping studies. To date, multiplex PCR sets have successfully been developed and applied in many aquaculture species, including(Nie., 2012),(Li., 2010; Liu., 2017) and(Li., 2016).
In the present research, we described the development and characterization of novelpolymorphic microsatellites as an important tool for future genetic research. Moreover, based on three primers PCR method (Blacket., 2012), we developed five sets of multiplex PCRs from these new microsatellites, and also tested the efficiency of these markers for parentage assignment in a cultured population.
2 Material and Methods
2.1 Sample Collection and DNA Extraction
Thirtyindividuals used for microsatellites development were captured from Haiyi Aquaculture Cooperation, Yantai, Chinain 2017. Samples used for parentage assignment were also collected in late July, 2018 from Yantai. One hundred and seventy-three brood stocks (with unknown sexes) were selected for natural spawning. Four hundred and eighty-six D-larvae were collected at 48-h post-fertilization and then were preserved in 95% ethanol. For brood stock, genomic DNA was extracted from the adductor muscle using the phenol-chloroform procedure (Li., 2006). The extraction of genomic DNA from offspring was performed by the Chelex extraction method (Li., 2003). After extraction, genomic DNA of each sample was assessed by electrophoresis using 0.8 % agarose gel.
2.2 Development and Characterization of Microsatellites
To obtain microsatellite marker resources, we selected two individuals and performed Restriction-site Associated DNA sequencing (RAD-seq) using the Illumina HiSeq 2500 platform. DNA extraction, library preparation, amplification, and sequencing were carried out by Genedenovo (Guangzhou, China). The Primer3 software (Untergasser., 2012) was used to design the primers. One hundred and sixty primer pairs were randomly selected to test the polymorphism in thirty individuals of. Amplification of each locus was performed in 10μLreaction solution containing 0.5U TaqDNA polymerase (Takara), 0.2mmolL−1of each dNTP, 1× PCR buffer, 2mmolL−1of MgCl2, 1μmolL−1of each primer and about 50ng template DNA. The conditions of PCR were as follows: initial denaturation at 94℃ for 3min, followed by 32 cycles of denaturation at 94℃ for 30s, annealing (annealing temperatures for different primer pairs are described in Table 1) for 45s, and extension at 72℃ for 45s per cycle, with a final extension at 72℃ for 5min. After amplification, PCR products were separated by 6% denaturing poly- acrylamide gels, and visualized by silver staining. A 10bp ladder was used as a reference marker for allele size determination. For each microsatellite locus, the number of alleles (), observed () and expected heterozygosities (), as well as the polymorphic information content () were calculated using the program CERVUS 3.0 (Kali- nowski., 2007). Tests for Hardy-Weinberg equilibrium (HWE) were performed with GENEPOP 4.0 (Rou- sset, 2008).
2.3 Multiplex PCR and Genotyping
Based on the polymorphism of loci and the efficiency of amplification, the best fifteen microsatellites that showed clear PCR products were selected for multiplexing. Under the criteria of non-overlapping loci, these loci were allocated to multiplex sets and the number of loci suitable for simultaneous amplification was increased as many as possible. The universal primer M13-tail was labeled with different fluorescent dyes (VIC and FAM) so that the amplification products can be differentiated by capillary se- paration. At the same time, the proper primer concentration, annealing temperature and cycle times of each multiplex PCR were then optimized with 8 samples randomly selected from the above 30 individuals of. Multiplex PCRs were performed in 10μL reaction solution containing 0.25U TaqDNA polymerase (Takara), 0.2mmolL−1of each dNTP, 1× PCR buffer, 2mmolL−1of MgCl2, 0.06 or 0.08μmolL−1forward primer, 0.15 or 0.20μmolL−1universal primer, 0.15 or 0.20μmolL−1reverse primer, and about 50–100ng template DNA. The conditions of PCR were performed as follows: initial denaturation at 94℃ for 3min; followed by 35 cycles of denaturation at 94℃ for 30s, 60s at the optimal annealing temperature, and 72℃ for 75s; then 8 cycles of denaturation at 94℃ for 30s, 53℃ for 60s, 72℃ for 75s, with a final extension at 72℃ for 10min. After amplification, PCR products were mixed with formamide and GeneScan 500- LIZ size standard (Applied BiosystemsTM Carlsbad, CA, USA) [0.5µL PCR product, 9.5µL Hi-Di Formamide (App-liedBiosystemsTM), 0.1µL GS500-LIZ]. After 5min of de- naturation at 94℃, amplification products were rapidly cooled and detected by an ABI-3130xl Genetic Analyzer. Allele sizes were estimated with the program Gene-Map- per v4.0.
