Antioxidant and antibacterial activities and identification of bioactive compounds of various extracts of Caulerpa racemosa from Algerian coast
Louiza Belkacemi✉, Mahmoud Belalia, Ali C. Djendara, Youcef BouhaddaLaboratoire de Technologie Alimentaire et de Nutrition, Site II EX-INES de Chimie, Chemin des Crêtes, BP 88, Abdelhamid IbnBadis University,Mostaganem, Algeria
2Laboratoire de Structure, Elaboration et Application des Matériaux Moléculaires, Site II EX-INES de Chimie, Chemin des Crêtes, BP 188,Abdelhamid IbnBadis University, Mostaganem, Algeria
3Laboratoire de Chimie Physique des Biomolécules et Interfaces Biologiques, B.P. 305, Route de Mamounia, Mustapha Stambouli University Mascara,Algeria
ABSTRACT Objective: To evaluate the antibacterial and antioxidant activities and to identify the volatile bioactive compounds present in different crude extracts of the seaweed Caulerpa racemosa var. cylindracea.Methods: Caulerpa racemosa harvested from the intertidal zone of Mostaganem coast (N 35°54'37.94", E 0°3'17.37") was subjected to Soxhlet extraction using methanol, chloroform, and hexane solvents.Antioxidant properties were assessed by using 2,2'-diphenyl-1-picrylhydrazyl (DPPH), 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and β-carotene bleaching assays. The antibacterial activity was evaluated on six standard bacterial strains using the agar disc diffusion method. The GC-MS analysis was performed using non-polar and polar capillary columns.Results: The chloroform extract of Caulerpa racemosa exhibited higher contents of polyphenols [(123.91±1.46) mg gallic acid equivalent/g dry extract] and tannins [(59.28±5.43) mg catechin equivalent/g dry extract] (P<0.001) and was the most effective in scavenging DPPH [(1.98±0.08) mg/mL] and ABTS [(1.66±0.05) mg/mL]radicals. The hexane extract displayed the best antibacterial activity against Staphylococcus aureus, Bacillus cereus, and Pseudomonas aeruginosa, producing inhibition zones of (11.16±0.76), (9.00±0.00)and (9.33±1.15) mm, respectively. The l-(+)-ascorbic acid 2,6-dihexadecanoate and 4-hydroxy-2methylproline were among the most abundant volatile compounds. Besides conventional fatty acids, cis-10-heptadecenoic acid, nonahexacontanoic acid, and dodecanoic acid, 3-hydroxy- were identified. Two phytosterols were identified: stigmast-5-en-3-ol- (12.9%) and stigmast-5-en-3.beta.-ol,(24S)- (4.57%).Conclusions: The preliminary identification of the volatile compounds reveals the presence of some new bioactive components not reported previously in Caulerpa racemosa from other geographical areas.Some of these compounds possess an interesting potential for pharmaceutical/nutraceutical applications.
KEYWORDS: Caulerpa racemosa var. cylindracea; West Algerian coast; Biological activities; Clionasterol; 4-Hydroxy-2methylproline
1. Introduction
The marine environment is an excellent reservoir of bioactive natural products[1,2]possessing structural features that are not found on land[3].As a result, marine organisms contain highly bioactive metabolites that could lead to the development of new pharmaceutical agents.
Marine macroalgae are a various group of marine organisms that have been widely investigated for their secondary metabolites content.They represent one of the richest sources of natural antioxidants among marine resources[4]. In fact, seaweeds are rich in bioactive compounds such as polyphenols, terpenoids, alkaloids, saponins,tannins and steroids known for their antioxidant properties[5,6].Antioxidant metabolites serve as sacrificial scavengers of reactive oxygen species (ROS)[7]. Thereby, seaweeds with their high content of antioxidant molecules can be used to ward off free radical[8]and thus prevent against ROS-mediated diseases such as diabetes mellitus,cardiovascular diseases, and cancer[9]. In fact, seaweeds are recognized for their effective management of diabetes by modulating key metabolic enzymes and increasing insulin sensitivity[10]. Besides, they exert anti-hyperlipidemic[11], anti-inflammatory[4]and antitumor[12]activities. Seaweed extracts were also considered to have antifungal[13]and antibacterial[14]activities. Indeed, seaweeds provide a great variety of metabolites such as polysaccharides, polyunsaturated fatty acids,phlorotannins, and other phenolic compounds that display a significant inhibitory action against human pathogens[14]with resistance to antibiotics[15,16].
Nevertheless, antioxidant and antibacterial activities depend on algal species[15]and their environment[17]. Indeed, metabolite concentrations may vary according to environmental factors such as seasonal periods, temperature, light, salinity and geographical location[17].So, by colonizing new geographical areas, the invasive species could modify their metabolomic profile as an adaptative response to the new environmental conditions.
