Characteristics of a Novel Tyrosinase Gene Involved in the Formation of Shell Color in Hard Clam Meretrix meretrix
YAO Hanhan, CUI Baoyue, LI Xiaoying, LIN Zhihua, and DONG Yinghui, *
Characteristics of a Novel Tyrosinase Gene Involved in the Formation of Shell Color in Hard Clam
YAO Hanhan1), CUI Baoyue1), LI Xiaoying2), LIN Zhihua1), and DONG Yinghui1), *
1),,,315100,2),,222000,
Hard clam () has a rich shell color variation among individuals from light yellow to bluish gray, brown red, and black, which may associating with melanin. Tyrosinase (Tyr) is a key rate-limiting enzyme for the biosynthesis of melanin,which affects the dark color of animal skin, hair, fur, scales and feathers. Here, we isolated and characterized the full-length cDNA sequence oftyrosinase gene () which encodes a protein with 689 amino acids. Sequence characteristics and phylogenetic analysis showed that there are a variety ofgenes in mollusks, which can be divided into different categories. The abundance oftranscript was detected in six tissues including mantle, adductor muscle, digestive gland, foot, gill and siphon of adultwith qRT-PCR.The resultsshowed a higher expression specifically in the mantle, digestive gland and siphon, which was consistent with the phenotypic color difference. In the embryos and larvae at different developmental stages, high expression was found in the trochophore larvae and juvenile clams. Among threestrains with different shell colors, the expression level ofand total melanin content in the mantle edge both showed significant differences, suggesting thatis involved in the formation of shell color. The results obtained in this study will improve our understanding of molluscan tyrosinase gene function and molecular mechanism of shell color pattern determination.
; shell color; tyrosinase; melanin
1 Introduction
Tyrosinase (TYR, EC 1.14.18.1) is one of the type-3 cop- per-containing enzymes found in most bacteria, fungi, plants and animals (Yu., 2014). Tyrosinase is known to be a key rate-limiting enzyme in melanogenic pathway, which catalyzes both the initial hydroxylation of monophenols and the oxidation of ο-diphenols to ο-quinones in melanin biosynthesis through a series of biochemical re- actions (Giebel., 1991). Tyrosinase family genes in invertebrates are involved in diverse biological processes such as pigment formation (Sugumaran, 2002), innate immunity (Soderhall and Cerenius, 1998; Kanost., 2004), wound healing (Anderson, 2010) and sclerotization of cuticles (Marmaras., 1996). In molluscan bivalves, a few of tyrosinase genes have been isolated and further functionally analyzed recently. For example, in the pearl oyster (), four tyrosinase-like genes including,,and, have been reported to associate with the formation of shell matrix, melanin synthesis and pigmentation of prismatic layer (Zhang., 2006; Nagai., 2007; Takgi and Miyashita, 2014); in Pacific oyster (), a large number of tyro-sinase isoforms have been identified, suggesting that they may be involved in immunity and early larval shell biogenesis (Huan., 2013; Yu., 2014; Zhang., 2016); in the scallop () and Manila clam (), the tyrosinase activity is detected to associate with antibacterial function (., 2005; Zhou., 2012); in Yesso scallop (), tyrosine-relating proteins function in both bio-mineralization and melanogenesis, which may be involvedin shell formation and pigmentation (Sun., 2015); and in the freshwater mussel (), the tyrosi- nase activity of mantle in purple mussels is higher than that in white mussels, indicating thatand-1 maycontribute to the formation of nacre color (Chen., 2017).
Hard clam (), a benthic marine bivalve, naturally habitats western Pacific coasts of China, Japan, Korea, Vietnam and India (Wang., 1993). Be- cause of its delicious taste and great commercial value, the clam has become a popular seafood and one of most commercially important maricultured shellfish in China. Highly polymorphic shell coloration inwas known long ago. In shellfish market, shell color usually affects consumer preference thus is an important factor to evaluate its quality and value (Nell, 2001; Brake., 2004). High quality individuals with bright black or red Shell color are favored by vast number of Chinese consumers. The rich shell color variation amongindividuals indicates the presence of intensive genetic variability, which have received wide attentions due to its association with its growth and nutritive components (Gu., 2014). The selection for shell color inhas already been documented, and shell color-relating mo- lecular markers have also been screened and analyzed (Zhu., 2011). Recently, a comparative transcriptome analysis among four different color morphs ofrevealed that Notch signaling pathway plays a crucial role in shell pigmentation and may be involved in shell color patterning (Yue., 2015). However, the knowledge of major controlling genes and detail molecular me- chanisms underlying shell color formation is extremely li- mited.
To investigate the shell color-relating genes and mole- cular mechanisms, we used threestrains with different shell colors and selected for five generations as experimental materials, characterized the full-length cDNAof tyrosinase encoding gene of(), and profiled its expression during embryonic/larval development and in adult tissues. We also analyzed the difference of gene expression and total melanin content in the mantle edge of these three strains. The results will deepen our understanding of molluscan tyrosinase gene function and provide a basis for further studies on the molecular me- chanism of shell color pattern determination.