2.4 Simulation and Parentage Assignment
We used CERVUS 3.0 to calculate the,,,and the non-exclusion probability of each microsatellite locus in this study. The genotypes of total 659 individuals, including 486 offspring and 173 candidate parents, were successfully analyzed using the optimized multiplex. The efficiency of the programin parentage assignment was validated. Similarly, the same program was also used to conduct the simulation and real parentage analysis based on the likelihood-based approach, and the parameters were as following: 10000 replication cycles, 50 to 300 candidate parents, 100% of the candidate parents sampled and genotyped, and strict 95% confidence level. We allowed 5% of typing errors in the assignment procedures, since this significantly reduces the impact of mismatches in parent-offspring relationships that may be caused by null alleles and mutations (Marshall., 1998).
2.5 Estimation of Effective Breeding Numbers (Ne)
The sibship reconstruction of the offspring and the inference of effective population size (N) were performed using COLONY 2.0 (Jones and Wang, 2010) without including parents. The analysis parameters were set as follows: diploid parents, both sexes polygamy, and 0.025 of the error rate of genotyping according to the suggestion of Wang (2004).
3 Results
3.1 Properties of Microsatellite Markers
A total of 24811 SSR repeats from the assembled contigs were obtained in the study, among which dinucleotide was the most frequent (59.05%), followed by mononucleotide (17.60%) and tetranucleotides (13.36%) (Fig.1). In the study, we selected and designed 160 primer pairs at random. A total of 46 polymorphic microsatellite loci wereamplified successfully, and the others were monomorphic or produced multi bands. The number of alleles ranged from 3 to 22 (Table 1), with the average of 12.The observed heterozygosity and expected heterozygosity varied from 0.133 to 1.000 and 0.445 to 0.949, respectively. Theof each locus had values between 0.399 and 0.929 with a mean of 0.780. Of the 46 polymorphic loci, 20 de- viated significantly from HWE after a Bonferroni correction.

Fig.1 Percentage of different microsatellite repeats in C. nippona.

Table 1 Characteristics of 46 microsatellite markers developed for Iwagaki oyster Crassostrea nippona

LociRepeat motifPrimer sequence (5’–3’)Ta(℃)Size range (bp)NaHoHePICPHWEGenBank accession number Cn53(TTA)8F: TTGCTGCAATTTGTTTGACG R: ATGCACAAAGGCTTTCATCC64291–315130.9000.9110.8870.0263 MK078328 Cn57(AG)17F: CAGGTGCTGCTTATTATGGAGA R: GCTTCCAGATGCTGCAAACT64199–231150.7670.9250.9030.0000*MK078329 Cn61(AG)26F: CAGTTTGGGGGAGAAATTCA R: CAGAAACACGAAAGTTCCGA62232–290160.5000.9100.8860.0000*MK078330 Cn65(TC)18F: ATGGGCGACAATAAAACAGG R: TATTGCAACAGCCGTGAGTC60269–321200.6670.9200.8980.0000*MK078331 Cn66(CT)26F: ACCAAGACTCGGTTTTGTGC R: GAAACCGATTCGTTAGCACC58247–291120.7670.8710.8400.0000*MK078332 Cn68(GA)25F: TTATCGGGAAAATGCTTTCG R: GTCTGTGCGTCTGGAGATCA62207–247130.8000.9170.8930.0000*MK078333 Cn72(AT)8F: TCAACTGGTCATTGCTGGTG R: TGCCTGCATGCGTATGTTAT62208–24060.5000.5240.4780.4734 