Two New Species of Free-Living Marine Nematodes of the Desmodoridae from Mangrove Wetlands of Xiamen Bay, China
ZHOU Xiping, ZENG Jiali, CAI Lizhe, FU Sujing, and TAN Wenjuan
Two New Species of Free-Living Marine Nematodes of the Desmodoridae from Mangrove Wetlands of Xiamen Bay, China
ZHOU Xiping1), 2), ZENG Jiali1), 3), CAI Lizhe3), 4), *, FU Sujing3), 4), and TAN Wenjuan3), 4)
1)School of Environmental Science and Engineering, Xiamen University Tan Kah Kee College, Zhangzhou 363105, China 2) Key Laboratory of Estuarine Ecological Security and Environmental Health, Tan Kah Kee College, Zhangzhou 363105, China 3) College of the Environment and Ecology, Xiamen University, Xiamen 361102, China 4) Key Laboratory of the Ministry of Education for Coastal and Wetland Ecosystems, Xiamen University, Xiamen 361102, China
Mangroves are unique in their biodiversity, but studies on their meiobenthic biodiversity in China are scarce. Despite the importance of mangroves, little work has been done on the classification of nematodes in mangrove wetlands. Fujian Province is the most northern point of China’s natural mangrove distribution, and it is also one of the provinces with the earliest constructed mangrove forest. In this paper, two new free-living marine nematode species of Desmodoridae from the Xiamen mangrove wetlands in China are described.sp. nov. is characterized by a cylindrical body, and smooth head capsule set off from the rest of the body. The cuticle is finely annulated and thickened at the midbody. Lateral ridges run from the posterior end of the pharynx to the middle of the tail. The amphid foveae is loop shaped and opens at the top with a double contour amphidial, pharynx with bipartite cuticularized internal cavity. There are 18 tubular precloacal supplements and tail with three small protuberances.sp. nov. is characterized by a relatively short and plump body with finely annulated cuticle, which is particularly obvious in the tail. The head is small and wide with intensive striates. The inner and outer labial sensilla are indistinct with short spicules and ventral apophysis, a gubernaculum with a block-shaped hook, a swollen conical-cylindrical tail and an absence of precloacal supplements.
sp. nov.;sp. nov.; free-living marine nematodes; mangroves
1 Introduction
Mangroves are unique in their biodiversity, but few studies have considered their meiobenthic biodiversity in China (Liu and Huang, 2012). Fujian Province is the northernmost province in China containing natural mangroves and was also one of the earliest provinces to undergo mangrove forest construction in China (Guo., 2014). The nematode diversity in Fujian Province was previously investigated, and new species were found and described from mangrove wetlands in Xiamen Bay (Li and Guo, 2016; Fu, 2018). Only 300 species have been described in detail in China; among them, 90 species are newly found (Shi, 2016). There is still much work to be done on nematode classification, especially in mang- rove wetlands.
We discovered two new species of the generaandin the mangrove forests of Xiamen Bay. The genusincludes six subgenera:Stekhoven, 1951;Filipjev, 1918;Filipjev, 1918;Timm, 1961;Chitwood, 1936; andCobb, 1933.contains 8 valid species.Filipjev, 1918 is characterized by a loop-shaped or round amphidialis, longitudinal lateral ridges (in some individuals), a pharynx bulb internal lining divided into two or three parts, and precloacal supplemental organs (in some individuals) (Gagarin and Tu, 2014). The genusDitlevsen, 1921, was most recently revised by Fonseca. (2006) and its latest species was described by Portnova (2009); Shi and Xu (2017) transferred the two reflexed ovaries species ofto, andnow contains 39 valid species.
Two new species,()sp. nov. andsp. nov., are described in this article.
2 Materials and Methods
2.1 Study Area
Samples of meiofauna were collected from the mangrove wetland of Tong’an Bay (24.65˚-24.70˚N, 118.20˚-118.30˚E) in summer and winter of 2014 in Xiamen Bay, Fujian Province.
2.2 Sampling Method and Sample Processing
Sediment core samples (2.9-cm inner diameter) were obtained, placed in plastic bottles and fixed with 5% for- maldehyde. The samples were washed and filtered through 500- and 42-μm mesh sieves, and the material retained on the 42-μm mesh sieve was collected.
The nematodes were extracted using the flotation technique in Ludox-TM with a specific gravity of 1.15-1.18 (De Jonge and Bouwman, 1977). Nematodes were extrac- ted from the samples under a stereoscopic microscope and transferred to a glycerin solution (McIntyre and Warwick, 1984) consisting of 9:1 (V:V) ethanol/glycerol in a cavity block. Then, the ethanol was gradually evaporated, and the nematode specimens were mounted permanently on slides.
Photographs and body measurements were taken with Nikon-50i microscopy equipment. Drawings were made by using a Wacom CTH-690 digital panel. Holotype and paratype specimens were deposited at the College of the Environment and Ecology, Xiamen University, China.
Measurements are in µm. Abbreviations are as follows: a=body length/maximum body diameter, b=body length/ esophagus length, c=body length/tail length, abd=anal body diameter, c’=tail length/abd, cbd=corresponding body diameter, V%=distance from vulval opening to the anterior end as percentage of total body length, outer labial setae%=outer labial setae length as a percentage of the cbd, and amphid%=amphid diameter as a percentage of the cbd.
3 Results
3.1 Species Description of Metachromadora xiamenensis sp. nov.
Order Desmodorida De Coninck, 1965
Family Desmodoridae Filipjev, 1922
Diagnosis (Modified from Tchesunov, 2014)
Body cylindrical. Cuticle distinctly annulated, without dots, but spines, fringes or longitudinal ornamentations may be present. No specialized ambulatory setae at anterior or posterior body end. Locomotion sinuous, typical for nematodes.
Genus Metachromadora Filipjev, 1918
Spiriniinae. Cuticle of head longitudinally striated. Cephalic setae short or papillae. Amphideal fovea strongly cuticularized. Buccal cavity with distinct dorsal tooth. Posterior pharyngeal bulb well-developed with a thick internal cuticular lining partitioned into two or three sections (Tchesunov, 2014).
Subgenus Metachromadoroides Timm, 1961
Diagnosis (Modified from Gagarin and Tu, 2014)
Cuticle annulated with longitudinal lateral ridges. Inner labial sensilla papilliform, outer labial and cephalic sensilla elongated papillae or short thick setae. Amphidial foveae on cuticular thickening, circular or loop-shaped with double contours. Posterior pharyngeal bulb well developed, with strongly cuticularized internal lining often divided into two or three parts. Precloacal supplemental organs in the shape of short, faintly cuticularized tubules present or absent.