2 Materials and Methods
2.1 Tissue Collection and Total RNA Extraction
For gene cloning and expressing, fresh and healthy adultindividuals with black spot shell color were obtained from Yinzhou Danyan Nursery Field in Ningbo, China. All clams were maintained in the aerated seawater at 20℃ for 3 days before use. A variety of tissues including mantle, adductor muscle, digestive gland, foot, gill and siphon were dissected from four adult black spot clams, immediately frozen in liquid nitrogen and then preserved at −80℃. Embryos/larvae of three parallel samples were collected at seven developmental stages including fertilized egg, 4-cell embryo, blastula, gastrulae, trocho- phore larvae, eyespots larvae and juvenile clam. To detect the difference ofexpression, we collected the mantle edge of three shell color (white shell: WS, black spot: BS, red shell: RS) strains from four individuals, which were developed by successive population selection (Fig.1).

Fig.1 Differences of shell color and mantle edge pigmentation among three Meretrix meretrix strains. A1, B1 and C1 are WS, BS and RS strains, respectively. A2, B2 and C2 are the mantle edge of WS, BS and RS clams, respectively. Arrows indicate the pigment band on the mantle edge of BS and RS clams.
Total RNA was extracted from these tissues and embryos/larvae using Trizol reagent (Invitrogen, USA). RNA integrity was examined by electrophoresis on a formaldehyde-denatured 1.2% agarose gel staining with ethidium bromide, and the quality and quantity were assessed by measuring the OD260and OD280with the method of ultraviolet spectrophotometry on NanoDrop ND-1000 (Thermo Scientific).
2.2 Isolation of the Full-Length cDNA of MmTyr
On the basis of 454 cDNA library of(Gen- Bank accession no. SRA021052), EST sequences ofwere retrieved. Primers for 5’-rapid amplification of cDNA end (RACE) (MmTyr-F1) and 3’-RACE MmTyr-R1 were designed (Table 1).
The full-length cDNA ofwas amplified under the following condition: 5 cycles of 94℃ for 30s, 72℃for 3min; 5 cycles of 94℃ for 30s, 70℃ for 30s and 72℃ for 3min; 25 cycles of 94℃ for 30s, 68℃ for 30s and a final extension at 72℃ for 3min. The PCR product was purified using Gel Extraction Kit (TIANGEN, Beijing, China). The purified PCR product was ligated into pMD18-T vector (TaKaRa, Dalian, China), and transformed into DH5α cells (TIANGEN, Beijing, China) following manu-facturer’s protocol with the positive clones sequenced. The full-length cDNA sequence was determined by joining together the sequences of the 3’ and 5’RACE products.
To confirm the accuracy of cloning and sequencing, the full-length cDNA was re-amplified with high fidelity polymerase (TaKaRa, Dalian, China) using primers MmTyr- F2 and MmTyr-R2 (Table 1), which were designed according to the cDNA sequence obtained. PCR product was cloned and sequenced following the procedure described above.
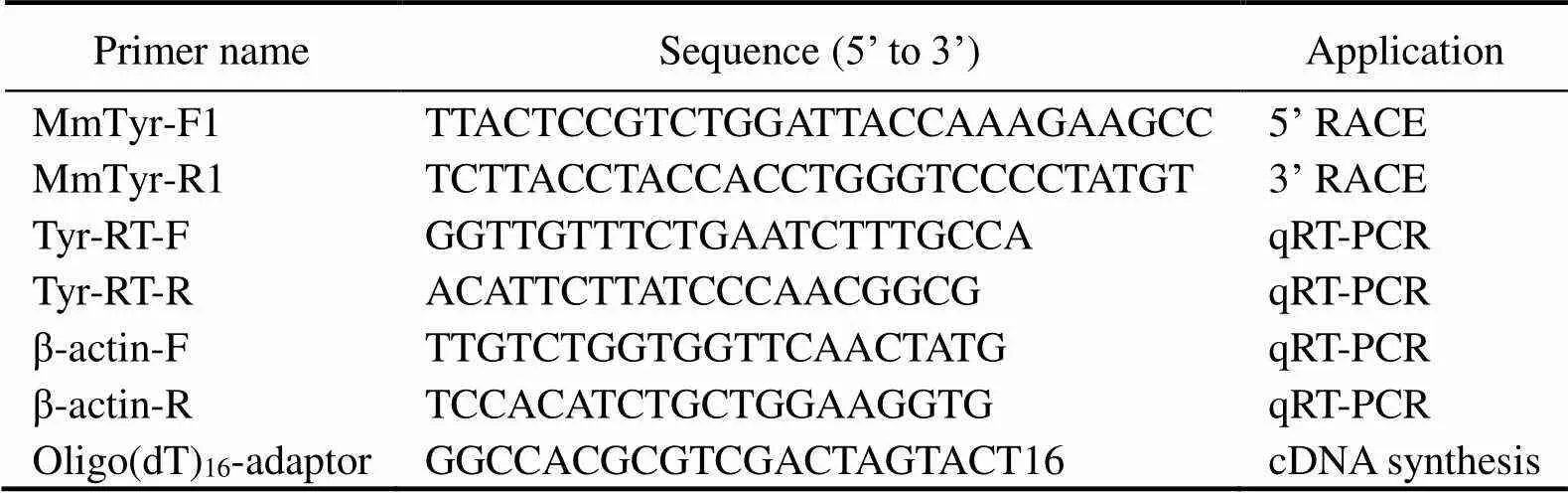
Table 1 Primers and their sequences
2.3 Sequence and Phylogenetic Analyses
The cDNA sequence were assembled and trimmed using BLAST algorithm (http://www.ncbi.nlm.nih.gov/blast/).The nucleotide and deduced amino acid sequences ofgene were analyzed to find the similarity with known ones. The deduced amino acid sequence was analyzed using the Simple Modular ArchitectureResearch Tool (SMART) (http://smart.embl-heidelberg.de) to predict protein domains. The presence and location of the signal peptide and the cleavage sites in the amino acid sequence were predicted with SignalP 4.0 server (http:// www.cbs.dtu.dk/services/SignalP/). The multiple sequence alignment of the sequences ofand other species was performed using the ClustalW2 multiple alignment program (http://www.ebi.ac.uk/Tools/msa/clustalw2/). An unrooted phylogenetic tree was constructed based on the protein sequence alignments with the neighbor-join- ing (NJ) method implemented in MEGA 5.0 software (http: //www.negasoftware.net/). The reliability of the branching was assessed using the bootstrap resampling (1000 pseudo- replicates).