MK078334 Cn77(GTCC)4F: ATTTGACGGCAGCAGAAATC R: CCAAGAGTTCAGTTTGCTGG56217–23750.3000.6930.6440.0000*MK078335 Cn79(AG)15F: CACGTGGAAGTGTGGAAGTG R: AATGGCGGCCTATTGAAAC62274–310120.7000.8780.8500.0004*MK078336 Cn88(CT)7F: TTGGACAACAACCTGGGAAT R: ATGTAACATGGCGGGAAAAG66189–19961.0000.7650.7160.0016 MK078337 Cn90(AG)13F: GCAAGTGAGCATGATTGTCC R: GGGCAGGTTTTTCTAATTTCG64247–289160.5670.9080.8850.0000*MK078338 Cn94(AG)28F: TCATTGGATTCCTTGAACACC R: AAACCTCCGGACGAAGAAGT64163–203150.6670.9240.9010.0000*MK078339 Cn96(AAT)5F: CATCGCTACATACCCCCTGT R: AACAGACACAGGGAAACGCT66251–28430.1330.5250.3990.0000*MK078340 Cn98(AG)13F: TGGATGACGAGCTAATGCAG R: TTCAGCTTGTGCCTCTGTTG66278–312210.9330.9490.9290.0000*MK078341 Cn101(TAT)7F: ATCGCAATGTATCTGCGTGA R: AAAAATGACCGTTTTGCCTG64268–27760.3330.5080.4690.0048 MK078342 Cn102(TGA)9F: ACTGGCATTCGTCGATAACC R: GTCTGCCATCTGCATCGTAA66224–23670.7000.7930.7470.0815 MK078343 Cn104(TGTC)6F: GTCACATGTTGTCCGTCGTC R: TTCAAGGATGCTGTGTTCCA66140–15860.7000.7640.7120.0594 MK078344 Cn105(AG)11F: AAGTGGCGGATCATATTGCT R: TAGTGCTTGACGCCATCTTG64137–17180.6000.7460.7010.0080 MK078345 Cn107(TTC)7F: TCACATTGCCTGTGGTGATT R: ATGACCTTGAAAGACGACCC60202–256121.0000.8820.8530.0000*MK078346 Cn108(AC)13F: AATTCAATGTTCGTCGGGAG R: GCTATTGAAGTTTTCCCGCA64250–28290.5000.5930.5550.0243 MK078347 Cn112(AT)8F: CCTCCGCTTCTTAGACGTTG R: CTGGAGGGAATGTGTGTCCT64242–25860.5000.7880.7380.0000*MK078348 Cn113(AG)7F: TGGAGGCATTTCTTGAGTCC R: GAGAGGCATTCGCAAAAGAG66176–232160.9330.8990.8740.5445 MK078349 Cn116(AACAT)4F: CGGCACTAAGAAACAAAGCA R: GTTTTCAATGCACATCACGG66225–23530.5670.6080.5170.1420 MK078350 Cn118(AG)21F: CGGATCAAATCCTTCAAAGC R: AATTGCAGAAGTTCCCGATG66241–265120.6670.8950.8690.0027 MK078351 Cn133(CAT)8F: TCACAACAAAAGGGTGCGTA R: AAAGCCTTGAAAAGTTCCCA64124–13350.9000.7380.6770.0298 MK078352 Cn145(CT)13F: TTCCATTGTGCATTCTGCAT R: CTGACGTTTCCCCTTCACTC60200–262150.9000.9020.8770.5315 MK078353 Cn150(CT)20F: TTGTCTCAGCATTCCATTCA R: TGGCCCGTAAATGAACTCTC60231–275170.7330.8880.8620.2722 MK078354 Cn151(TCT)10F: ATCGATGTGTGGATCCTTCC R: GGCGTCGTAAACATGGATTT63270–28450.5670.4550.4080.6479 MK078355 Cn160(CT)6F: AATGTCGCAAATTCCAAAGG R: GGCTCAGGAACAATGTGGAT63157–17360.6330.5320.4760.6221 MK078356
Notes: Ta, annealing temperature; Na, number of alleles;, observed heterozygosity;, expected heterozygosity;, polymorphic information content. * significantly deviated from Hardy-Weinberg equilibrium after sequential Bonferroni’s correction (<0.05/46).
3.2 Multiplex PCR and Parentage Assignments
Fifteen microsatellites were selected and allocated into five optimized multiplex PCR sets that work well for, each of which contains three microsatellite mark- ers (Table 2). Capillary electrophoresis showed that alleles in each multiplex set could be clearly identified(Fig.2). The polymorphism information and non-exclusion probability of each microsatellite locus were shown in Table 3. Overall, the polymorphism level of the microsatellite loci was moderate, with a mean of 5.7 alleles per locus and a meanof 0.575. Theandvaried from 0.169 to 0.814 and 0.236 to 0.804, respectively.