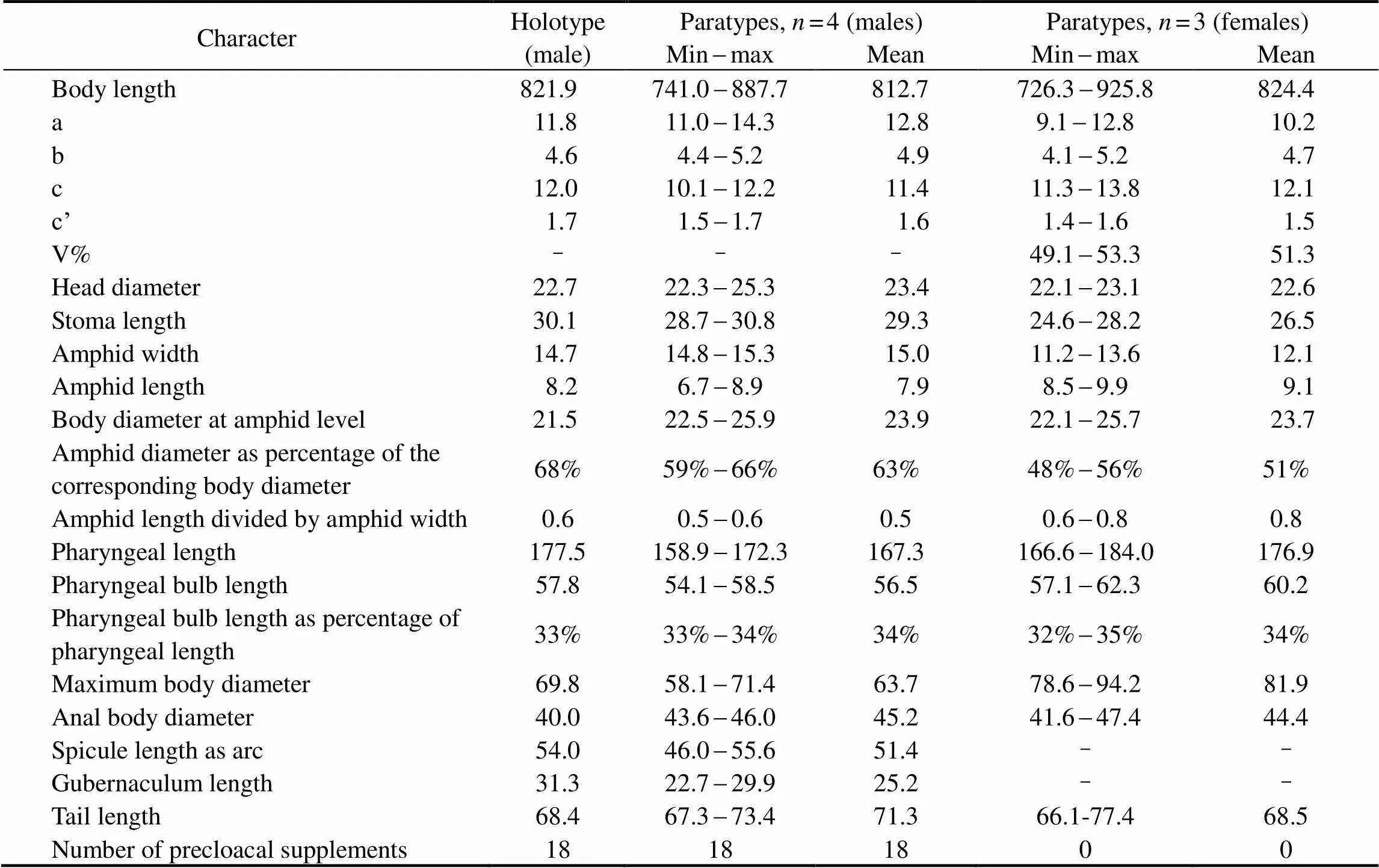
Table 1 Morphometrics of Metachromadoraxiamenensis sp. nov. (in μm)
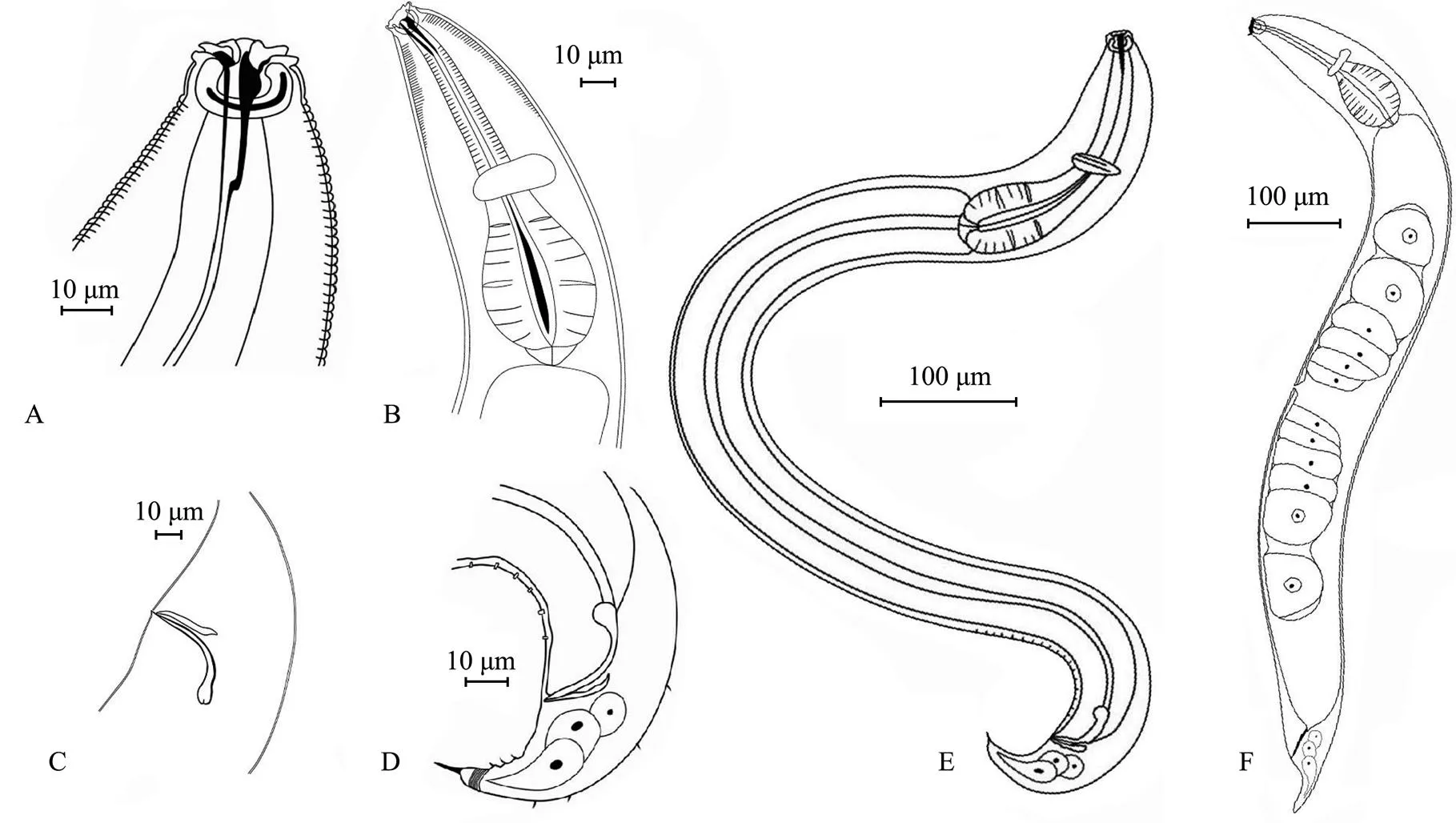
Fig.1 Metachromadoraxiamenensis sp. nov. A, male head; B, lateral view of female anterior end; C, male spicules and gubernaculum; D, male tail; E, entire view of male body; F, entire view of female body.