2.4 Quantitative Real-Time PCR (qRT-PCR) of MmTyr Transcripts
The expression oftranscript in the tissues of adults (4 sets of sample each tissue) and at early developmental stages (>500, 3 sets of sample each stage) were analyzed using quantitative real-time PCR (qRT-PCR) with three technical repeats each. Total RNA was extracted using the same method as was described above. The primers Tyr-RT-F andTyr-RT-R (Table 1) were used to amplify a 149bp fragment offrom cDNA template. The abundance of actin encoding gene transcript was amplified with primers β-actin-F and β-actin-R (Table 1) and used as the internal control.
All qRT-PCR were carried out in a total volume of 20μL containing 10μL of iTaq Universal SYBR Green Supermix (Bio-Rad), 7.2μL deionized water, 0.8μL of first strand cDNA and 1μL of forward and reverse primers each, and run on a 7500 Real-time System (Applied Biosystems, CA, USA). The amplification was performed using the following thermal cycling condition: initial denaturation at 94℃ for 20s, which was followed by 40 cycles of denaturing at 94℃ for 3s, annealing at 60℃ for 15s and extending at 72℃ for 10s. To compare theexpression levels in the mantle edge of clams with different shell colors, four individuals each shell color strain were analyzed.
2.5 Analysis of the Melanin Content in Mantle Edge
To investigate the relationship between total melanin content (TMC) in mantle and shell color, TMC of mantle edge ofwith three shell colors was examined by shellfish melanin ELISA kit (Shanghai Qiaodu, China) according to manufacturer’s instructions. Every analysis was conducted with three replicates. In each analysis, about 0.3g mantle sample was mixed with 500μL phy- siological saline solution. After grinding, the samples were homogenized and centrifuged at 3000rmin−1for 10min. The absorption of the extract was measured by reading the optical density (OD value) at 450nm in a microplate reader (Thermo Fisher Scientific, USA). Calibration curve (2=0.9900) was obtained with the standard melanin. TMC was calculated using the formula=0.025+0.0636 whereis the OD450nmvalue. The results of TCC were expressed in μgg−1tissue on a dry weight basis.
2.6 Statistical Analysis
All data of qRT-PCR were presented as mean±S.E. The results were based on the CT value of PCR product and the comparative CT method was used to analyze the expression level of. Statistical analysis was per- formed using software SPSS 19.0. An one way analysis of variance (ANOVA) was used to compare the difference of relativeexpression level among adult tissues, among different developmental stages and among different shell color strains. Multiple comparisons were conducted using the Student Newman Keuls test. Thevalues less than 0.05 and 0.01 were considered statistically significant and extremely statistically significant, respectively.
3 Results
3.1 Identification of MmTyr cDNA Sequences
The full-length cDNA sequence ofwas 2406bp (GenBank accession no. KP250881), which contained a 2070bp putative open reading frame (ORF) encoding 689 amino acid residues, 42bp of 5’ untranslated region (5’- UTR) and 297bp of 3’-UTR that included a stop codon (TAA), a putative polyadenylation consensus signal (AA TAAA) and a polyA tail (Fig.2A). The predicted molecu- lar weight of the mature MmTyrwas 78.25kDa, and its theoretical isoelectric point was 6.69. A signal peptide of 24 amino acids from the N-terminal region of MmTyr wasidentified using SignalP 4.0 server. Further analysis of the signal peptide revealed that MmTyr belonged to secreted form (subtype α). Structural and functional analysis indi- cated that MmTyr contained highly conserved domain and two highly conserved domains of tyrosinase family of proteins,., copper-binding domain CuA (168–185aa) and CuB (308–319aa) (Fig.2A), which are cogently related to the tyrosinase activities and melanin biosynthesis.
By comparing the encoded amino acids in multiple spe- cies, we found that the number of amino acids of Tyr fami- ly genes in mollusk varied between 445aa inTyrand 857aa ofTyr-3, while Tyr family genes in vertebrate changed within a small extent, rang- ing from 526aa inTyrp-1b to 543aa inTyr (Table 2).
3.2 Domain Structure Comparison and Phylogenetic Analysis
Comparison of Tyr domain structure ofand selected model species indicated that the domain structure of MmTyr was similar to that of other mollusks such asTyr andTyr-1 although there is a wide variation (Fig.2B). Compared to mollusks, the Tyr domain structure of vertebrate (,and) was relatively stable.
To investigate the relationship between MmTyr and other Tyr family members, a neighbor-joining phylogenetic tree was constructed with the conserved exon corresponding regions of Tyr proteins from various species (Fig.2C). Clearly, two distinct groups are shown in the obtained tree. The invertebrate Tyrs and Tyrp including Tyr, Tyr-1, Tyr-2, Tyr-3 and Tyrp-1 are clustered together while the vertebrate and sea squirt Tyr and Tyrp are clus- tered together. In the invertebrate group, Tyrs or Tyrp of mollusk are clustered into these two different groups with low bootstrap proportion supports, suggesting that the kinship between different species is not very close.