The green alga Caulerpa racemosa (C. racemosa), known for its biological activities[5,18], is among the invasive species of the Mediterranean basin. Widely distributed in tropical and subtropical regions, it was for the first time reported in Algeria in Algiers Bay in 2007 and then in the west Algerian coast, precisely in Arzew bay,Salamandre and Stidia stations (Mostaganem, Algeria) in 2010[19].
To our knowledge, the Algerian studies have looked at the ecology and the spread of C. racemosa, but none focused on its chemical composition and biological properties. In order to ascertain the metabolic adaptation of this seaweed to the Mediterranean Sea environment that could stimulate new bioactive metabolites synthesis,we evaluated the phytochemical composition and preliminarily identified the volatile bioactive compounds present in different crude extracts of C. racemosa var. cylindracea harvested from intertidal zone in the west coast of Algeria. Furthermore, we assessed the antioxidant and the antibacterial activities of different extracts looking for possible nutraceutical applications.
2. Materials and methods
2.1. Collection and extraction
C. racemosa var. cylindracea was harvested from the intertidal zone of Salamandre station (Latitude N 35°54’37.94”, Longitude E 0°3’17.37”) in Mostaganem coast (Algeria). After harvesting, the seaweed was washed to remove epiphytes and sand. After washing with distilled water, the samples were shade-dried to constant weight and then powdered. Dried C. racemosa powder was separately extracted with three different solvents, methanol, chloroform, and hexane, using the Soxhlet apparatus for 8 h at a temperature not exceeding the boiling points of each solvent. After filtration, the extracts were concentrated in a vacuum at 40 ℃ using a rotary evaporator. Finally, the extracts were weighted and the yield of each extract was determined as follow:
Extract % = (Weight of extract in grams/Weight of sample in grams)×100
2.2. Preliminary phytochemical screening
The methanol, chloroform, and hexane extracts were tested for the possible presence of polyphenols, flavonoids, tannins, coumarins,quinones, anthraquinones, sterols, saponins, terpenoids, basic and salt alkaloids, reducing sugars and amino acids. Tests were performed according to Harborne[20].
2.3. Determination of total phenolics, flavonoid, and condensed tannins content
2.3.1. Total phenolics content
Total phenolics content of algal extracts was determined using Folin-Ciocalteu reagent according to the method described by Farasat et al.[9]. Briefly, 200 µL of each extract were mixed with 1 000 µL of 1:10 Folin-Ciocalteu reagent followed by the addition of Na2CO3(800 µL, 7.5%). After incubation at room temperature for 2 h in the dark, the absorbance at 765 nm was recorded. Different concentrations of gallic acid (5-100 µg/mL) were used to establish the standard curve. Total phenolic content were expressed as mg gallic acid equivalent (GAE)/g dry extract.
2.3.2. Flavonoids content
The flavonoid content was determined using aluminum chloride as described by Cox et al.[21]. In brief, a 250 µL aliquot of each extract(1 mg/mL) and quercetin (10-100 µg/mL), used for the calibration curve, was mixed with 1.25 mL of double distilled water and 75 µL of 5% NaNO2solution. After 6 min, 150 µL of 10% AlCl3solution was added. After 5 min of incubation, 0.5 mL of 1 M NaOH solution was added to the mixture, and then the total volume was made up to 2.5 mL with double distilled water. The solutions were mixed well and the absorbance against blank was determined at 510 nm.The results were expressed as mg quercetin equivalent (QE)/g dry extract.
2.3.3. Total condensed tannin content
The determination of total condensed tannins was carried out according to the method followed by Belviso et al.[22]. A 50 µL aliquot of each extract and catechin (100-1 000 µg/mL), used for standard curve, was added to 3 mL of a 4% methanol vanillin solution and then 1.5 mL of concentrated hydrochloric acid (37%)was added to the mixture. The mixtures were kept for 15 min in the dark at room temperature, and the absorbances were measured at 500 nm. The results were calculated and expressed as mg catechin equivalent (CE)/g dry extract.
2.4. In vitro antioxidant assays
2.4.1. 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay
The DPPH radical scavenging assay was determined by the procedure described by Sanchez-Moreno et al.[23]. An aliquot of 50 µL of a solvent solution containing different extract/standard concentrations was added to 1 950 µL of 0.06 mM DPPH solution.The mixture was left in the dark for 30 min. The assay absorbance was measured at a wavelength of 517 nm. The inhibition percentage was calculated as follows: inhibition (%) = (Ac-As)/Ac×100;Where, Ac: absorbance of the control reaction; As: the absorbance in presence of the sample/positive control. The half-maximal inhibitory concentration (IC50) was calculated by linear regression analysis and expressed as a mean of three determinations. Ascorbic acid was used as positive control.