The simulation results using CERVUS 3.0 showed that with these five multiplexes, the total assignment success rate of 97% could be achieved even if the number of candidate parents increased to 300 (Fig.3). In actual parentage assignments, the parentage analysis performed with a cultured population (173 parents and 486 offspring) de- monstrated that 80% of all offspring were correctly assigned to their parental pair using three multiplex PCRs with the highest polymorphic information, and 96% of all offspring were allocated to their most likely parents based on all five sets (Fig.4), which was similar to the simulation results. Among 486 offspring, 21 individuals (4%) failed to be assigned to their parents because more than 20% of the loci were not amplified. The most likely reason was that the DNA quanlity was poor.

Table 2 Reaction conditions of five multiplex PCRs
Note: Ta, annealing temperature.

Fig.2 Capillary electrophoresis of five multiplex sets. Horizontal axis shows the size range of each locus in base pairs (bp).

Table 3 Characteristics of the five multiplex PCRs in Crassostrea nippona
Notes: Na, number of alleles;, observed heterozygosity;, expected heterozygosity;, polymorphic information content; NE-1P, average non-exclusion probability for one candidate parent; NE-2P, average non-exclusion probability for one candidate parent given the genotype of a known parent of the opposite sex; NE-PP, average non-exclusion probability for a candidate parent pair;(Null), frequency of null allele.
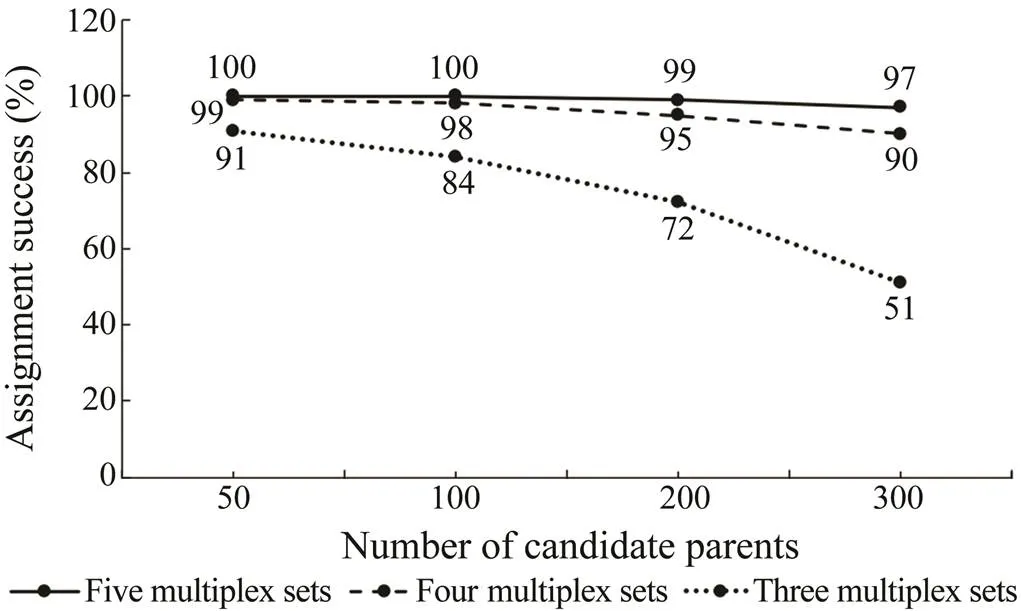
Fig.3 Assignment success rate of simulated genotype data at the 95% confidence level. Each multiplex was added in decreasing order of average polymorphic information con- tent (PIC).

Fig.4 Cumulative assignment success rates of simulated and real genotype data at the 95% confidence level. Each multiplex was added in decreasing order of average polymorphic information content (PIC).
3.3 Effective Breeding Number (Ne) Estimation
The results of sibship reconstruction showed that the inferred number of male and female parents were 70 and 63, respectively. We reconstructed full-sib families with COLONY 2.0, indicating that the number of full-sib fa- milies were 122 and the number of offspring per family were between 1 to 20. The effective population size (N) was estimated as 80.