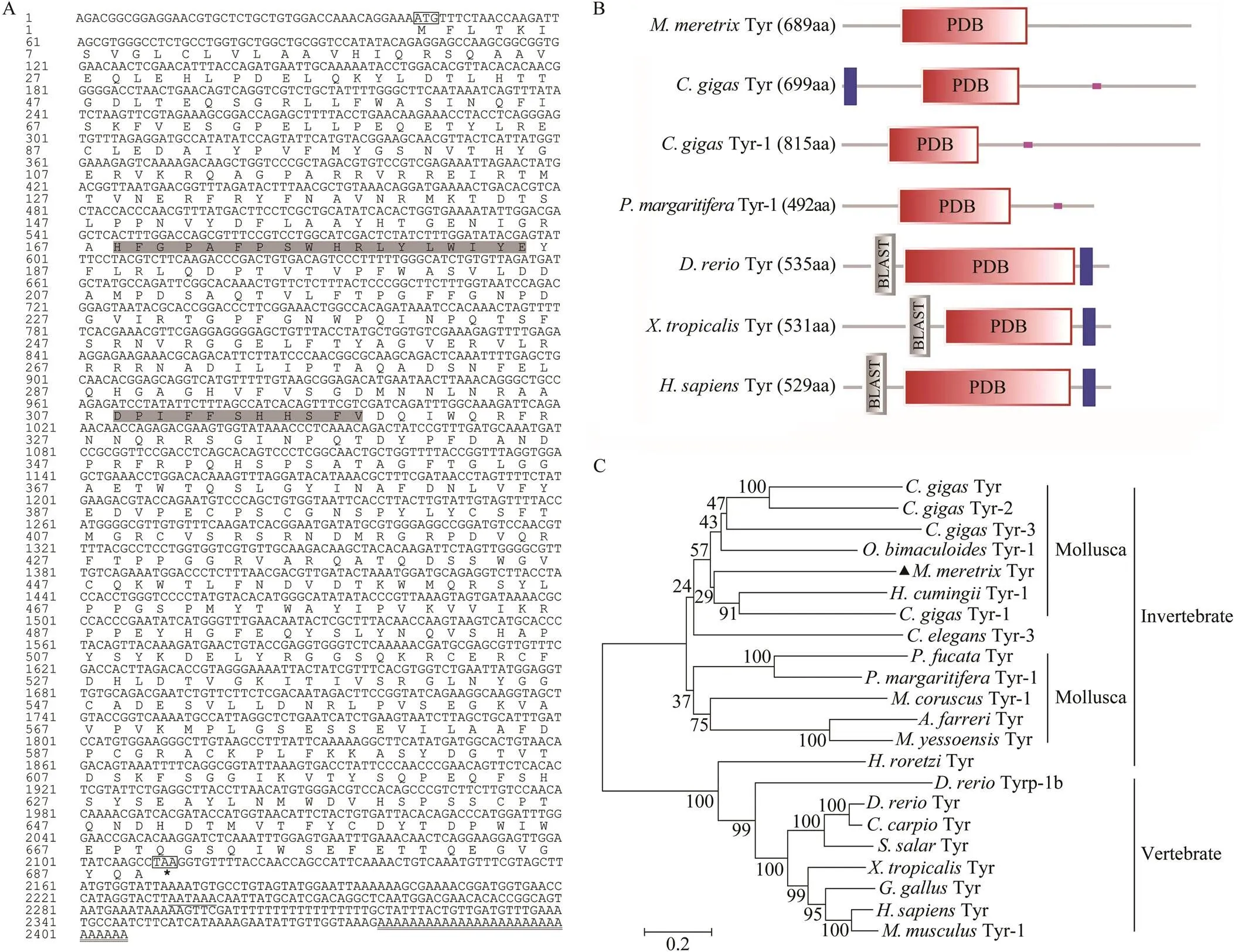
Fig.2 Sequence characteristics and phylogenetic analysis of MmTyr. A. The full-length cDNA sequence of MmTyr and its deduced amino acid sequence. The boxes are the start codon and the stop codon. The asterisk indicates the end of the protein translation, the underlined part is the polyadenylation signal, and the bold underlined part is polyA tail. The two shaded gray parts are tyrosinase copper-binding domain CuA (168–185aa) and CuB (308–319aa). B. Comparison of domain structure of Tyrs of M. meretrix and selected model species. C. Phylogenetic analysis of Tyr proteins from different species with neighbor-joining method implemented in MEGA6.0 software. The abbreviation and the GenBank accession numbers used to construct the phylogenetic tree are listed in Table 2.