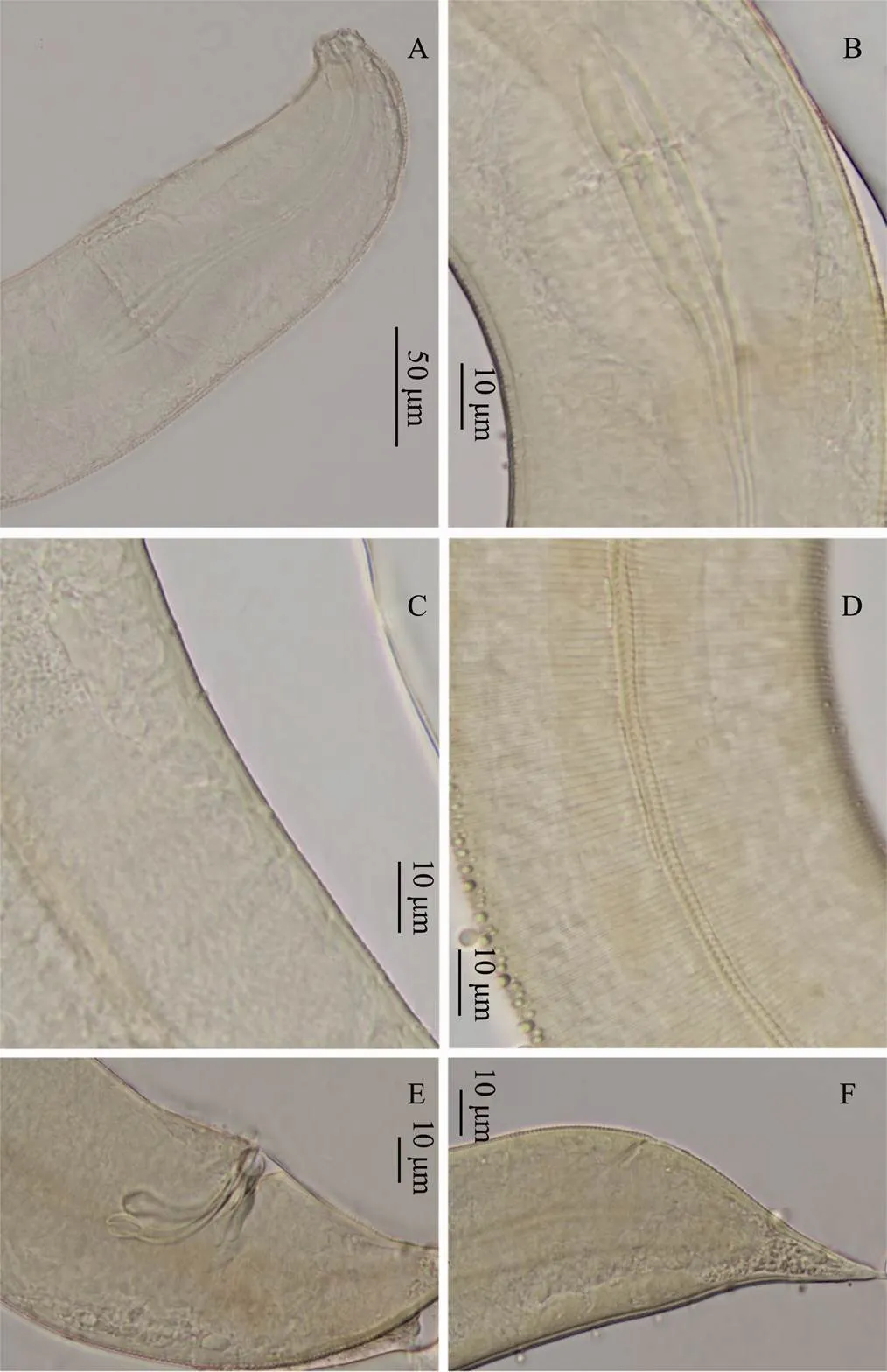
Fig.2 Metachromadora xiamenensis sp. nov. A, head of male, showing buccal cavity and labial sense organ; B, finely annulated cuticle of male, with an esophageal bulb; C, eighteen precloacal supplements of male; D, lateral punctuation of female; E, male spicules and gubernaculum; F, tail of female.
Type species
,1961
sp. nov. (Figs.1, 2, Table 1)
Type material
Male holotype, slide 201402E311. Four male paratypes, slides 201402E311, 201402E312, 201402E211, and 201 402D102. Five female paratypes, slides 201402E306, 201402E308, 201402E312, 201402E314, and 201402E 314. All the specimens were collected from the muddy surface sediment layer (0-10cm) in an artificial mangrove wetland located in Tong’an Bay in Xiamen. The mangrove species were mainly, also includes,and.
Etymology
This species is named after the city Xiamen, where it was found.
Measurements
The morphometric characteristics of the holotype and paratypes are given in Table 1.