4 Discussion
Microsatellites are one of the most widely used DNA markers in aquaculture genetics (Liu and Cordes, 2004). The traditional methods of developing genomic microsatellite markers, such as the hybrid capture method (Os- trander., 1992) and the use of published data (Li., 2009), are either time-consuming or highly dependent on available resources. With the rapid development on next- generation sequencing technologies, the identification and characterization of large numbers of microsatellites in non-model species have become easier and faster. Next- generation sequencing technique provides a more convenient and economical method for obtaining genetic markers. In this study, a set of 46 polymorphic microsatellite markers were developed and characterized based on RAD-seq. According to the criterion defined by Botstein. (1980), there are 41 loci with high information content (>0.5), and the remaining 5 loci have moderate information content (0.5<<0.25). Among 46 loci developed here, 20 loci were significantly deviated from HWE after sequential Bonferroni correction. Many factors can cause the problem, including lack of random mating, sampling effect, and null alleles (Sekino., 2003).
In order to facilitate large-scale population research, five multiplex PCRs were developed and optimized in the study based on a three-primer-PCR approach, involving a fluorescently labelled universal primer, a specific forward primer with 5’universal primer sequence tails,and a normal specific reverse primer (Blacket., 2012). Such method not only obtains a level of multiplexing and PCR efficiency similar to microsatellite fluorescent detection assays using direct labeling primers, but also dramatically reduces the cost of the experiment (Blacket., 2012).Multiple PCRs developed in this study show relatively high efficiency of parentage assignment. Pedigree analysis of real offspring showed that 80% of the offspring were explicitly assigned to their parents when using three multiplex PCRs. This result is slightly different from previous studies on marine bivalves (Li., 2010; Nie., 2012), which found that fewer multiplexes were needed to achieve the same assignment success rate. There are many reasons for this difference, such as the slightly lower, the more potential parents and offspring, and unknownparents genders.
The sibship reconstruction revealed that not all candidate parents were involved in the reproduction and the parents participating in reproduction had unequal reproductive contribution. The difference of parental reproductive contribution is the result of various factors, including fertility rate and survival rate of offspring. In this study, the effective size of a populationNwas smaller than the actual number of individuals. Boudry. (2002) believed that this phenomenon was caused by many factors such as overlapping generations, non-random mating and unbalanced sex ratio. The effective population size is a very important parameter for breeding populations, and its reduction will result in increased inbreeding risk and decrease of genetic diversity of offspring. Thus, in order to reduce the risks of inbreeding and avoid genetic degeneration of hatchery-produced seed, the effective po- pulation size per generation in a hatchery population should be increased by increasing the number of parents and e- qualizing the sex ratio.
In summary, for the first time, we developed and characterizedthenovel polymorphic microsatellites for. Additionally, five multiplex PCR assays were developed and validated from these new microsatellites using universal primers labeled with fluorescent dyes. As the aquaculture ofis still in its infancy, this study will help to formulate effective management strategies for artificial seeding and breeding. Moreover, the five sets of multiplex PCRs described here can not only be applied to parentage assignment, but also provide an important tool for marker-assisted breeding and population genetic analysis in.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 31772843), the Natural Sci- ence Foundation of Guangxi Province (No. AA17204080-4), and the Fundamental Research Funds for the Central Uni- versities (No. 201762014).
Blacket, M. J., Robin, C., Good, R. T., Lee, S. F., and Miller, A. D., 2012. Universal primers for fluorescent labelling of PCR fragments–An efficient and cost-effective approach to genotyping by fluorescence., 12: 456- 463.
Botstein, D., White, R. L., Skolnick, M., and Davis, R. W., 1980. Construction of a genetic linkage map in man using restriction fragment length polymorphisms., 32: 314-331.
Boudry, P., Collet, B., Cornette, F., Hervouet, V., and Bonhom- me, F., 2002. High variance in reproductive success of the Pacific oyster (, Thunberg) revealed by microsatellite-based parentage analysis of multifactorial crosses., 204: 283-296.
Itoh, N., Tun, K. L., Komiyama, H., Ueki, N., and Ogawa, K., 2004. An ovarian infection in the Iwagaki oyster,, with the protozoan parasite., 27: 311-314.
Jones, O. R., and Wang, J., 2010. COLONY: A program for par- entage and sibship inference from multilocus genotype data., 10: 551-555.