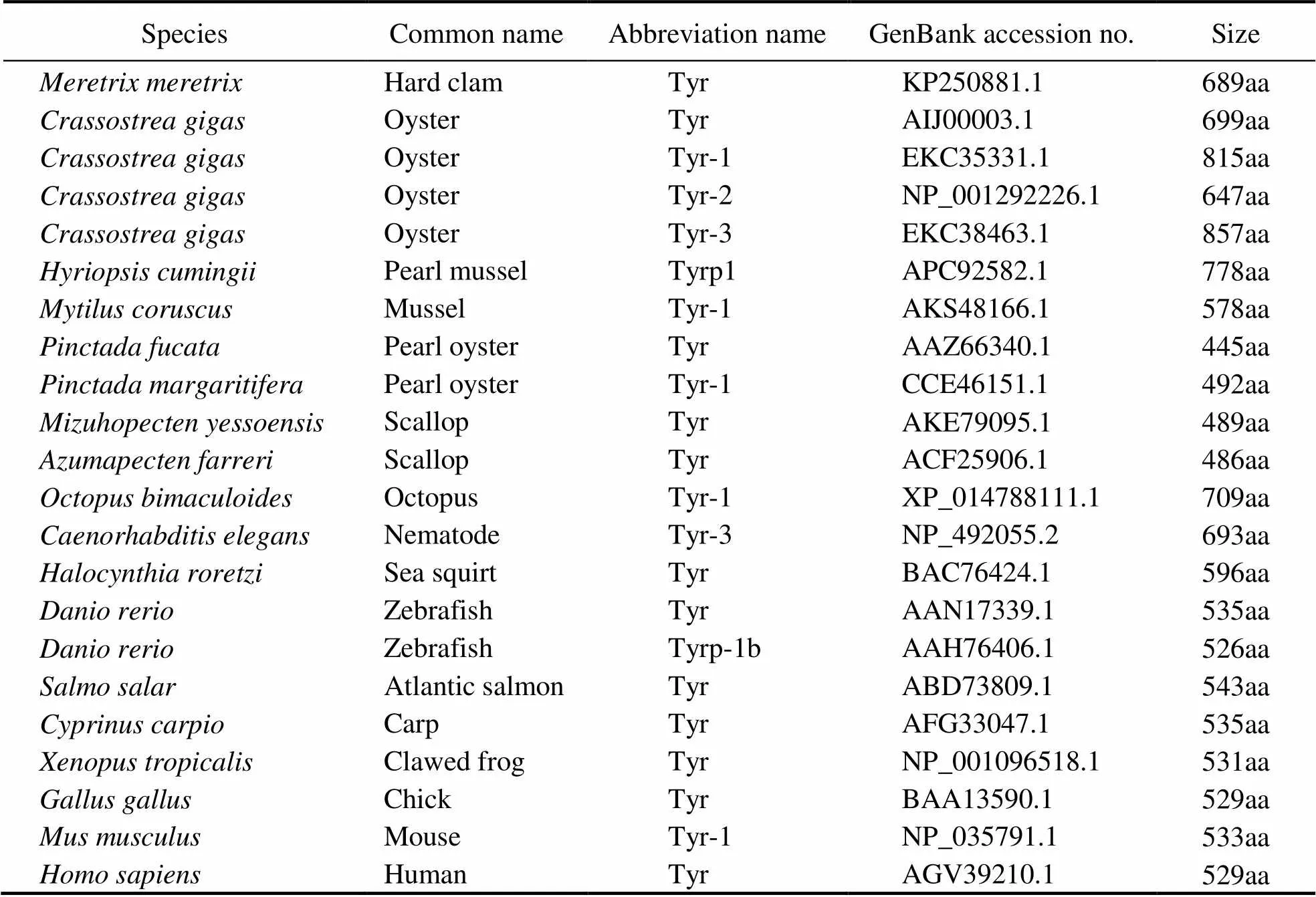
Table 2 Phylogenetic analysis of Tyr amino acid sequences of different species
3.3 Differential Expression of MmTyr Among Embryos, Larvae, and Adult Tissues
Mantle, adductor muscle, digestive gland, foot, gill and siphon from black spot clams were chosen to check theexpression differences. The colors of digestive gland and siphon were black, and there was an obvious pigment line on the mantle edge of black spot strains, while the colors of other tissues were all milk white. Our results indicated thatis differentially expressed in varied tissues. In all six examined tissues, the predominant trans- cription was found in the mantle, digestive gland and siphon, while the very weak expression was observed in other tissues including gill, foot and adductor muscle, which is consistent with the color differences between tis- sues (Fig.3A).
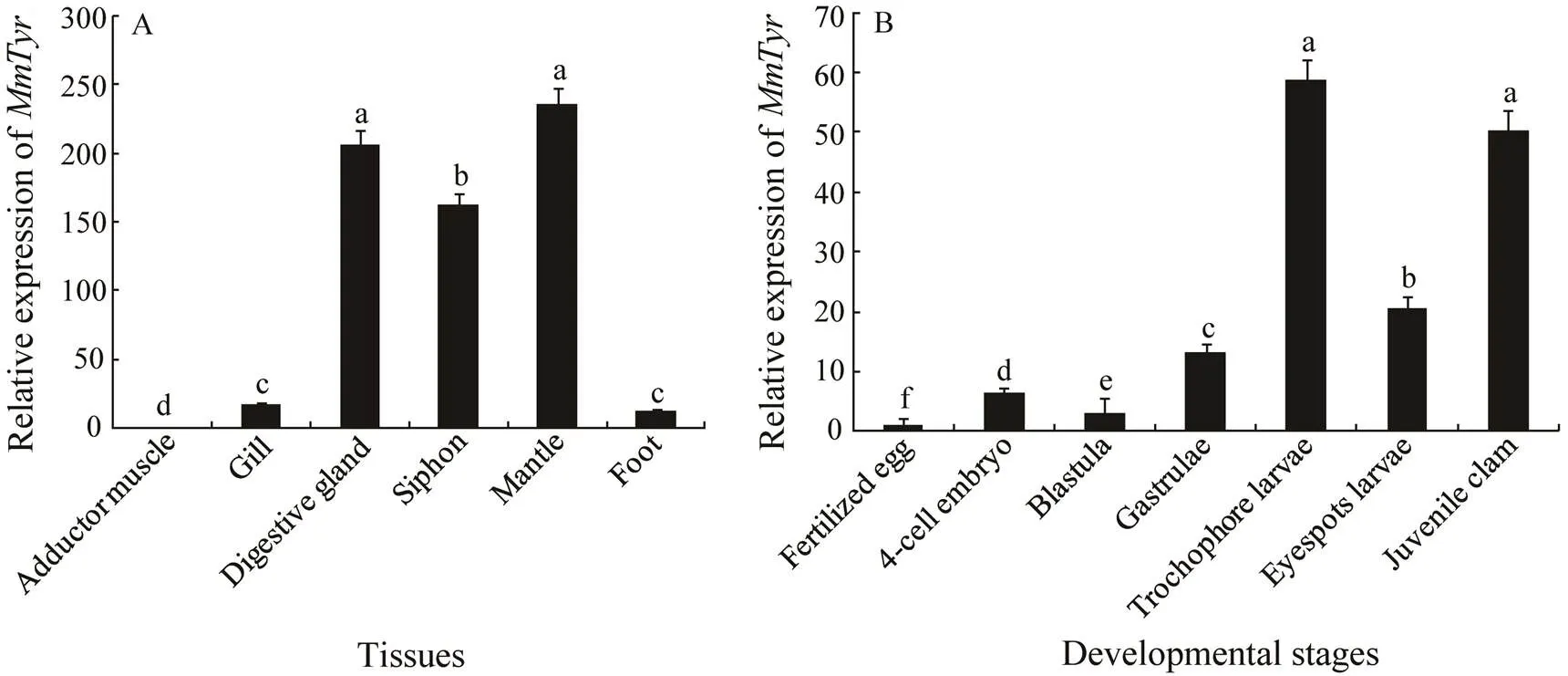
Fig.3 Relative expression of MmTyr gene in four adult tissues (n=4) and seven early developmental stages (n>500). The method of 2−ΔΔct was used to analyze the expression level of MmTyr, and the results are presented as mean±S.E. The data were assessed using ANOVA. The Tukey Honest Significant Difference was performed on each data set, and Superscript letters represent statistically significant differences at P<0.05.
transcript in all embryos and larvae showed that higher expression level is in the trochophore larvae and juvenile clams while lower expression level is in larvae at early developmental stages (Fig.3B).
3.4 Differential Analysis of MmTyr Expression and Total Melanin Content in Mantle Edge of Clams with Three Shell Colors
We compared the expression ofin mantle edge of strains with white shell, black spot shell and red shell, respectively (Fig.4A). A significant difference was detected between the black spot strain and white shell strain or red shell strain (<0.05), and no significant difference was found between the red shell strain and white shell strain. From the quantity ofexpression, the mantle edge of black spot clams is 8.0-fold of that in white shell clams, and 2.7-fold of that in red shell clams, which implies thatis involved in the formation of shell colors.
Total melanin content in mantle edge of three shell color strains was also tested with melanin ELISA kit. The result showed that the highest content of melanin is in the mantle edge of black spot clams, which was consistent with the detected with qRT-PCR (Fig.4B).