Description
Male. Body cylindrical, smooth head capsule set off from the rest of the body. Cuticle finely annulated, with a thickness of 1 μm at midbody. Lateral ridges beginning at the posterior end of the pharynx and extending to the middle of the tail. Somatic setae short and sparse, irregularly spaced. Labial sensilla indistinct, with four cephalic sensilla in the shape of large elongated papillae that are 2.5-2.7μm long (16%-18% of the corresponding lip areawidth). Amphids 14.8-15.3μm (59%-66%cbd) and loop- shaped, doubly contoured with an open top. Amphids situated in the labial region, extending to the end of the head capsule and surrounded by a widened cuticular ring. Stoma 28.7-30.8μm deep, with a strong dorsal tooth positioned in the anterior part. Pharynx cylindrical, 158.9-172.3μm long, posteriorly enlarged with a well-developed bipartite basal bulb, the internal lining of which is strongly cuticularized. Bulb length 33%-34% of the total pharynx length. Nerve ring indistinct, with small cardia.
Male with one anterior testis, positioned to the right of the intestine. Eighteen tubular precloacal supplements pre- sent. Paired curved spicules 46.0-55.6μm long (1.0-1.4 abd), distinctly cephalated. Gubernaculum with middle piece and double dorsal apophyses. Tail 67.3-73.4μm long, 1.5-1.7 anal diameter, with a finger-like tip. Three small protuberances with short setae positioned on the ventral side of the tail. Caudal glands present, but poorly visible.
Females. Females similar to males. Reproductive system didelphic, amphidelphic with reflexed ovaries. Anterior ovary positioned to the left of the intestine, posterior ovary to the right of the intestine. Vulva with thick walls, situated at the median body.
Differential diagnosis
()sp. nov. is characterized by loop- shaped amphidial foveae with an open top and double contours, a pharynx with a bipartite cuticularized internal cavity, arcuate spicules, proximal end strongly cephalated, 18 tubular precloacal supplements, and a short, conical tail with three small protuberances.
This new species is morphologically similar to()(Gagarin and Tu, 2014) in the shape of the amphid and the head sensilla pattern, and has a similarly armed tail in males.sp. nov. is also similar toin thelength, a, b and c values, but A% (59-66% in()sp. nov.41.6% in), with longer spicules (74 µm in()sp. nov.46-56µm in), and different numbers of the internal lining of the pharyngeal bulb (2 in()sp. nov.. 3 in). However, the new species can be distinguished from the other species by the presence of 18 precloacal supplements, a longer and thinner tail (c = 10.1-12.2 and с’ =1.5-1.7 in()sp. nov.. c =15.3-19.9 and с’ =1.0-1.9 in())and wider lip area.
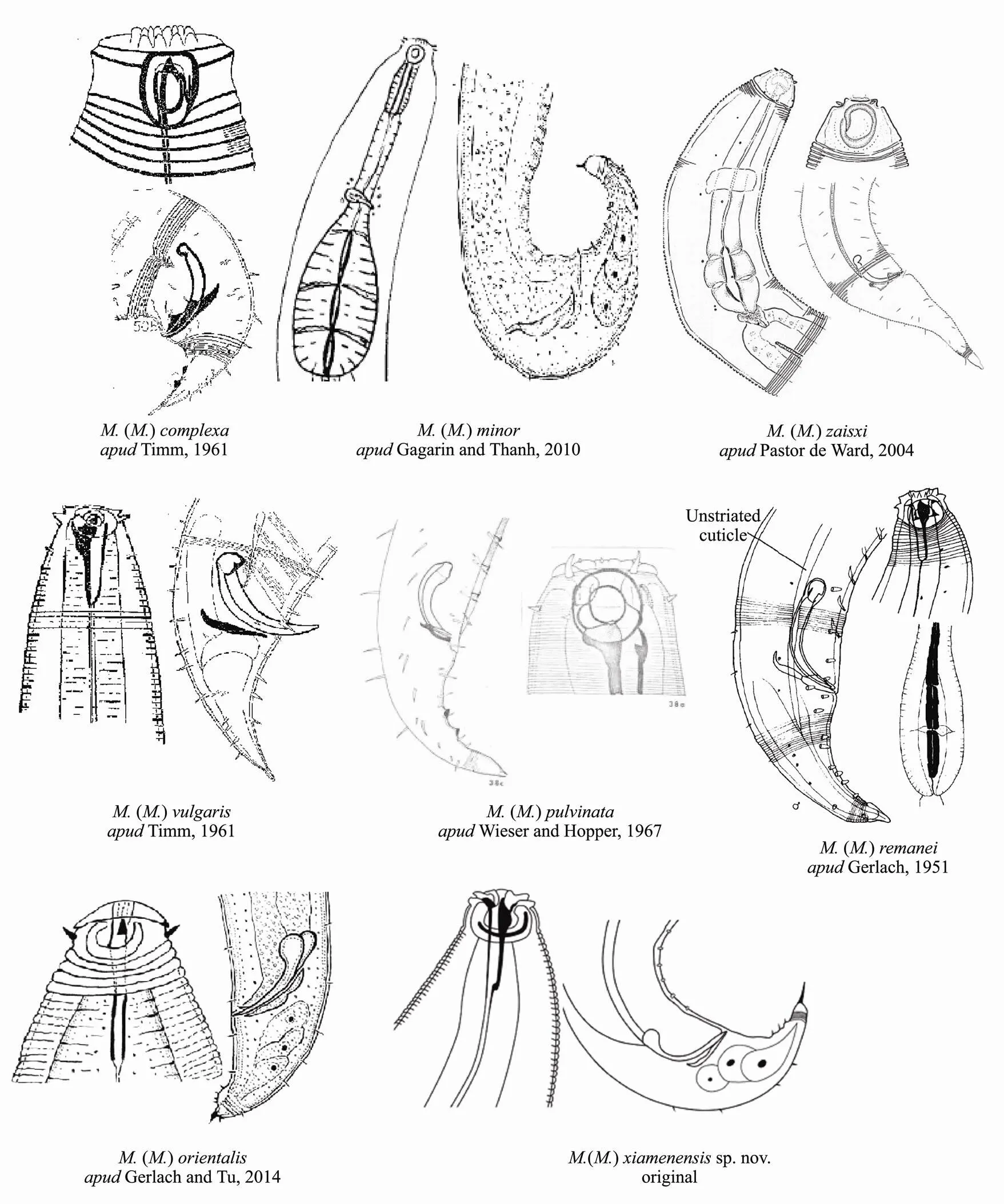
Fig.3 Pictorial key to the species of Metachromadora (Metachromadoroides).
The composite differentiating characters for all male() species are provided to aid in identification (Table 2).