Kalinowski, S. T., Taper, M. L., and Marshall, T. C., 2007. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment., 16: 1099-1106.
Li, N., Li, Q., Kong, L. F., and Yu, H., 2016. Development of three multiplex PCR primer sets for ark shell () and their validation in parentage assignment., 15: 311-317.
Li, Q., Liu, S. K., and Kong, L. F., 2009. Microsatellites within genes and ESTs of the Pacific oysterand their transferability in five otherspecies., 12: 15-16.
Li, Q., Park, C., and Kijima, A., 2003. Allelic transmission of microsatellites and application to kinship analysis in newly hatched Pacific abalone larvae., 69: 883- 889.
Li, Q., Yu, H., and Yu, R. H., 2006. Genetic variability assessed by microsatellites in cultured populations of the Pacific oyster () in China., 259: 95-102.
Li, R. H., Li, Q., Cornette, F., Dégremont, L., and Lapègue, S., 2010. Development of four EST-SSR multiplex PCRs in the Pacific oyster () and their validation in pa- rentage assignment., 310: 234-239.
Liu, T., Li, Q., Song, J. L., and Yu, H., 2017. Development of genomic microsatellite multiplex PCR using dye-labeled uni- versal primer and its validation in pedigree analysis of Pacific oyster ()., 16: 151-160.
Liu, Z. J., and Cordes, J. F., 2004. DNA marker technologies and their applications in aquaculture genetics., 238: 1-37.
Li, W. J., 2007. Biology and cultivation of oyster., 26: 689-690 (in Chinese with Eng- lish abstract).
Marshall, T. C., Slate, J., Kruuk, L. E. B., and Pemberton, J. M., 1998. Statistical confidence for likelihood-based paternity inference in natural populations., 7: 639-655.
Nie, H. T., Li, Q., and Kong, L. F., 2012. Development of four multiplex PCRs in the Zhikong scallop () and their validation in parentage assignment., 44: 96-101.
Ostrander, E. A., Jong, P. M., Rine, J., and Duyk, G., 1992. Construction of small-insert genomic DNA libraries highly enriched for microsatellite repeat sequences., 89: 3419-3423.
Porta, J., Porta, J. M., Martínez-Rodríguez, G., and del Carmen Alvarez, M., 2006. Development of a microsatellite multiplex PCR for Senegalese sole () and its application to broodstock management., 256: 159-166.
Rousset, F., 2008. GENEPOP’007: S complete re-implementa- tion of the GENEPOP software for Windows and Linux., 8: 103-106.
Sekino, M., Hamaguchi, M., Aranishi, F., and Okoshi, K., 2003. Development of novel microsatellite DNA markers from the Pacific oyster., 5: 227- 233.
Untergasser, A., Cutcutache, I., Koressaar, T., Ye, J., Faircloth, B. C., Remm, M., and Rozen, S. G., 2012. Primer3–New capabi-
lities and interfaces., 40: e115-e115.
Wang, J., 2004. Sibship reconstruction from genetic data with typing errors., 166: 1963-1979.
Yoon, H. S., Jung, H. T., and Choi, S. D., 2008. Suminoe oyster () culture in Korea., 27: 505-508.
Yuan, M. Y., Mao, L. J., and Zhou, W., 2008. Chromosome karyotypic analysis of oyster., 23: 318-320 (in Chinese with English abstract).
April 24, 2019;
June 10, 2019;
September 12, 2019
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2020
E-mail: qili66@ouc.edu.cn
E-mail: 310687878@qq.com
(Edited by Qiu Yantao)
 Journal of Ocean University of China2020年1期
Journal of Ocean University of China2020年1期
- Journal of Ocean University of China的其它文章
- Circulation and Heat Flux along the Western Boundary of the North Pacific
- System Reliability Analysis of an Offshore Jacket Platform
- The Mineral Composition and Sources of the Fine-Grained Sediments from the 49.6˚E Hydrothermal Field at the SWIR
- Research Progress of Seafloor Pockmarks in Spatio-Temporal Distribution and Classification
- Application of the Static Headland-Bay Beach Concept to a Sandy Beach: A New Elliptical Model
- Climatology of Wind-Seas and Swells in the China Seas from Wave Hindcast