Fig.4 Comparative analysis of expression of MmTyr (n=3) and total melanin content in three shell color strains (n=3). A. The method of 2−ΔΔct was used to analyze the expression level of MmTyr, and the results are presented as mean±S.E. The data were assessed using ANOVA. The Tukey Honest Significant Difference was performed on each data set, and significant differences between WS and BS (or RS) are indicated with an asterisk for P<0.05, and with two asterisks for P<0.01. B. TMC of mantle edge of three shell colors M. meretrix with three repetitions was examined, and multiple comparisons were conducted using the Student Newman Keuls test. Significant differences between WS and BS (or RS) are indicated with an asterisk if P<0.05, and with two asterisks if P<0.01.
4 Discussion
In mollusks, the most common cause of shell color formation is the presence of biological pigments including melanin, porphyrins and polyenes or carotenoids (Williams, 2017). Melanin including black to brown eumelanin and yellow to reddish-brown pheomelanin have been considered a class of essential compounds in the pigments of molluscan shells. However, they are particularly difficult to be isolated and characterized due to their intractable chemical properties, the presence of tightly bound proteins and metal ions, and the lack of effective methods for the identification of their chemical forms (Wakamatsuand Ito, 2002; Wakamatsu., 2003). Some researchers have already found that the secretory cells of the mantle edge from mollusk contribute to the formation of pigmented prismatic shell layer and are involved in melanin production (Comfort, 1951; Jolly., 2004; Nagai., 2007). Recently, the melanin pigment from Yesso scallopis isolated from the brown-pigmented shells using hydrochloric acid method, and the types and ratios are characterized by spectrophotometry (Sun., 2017). In the present results, we also observed there is an obvious pigment line on the mantle edge of red and blackindividuals, likeand(Jolly., 2004; Brake., 2004), but not of whiteindividuals (Fig.1). Extant research has revealed that mantle edge pigmentation and shell pigmentation are positively correlated (<0.0001) in(Brake., 2004). Therefore, it is speculated that the mantle pigments may be directly related to the formation of shell color. Further results on the total melanin content in mantle edge of three shell color strains showed that the pigment content of black spot individuals is significantly higher than that of red shells and white shells, indicating that melanin is one of the most key shell pigments.