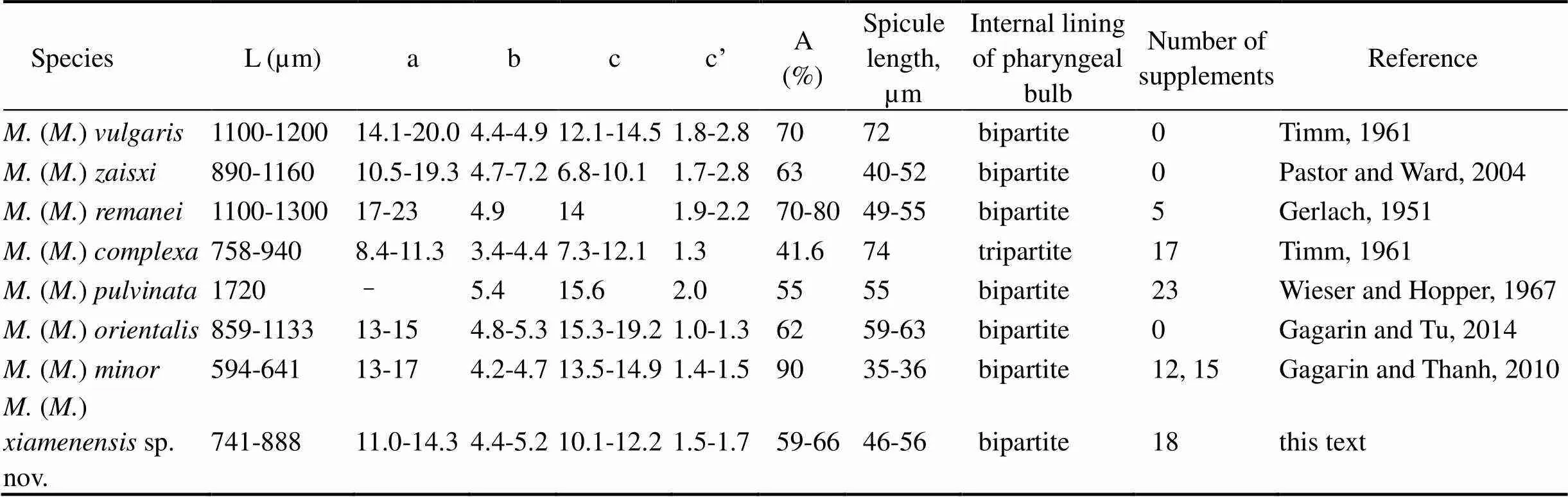
Table 2 Differential characters of males among Metachromadora species
Notes: A=amphid diameter as a percentage of the corresponding body diameter.Spicule/cloaca is the ratio of spicule length to body diameter at the cloaca level.
3.2 Species Description of Molgolaimus euryformis sp. nov.
GenusDitlevsen, 1921
Diagnosis (Modified from Fonseca., 2006)
Cuticle finely annulated. Amphid situated behind the narrowing part of the head. Inner labial and outer labial sensilla small, hard to observe. Cephalic setae close to the cephalic constriction. Buccal cavity small and narrow with small teeth. Esophagus cylindrical with a pronoun- ced posterior spherical bulb, heavily sclerotized at the bulb. Spicules of different lengths and shapes from short and bent to long and straight. Gubernaculum with or without apophysis. Precloacal supplements often present. Tail of varying shape and length, from short and conical to elongate and slender, cylindrical posteriorly.
Type species
Ditlevsen, 1921
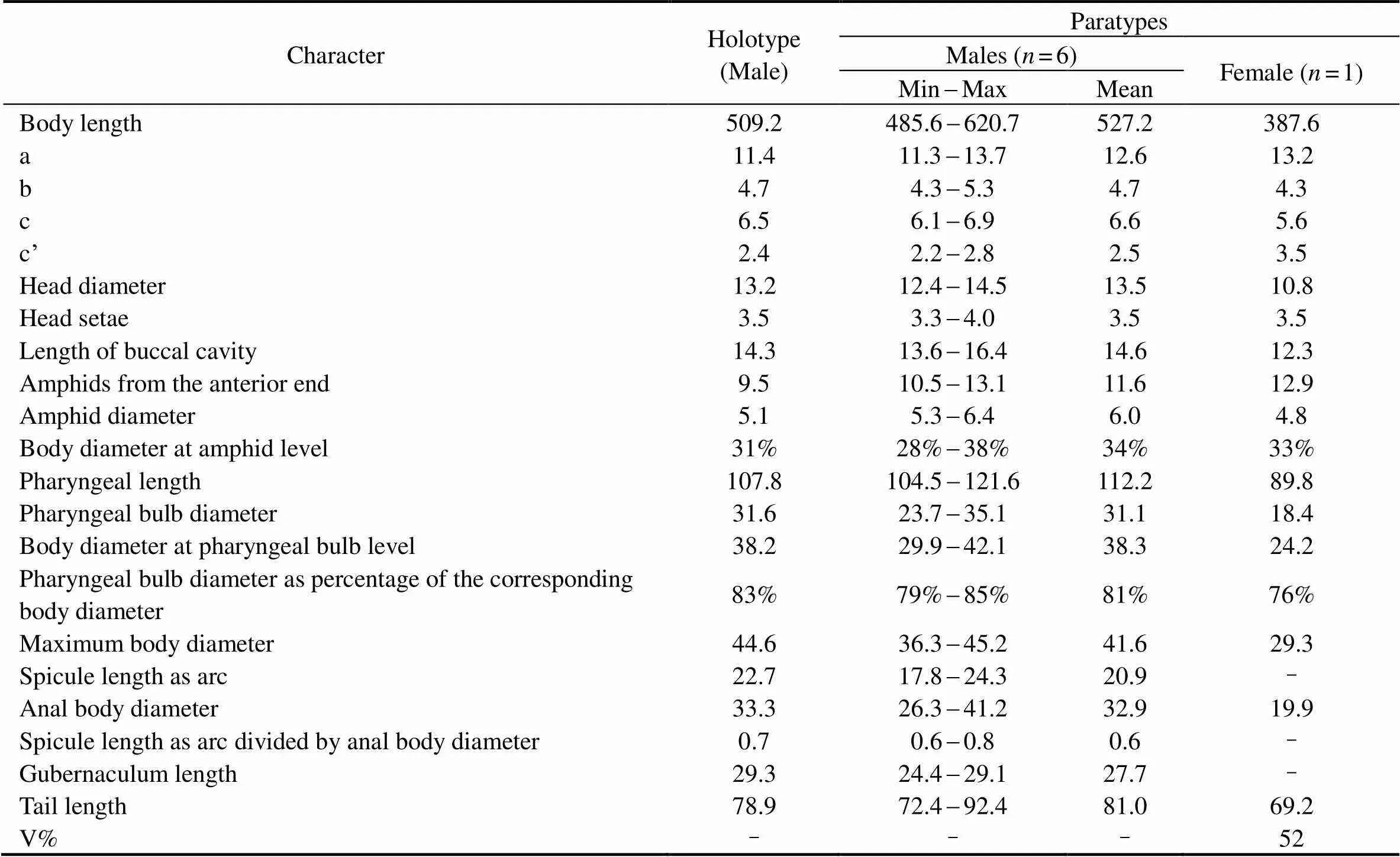
Table 3 Morphometrics of Molgolaimus euryformis sp. nov. (in μm)
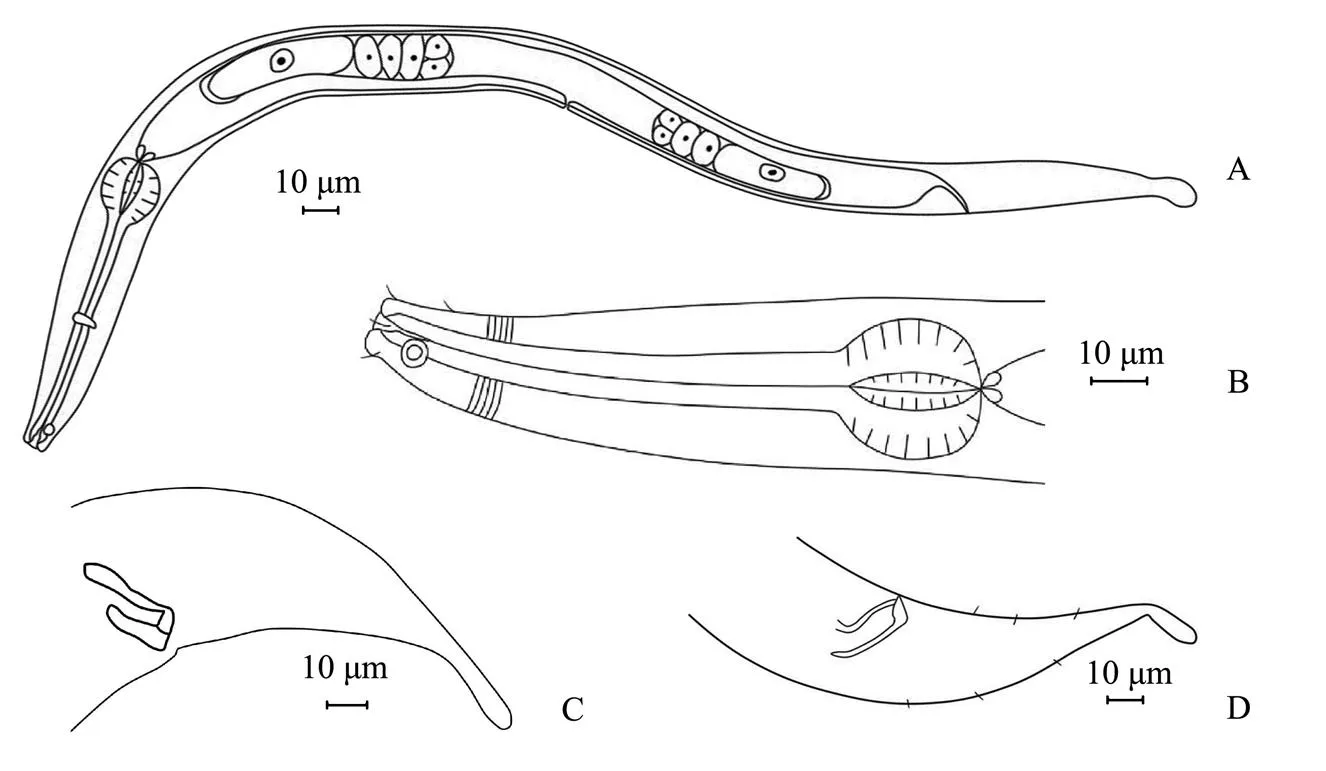
Fig.4 Molgolaimus euryformis sp. nov. A, entire view of female body; B, male anterior region; C, structure of spicules and gubernaculum; D, lateral view of male tail.
Molgolaimuseuryformis sp. nov.
Type material
Male holotype, slide 201408C203. Five male paratypes, slides 201408C203, 201408C208, 201408B211, 201402 F210, and 201408C207. One female paratype, slide 2014 02C301.
Etymology
This species is named for its lower ‘a value’ compared to that of other species in the genus.
Measurements
The morphometric characteristics of the holotype and paratypes are given in Table 3.
Description
Male. Body relatively short, cylindrical. Cuticle finely annulated, particularly obvious on the tail. Head small and wide with intensive striation, separated from body by small narrowing before head setae. Inner and outer labial sensilla indistinct. Four cephalic setae approximately 3-4 µm long (30% of head diameter). Amphid fovea circular, 5-6µm in diameter (28%-38% of cbd), located 11-13µm from the anterior end. Buccal cavity small with teeth. Esophagus forms a pronounced spherical bulb posteriorly with a 31-µm diameter. Cardia is small and extended, and the glandular body lies posterior to the cardia. Excretory pore not observed. Reproductive system monarchic, outstretched testis situated to the left of the intestine. Spicules short with ventral apophysis, 20.9µm in length (0.6-0.8abd). Gubernaculum parallel to spicule, with a block-shaped hook on the terminus. Tail conical along 3/4 of its length, cylindrical posteriorly with a swollen tip, 2.2-2.8 anal diameter.
Female. Similar to male, reproductive system didelphic with reflexed ovaries. The ovaries situated to the left of the intestine. Anterior ovary slightly longer than the posterior ovary.
Differential diagnosis
sp. nov. is characterized by its short and relatively plump body (L=485.6-620.7µm, a=11.3-13.7), relatively short head setae, ventral apophysis (17.8-24.3μm), gubernaculum with a block-shaped hook, conical-cylindrical shape with a swollen tail tip and absence of precloacal supplements.