The complete molecular pathway for the biosynthesis of melanin in mollusks is still not well understood. Tyrosinase, as a key rate-limiting enzyme, catalyzes the ini- tial and rate-determining step reaction in the melanosome, and plays an important role in the regulation and production of melanin (Korner and Pawelek, 1982). Tyrosinase genes have been identified, which specially express at significantly higher levels in mantle tissue of many bivalve species (Feng., 2015; Lemer., 2015; Sun., 2015), implying that tyrosinases play distinctive roles in melanogenesis in shells or pearl. Previous genetic studies found that the tyrosinase gene family has undergone an expansion and has different physiological functions in mollusks. In, at least four tyrosinase genes (,,,) express in mantle.expresses in both pallial and edge region and seems to be involved in melanin biosynthesis;expresses specially in the pallial region and may form the nacreous layer;is transcribed in the mantle pallial and participated in the synthesis of melanin; andexpresses at the middle fold and is most likely involved in periostracum formation (Zhang., 2006; Nagai., 2007; Takgi and Miyashita, 2014). Of thetyrosinase genes,anddisplay a completely different expression pro-file anddifferentially expresses at the highest level in mantle edges, suggesting thatplays a critical role in the formation of pigmentation (Yu., 2014). Ofthetyrosinase genes,and- 1 mainly express in the mantle. The expression ofis higher in the purple mussels and involved in nacre formation while the expression of-1 is higher in the white mussels and may be involved in periostracum and nacreous layer formation (Chen., 2017). In, our results showed thatdifferentially expresses at the highest level in mantle, similar to that ofin(Zhang., 2006)and2in(Yu., 2014). Somay be involved in the formation of shell and pigmentation. Moreover, the significant high expression ofwas also found in digestive gland and siphon, which are rich in melanin, suggesting thattakes part in the synthesis of melanin pigmentation in several tissues. Furthermore, the results ofexpression in mantle edge of three strains in black spot clams is 8.0-fold of that in white shell clams, and 2.7-fold of that in red shell clams, which implies that it is directly involved in the formation of shell colors in this species.
Although the roles of tyrosinase genes in adult shells have been reported in many molluscan bivalves (Zhang., 2006; Nagai., 2007; Takgi and Miyashita, 2014; Chen., 2017), their roles during early development are rarely investigated. Huan(2013) detected the expres- sion of tyrosinase gene of() during early larval stages by whole mounthybridization and electron scanning microscopic observation and found that it expresses from trochophore to early D-veliger, thus spe- culated thatspecially functions in initial phase of the oyster larval shell biogenesis. In this study, we detected the obvious expression ofin the gastrulae, and observed the highest level of expression in trocho- phore larvae and juvenile clams, which is significantly higher than that at other developmental stages but lower than that in eyespots larvae. These results suggested thatis also involved in the biomineralization and formation of larval shell of this species.
Acknowledgements
This work was supported financially by the National Key Research and Development Program of China (No. 2018YFD0901404), the National Natural Science Foundation of China (No. 31772846), the Zhejiang Provincial Natural Science Foundation (No. LY16C190004), the Ge- neral project of Zhejiang Provincial Educational Department (No. Y201329410), the Open-End Funds of Jiangsu Key Laboratory of Marine Biotechnology (No. HS16001), and the Modern Agro-Industry Technology Research System (No. CARS-49).
Anderson, S. O., 2010. Insect cuticular sclerotization: A review., 40 (3): 166-178.
Brake, J., Evans, F., and Langdon, C., 2004. Evidence for genetic control of pigmentation of shell and mantle edge in selected families of Pacific oyster,., 229: 89-98.
Chen, X. J., Liu, X. J., Bai, Z. Y., Zhao, L. T., and Li, J. L., 2017.and-1 of, novel tyrosinase and tyrosinase-related protein genes involved in nacre color formation.,204: 1-8.
Comfort, A., 1951. The pigmentation of molluscan shells., 26: 285-301.
Cong, R., Sun, W., Liu, G., Fan, T., Meng, X., Yang, L., and Zhu, L., 2005. Purification and characterization of phenoloxidase from clam., 18 (1): 61-70.
Feng, D. D., Li, Q., Yu, H., Zhao, X. L., and Kong, L. F., 2015. Comparative transcriptome analysis of the Pacific oystercharacterized by shell colors: Identification of genetic bases potentially involved in pigmentation.e, 10 (12): e0145257.
Giebel, L. B., Strunk, K. M., and Spritz, R. A., 1991. Organization and nucleotide sequences of the human tyrosinase gene and truncated tyrosinase-related segment., 9 (3): 435- 445.
Gu, X. F., Lin, Z. H., Dong, Y. H., and Yao, H. H., 2014. Analysis and evaluation of general nutritive components of clams () with three kinds of shell colors and decorative patterns.,26 (12): 3850-3857.
Huan, P., Liu, G., Wang, H. X., and Liu, B. Z., 2013. Identification of a tyrosinase gene potentially involved in early larval shell biogenesis of the Pacific oyster., 223 (6): 389-394.
Jolly, C., Berland, S., Milet, C., Borzeix, S., Lopez, E., and Dou- menc, D., 2004. Zona localization of shell matrix protein in mantle(Mollusca, Gastropoda)., 6 (6): 541-551.
Kanost, M. R., Jiang, H., and Yu, X. Q., 2004. Innate immune responses of a lepidopteran insect,., 198 (1): 97-105.
Korner, A., and Pawelek, J., 1982. Mammalian tyrosinase catalyzes three reactions in the biosynthesis of melanin., 217 (4565): 1163-1165.