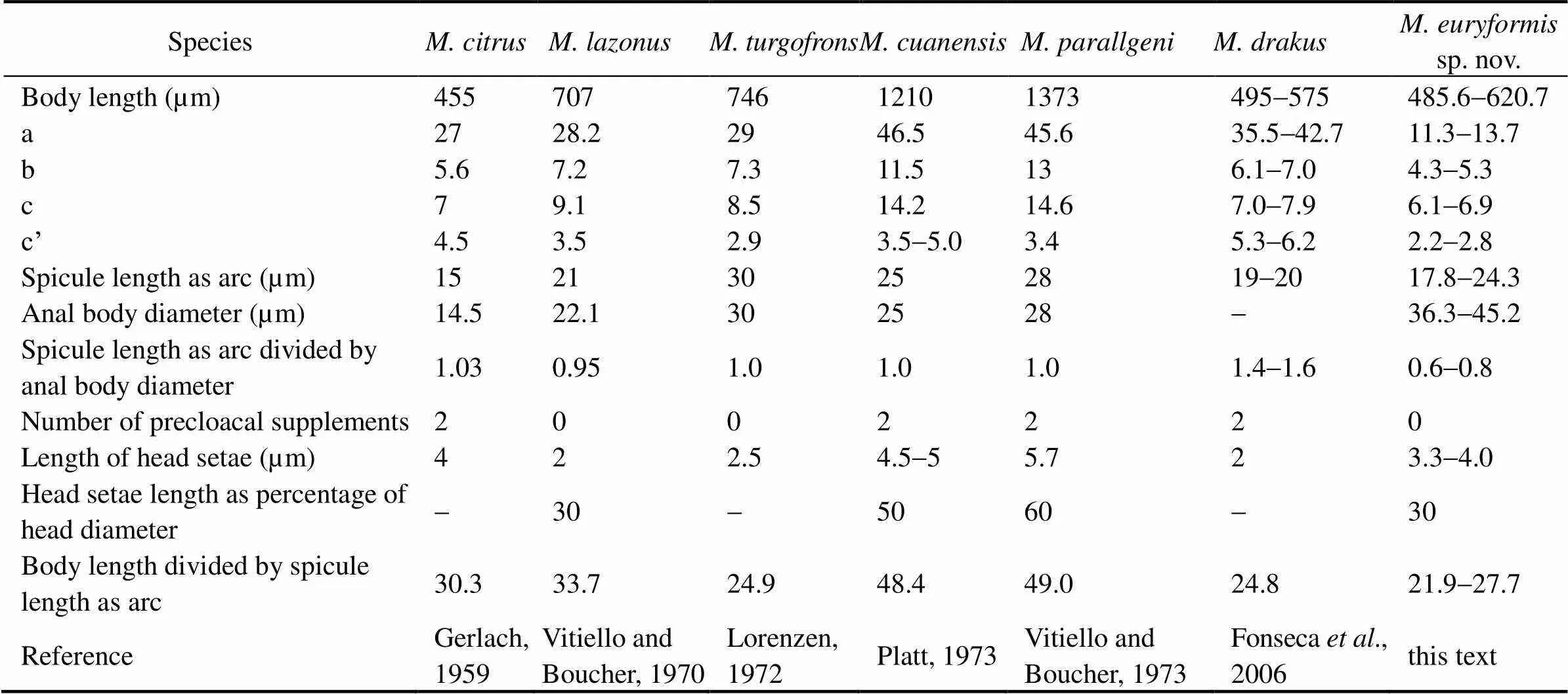
Table 4 Comparison of Molgolaimus euryformis sp. nov. with allied species (male)
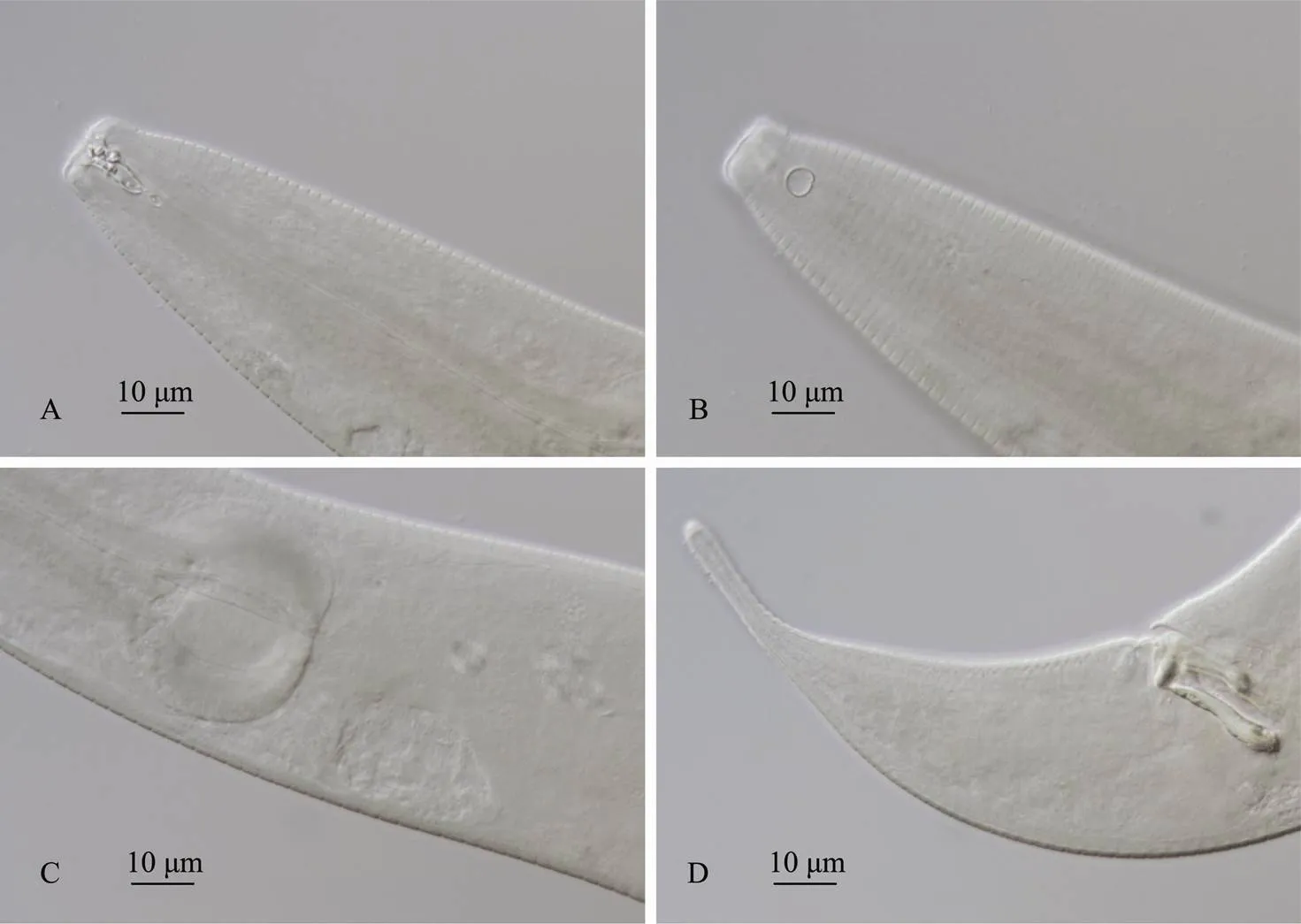
Fig.5 Molgolaimus euryformis sp. nov. A, anterior body of male, showing the buccal cavity and cephalic setae; B, anterior body of male with amphids; C, spherical esophageal bulb of male; D, spicules, gubernaculum and tail with swollen tip.
Based on the morphological parameters, the new species belongs to group 1a in the identification key (Fon- seca, 2006), members of which are characterized by short spicules (<35μm) and a spicule to abd ratio less than 1.
Among the species in the group ((Gerlach,1959),(Vitiello, 1971),(Loren- zen, 1972),(Platt, 1973) and(Vitiello, 1973)),sp. nov. has the smallest value and spicule/abd ratio. This species is most closely related toandbut differs from them by its conical-cylindrical body with a swollen tail tip and short spicules with ventral apophysis.
The new species resembles(Fonseca, 2006) in the ratio of body length to spicule length. How- ever,differs fromsp. nov. by its slim body, its spicule form (short and without ventral apophysis) and the presence of only one precloacal supplement.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (No. 41606119), the Provincial Natural Science Foundation of Fujian (No. 2017J05068), and the New Century Outstanding Talents Support Program for Institutions of Higher Learning in Fujian Province and Building Ecological Security for Urban Agglomeration and Integrating Ecological Restoration Techniques for Coastal Zones in Fujian River Deltas (No. 2016YFC0502900). The authors are also grateful to many postgraduates and undergraduates for their help during the field samplings and laboratory analysis. We are also very grateful to Prof. Yong Huang and Prof. Yuqing Guo for their help in the improving this manuscript respectively.
De Jonge, V. N., and Bouwman, L. A., 1977. A simple density separation technique for quantitative isolation of meiobenthos using the colloidal silica Ludox-TM., 42: 143-148.
De Ward, C. T., 2004. New species of(Nematoda, Comesomatidae) and(Nematoda, Desmo- doridae) from Patagonia, Chubut, Argentina., 542 (1): 1-15.
Fonseca, G, Vanreusel, A., and Decraemer, W., 2006. Taxonomy and biogeography ofDitlevsen, 1921 (Nema- toda: Chromadoria) with reference to the origins of deep sea nematodes., 18 (1): 23-50.
Gagarin, V. G., and Tu, N. D., 2014. Two new species of free- living nematodes (Nematoda and Chromadorea) from mangrove thicket in Vietnam., 7 (4): 338- 347.
Gerlach, S. A., 1951. Nematoden aus der familie der chromado- ridae von den deutschen Küsten., 8: 106-132.
Gerlach, S. A., 1959. Neue meers-nematoden aus dem Supra- litoral der deutschen Küsten. Internationale revue der gesamten., 44 (1-4): 463-467.
Guo, Y., Chang, Y., Chen, Y., Li, Y., and Liu, A., 2015. Descrip- tion of a marine nematodesp. nov. (Comesomatidae) from mangrove forests of Quanzhou, China, with a pictorial key tospecies., 14 (6): 1111-1115.
Fu, S. J., Zeng, J. L., Zhou, X. P., Tan, W. J., and Cai, L. Z., 2018. Two new species of free living nematodes of genus(Nematoda: Enoplida: Tripyloididae) from mangrove wetlands in the Xiamen Bay, China., 37 (10): 168-174, DOI: 10.1007/s13131-018-0000- 01321-2.
Li, Y. X., and Guo, Y. Q., 2016. Two new free-living marine nematode species of the genus(Anoplostoma- tidae) from the mangrove habitats of Xiamen Bay, East China Sea.,15 (1): 11-18,DOI: 10.1007/s11802-016-2896-x.
Liu, J. L., and Huang, B., 2012. Progress in the studies of the meiofauna in mangrove ecosystem., 36 (10): 118-122 (in Chinese with English abstract).
Lorenzen, S.,1972. Die nematodenfauna im verklappungsgebiet für industrieabwässer nordwestlich von helgoland: II. Desmodorida und chromadorida.,187: 287-302.
McIntyre, A. D.,and Warwick, R. M., 1984. Meiofauna techniques. In:. Holme, N. A., and McIntyre, A. D., eds., Blackwell, Oxford, 217-244.
Pastor, C. T., and Ward, D., 2004. New species of(Nematoda, Comesomatidae) and(Nematoda, Desmodoridae) from Patagonia, Chubut., 542: 1-15.
Platt, H. M.,1973. Freeliving marine nematodes from Strangford Lough, Northern Ireland., 14 (3): 295-321.
Portnova, D., 2009. Free-living nematodes from the deep-sea Håkon Mosby Mud Volcano, including the description of two new and three known species., 2096: 197-213.
Shi, B., 2016. Taxonomy of nematodes and community structure of meiofauna in various marine habitats. PhD thesis. Univer-
sity of Chinese Academy of Sciences, Qingdao.
Shi, B., and Xu, K., 2017.sp. nov. in intertidal sediment from the East China Sea, with transfer of twospecies to(Nematoda, Desmodorida)., 97 (6): 1335-1342.
Tchesunov, 2014.Desmodorida De Coninck, 1965. In:Vol. 2. Schmidt-Rhaesa, A., ed., De Gruyter, Berlin, Boston, 399-420.
Timm, R. W., 1961.The marine nematodes of the Bay of Bengal., 1(1): 1-88.
Vitiello, P.,1970. Nématodes libres marins des vases profondes du Golfe du Lion. III. Monhysterida, Araeolaimida, Desmodorida., 2: 647-690.
Vitiello, P., 1973. Nouvelles espèces de Desmodorida (Nematoda) des côtes de Provence., 5: 137-146.
Vitiello, P., and Boucher, G., 1971. Nouvelles espèces de Chromadorida (Nematoda) des vases terrigènes méditerranéennes., 96 (2): 187- 196.
Wieser, W., and Hopper, B., 1967. Marine nematodes of the east coast of North America. I Florida., 135 (5): 239-344.
December 24, 2018;
August 2, 2019;
November 6, 2019
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2020
. E-mail: cailizhe@xmu.edu.cn
(Edited by Ji Dechun)
 Journal of Ocean University of China2020年1期
Journal of Ocean University of China2020年1期
- Journal of Ocean University of China的其它文章
- Circulation and Heat Flux along the Western Boundary of the North Pacific
- System Reliability Analysis of an Offshore Jacket Platform
- The Mineral Composition and Sources of the Fine-Grained Sediments from the 49.6˚E Hydrothermal Field at the SWIR
- Research Progress of Seafloor Pockmarks in Spatio-Temporal Distribution and Classification
- Application of the Static Headland-Bay Beach Concept to a Sandy Beach: A New Elliptical Model
- Climatology of Wind-Seas and Swells in the China Seas from Wave Hindcast