Lemer, S., Saulnier, D., Gueguen, Y., and Planes, S., 2015. Identification of genes association with shell color in the black- lipped pearl oyster,., 16 (1): 568.
Marmaras, V. J., Charalambidis, N. D., and Zervas, C. G., 1996. Immune response in insects: The role of phenoloxidase in de- fense reactions in relation to melanization and sclerotization., 31 (2): 119- 133.
Nagai, K., Yano, M., Morimoto, K., and Miyamoto, H., 2007. Tyrosinase localization in mollusc shells.,146 (2): 207-214.
Nell, J. A, 2001. The history of oyster farming in Australia., 63 (3): 14-25.
Soderhall, K., and Cerenius, L., 1998. Role of the prophenoloxidase-activating system in invertebrate immunity., 10 (1): 23-28.
Sugumaran, M., 2002. Comparative biochemistry of eumelano- genesis and the protective roles of phenoloxidase and melanin in insects., 15 (1): 2-9.
Sun, X., Yang, A., Wu, B., Zhou, L., and Liu, Z., 2015. Charac- terization of the mantle transcriptome of Yesso scallop (): Identification of genes potentially involved in biomineralization and pigmentation., 10: e0122967.
Sun, X., Wu, B., Zhou, L., Liu, Z., Dong, Y., and Yang, A., 2017. Isolation and characterization of melanin pigment from Yesso scallop., 16 (2): 279-284.
Takgi, R., and Miyashita, T., 2014. A cDNA cloning of a novel alpha-class tyrosinase of: Its expression analysis and characterization of the expressed protein., 2014: 1-9.
Wakamatsu, K., and Ito, S., 2002. Advanced chemical methods in melanin determination., 15 (3): 174-183.
Wakamatsu, K., Fujikawa, K., Zucca, F. A., Zecca, L., and Ito, S., 2003. The structure of neuromelanin as studied by chemical degradative methods., 86 (4): 1015-1023.
Wang, R. C., Wang, Z. P., and Zhang, J. Z., 1993.. Qingdao Ocean University Press, Qingdao, 322-324.
Williams, S. T., 2017. Molluscan shell colour., 92 (2): 1039-1058.
Yu, X., Yu, H., Kong, L. F., Guo, F. G., Zhu, G., and Li, Q., 2014. Molecular cloning and differential expression in tissues of a tyrosinase gene in the Pacific oyster., 41 (8): 5403-5411.
Yue, X., Nie, Q., Xiao, G. Q., and Liu, B. Z., 2015. Transcriptome analysis of shell color-related genes in the clam., 17 (3): 364-374.
Zhang, C., Xie, L. P., Huang, J., Chen, L., and Zhang, R. Q., 2006.A novel putative tyrosinase involved in periostracum formation from the peal oyster ()., 342 (2): 632-639.
Zhang, G., Fang, X., Guo, X., Li, L., Luo, R., Xu, F., Yang, P., Zhang, L., Wang, X., Qi, H., Xiong, Z., Que, H., Xie, Y., Holland, P. W., Paps, J., Zhu, Y., Wu, F., Chen, Y., Wang, J., Peng, C., Meng, J., Yang, L., Liu, J., Wen, B., Zhang, N., Huang, Z., Zhu, Q., Feng, Y., Mount, A., Hedgecock, D., Xu, Z., Liu, Y., Domazet-Lošo, T., Du, Y., Sun, X., Zhang, S., Liu, B., Cheng, P., Jiang, X., Li, J., Fan, D., Wang, W., Fu, W., Wang, T., Wang, B., Zhang, J., Peng, Z., Li, Y., Li, N., Wang, J., Chen, M., He, Y., Tan, F., Song, X., Zheng, Q., Huang, R., Yang, H., Du, X., Chen, L., Yang, M., Gaffney, P. M., Wang, S., Luo, L., She, Z., Ming, Y., Huang, W., Zhang, S., Huang, B., Zhang, Y., Qu, T., Ni, P., Miao, G., Wang, J., Wang, Q., Steinberg, C. E., Wang, H., Li, N., Qian, L., Zhang, G., Li, Y., Yang, H., Liu, X., Wang, J., Yin, Y., and Wang, J., 2012. The oyster genome reveals stress adaptation and complexity of shell formation., 490 (7418): 49-54.
Zhou, Z., Ni, D. J., Wang, M. Q., Wang, L. L., Shi, X. W., Yue, F., Liu, R., and Song, L. S., 2012. The phenoloxidase activity and antibacterial function of a tyrosinase from scallop., 33 (2): 375-381.
Zhu, D. L., Lin, Z. H., Dong, Y. H., and Yao, H. H., 2011. Genetic variation analysis of four strains ofthat have different shell colors and decorative patterns., 42 (3): 374-379.
April 16, 2019;
June 24, 2019;
November 27, 2019
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2020
. E-mail: dongyinghui118@126.com
(Edited by Qiu Yantao)
 Journal of Ocean University of China2020年1期
Journal of Ocean University of China2020年1期
- Journal of Ocean University of China的其它文章
- Circulation and Heat Flux along the Western Boundary of the North Pacific
- System Reliability Analysis of an Offshore Jacket Platform
- The Mineral Composition and Sources of the Fine-Grained Sediments from the 49.6˚E Hydrothermal Field at the SWIR
- Research Progress of Seafloor Pockmarks in Spatio-Temporal Distribution and Classification
- Application of the Static Headland-Bay Beach Concept to a Sandy Beach: A New Elliptical Model
- Climatology of Wind-Seas and Swells in the China Seas from Wave Hindcast