2.4.2. 2,2’-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging assay
The ABTS radical scavenging assay was performed according to Re et al.[24]. ABTS was dissolved in water to yield a 7 mM concentration. ABTS radical cation (ABTS˙+) was produced by reacting ABTS stock solution with 2.45 mM potassium persulfate solution (1:1) and kept in the dark at room temperature for 12-16 h before use. For the study, the ABTS solution was diluted with ethanol to an absorbance of (0.70 ± 0.02) measured at 734 nm. An aliquot of 100 µL of a solvent solution containing different extracts/standard concentrations was added to 1 900 µL of ABTS solution.The absorbance was measured at 734 nm exactly 6 min after initial mixing. All assays were determined in triplicate and the inhibition percentage was calculated as follows: inhibition (%) = (Ac-As)/Ac×100; Where, Ac:ABTS absorbance of the control reaction; As: the ABTS absorbance in presence of the sample/standard. Trolox was used as positive control.
2.4.3. β-carotene bleaching (BCB) assay
The BCB assay was determined according to Kulisic et al.[25].The β-carotene (0.1 mg) was added to a boiling flask together with linoleic acid (20 mg) and Tween 40 (100 mg), all dissolved in chloroform. After evaporation to dryness, under vacuum at 40 ℃ by a rotary evaporator, oxygenated distilled water (50 mL) was added and the mixture was emulsified for 1 min in a sonicator to form emulsion A. An aliquot of 100 µL of solvent stock solution of each extract/positive control was mixed with 2.4 mL of emulsion A. Prior to incubation at 50 ℃, the absorbance of all samples was taken (t= 0 min) against the blank at 470 nm. After 120 min of incubation, the absorbance was read at 470 nm. All determinations were performed in triplicate. The percentage inhibition was calculated from the data with the following formula: % inhibition= [(AA120-AC120) / (AC0-AC120)]×100; where AA120is the absorbance of the extract at t=120 min, AC120is the absorbance of the control at t=120 min, and AC0is the absorbance of the control at t=0 min. Tocopherol was used as positive control.
2.5. Antibacterial activity of C. racemosa extracts
The methanol, chloroform and hexane extracts of C. racemosa were tested against the standard Gram-positive and Gram-negative pathogens: Bacillus cereus (B. cereus) (ATCC 10876), Enterococcus faecalis (ATCC 29212), Staphylococcus aureus (S. aureus) (ATCC 25923), Escherichia coli (ATCC 25922), Pseudomonas aeruginosa(P. aeruginosa) (ATCC 27853) and Proteus mirabilis (ATCC 35659).The sensitivity of these bacteria to the three extracts was assessed using the diffusion method reported by Bauer et al.[26]. The extracts were dissolved in dimethyl sulfoxide to final concentrations of 100 mg/mL. Sterile discs (6 mm in diameter) were impregnated with 10µL of the extract solutions. Negative controls were prepared on discs impregnated with dimethyl sulfoxide (solvent control). Penicillin G (10 µg), gentamicin (10 µg) and cefazolin (30 µg) were used as positive controls. Bacterial inocula were prepared from 18 h cultures,and the optical density of the bacterial suspension was adjusted to a turbidity corresponding to 0.08-0.1 by measuring the absorbance at 625 nm using a spectrophotometer. The discs impregnated with each extract were applied on Petri dishes poured with Mueller-Hinton agar, and the plates were left for 30 min at room temperature to allow diffusion of the extracts. After 24 h of incubation at 37 ℃, the antibacterial activity was estimated by measuring the inhibition zone radius (mm) in the three replicates.
2.6. Gas chromatography-mass spectrometry (GC-MS)analysis
The GC-MS analysis was performed using a Shimadzu GCMS TQ8030. In order to characterize both non-polar and polar volatile compounds, two different columns have been used: a nonpolar capillary column [Crossbond 100% dimethyl polysiloxane,30 m, 0.25 mm ID, 0.25 µm df, RTX-1 (Restek, USA)] and a polar capillary column [Crossbond carbowax polyethylene glycol, 30 m,0.32 mm ID, 0.25 µm df, Stabilwax (Restek, USA)]. Injection mode was splitless at a split ratio: 40.0. Helium was used as a carrier gas at a flow rate of 1.0 mL/min. The GC oven was initially at 50 ℃ and was held for 5 min after injection for both columns, followed by temperature ramping at 10 ℃/min up to a final temperature of 230 ℃and 250 ℃ respectively for the polar and non-polar column. The fragmentation was carried out by electron impact under a field of 70 eV. The scanning range was from 40 Da to 600 Da. The total run time was approximately 23 and 28 min for the polar and non-polar column, respectively. Peak identifications were tentatively performed by comparison with the NIST11 library (NIST 11- MassSpectral Library, 2011 version).
2.7. Statistical analysis
All the assays were performed three times (n=3) and the results were expressed as mean ± standard deviation (SD). The assays were compared by using one-way analysis of variance (ANOVA),followed by Tukey’s post-hoc test. P<0.05 indicates statistical significance. All statistical analyses were performed using SEES Statistic 20.0 software.
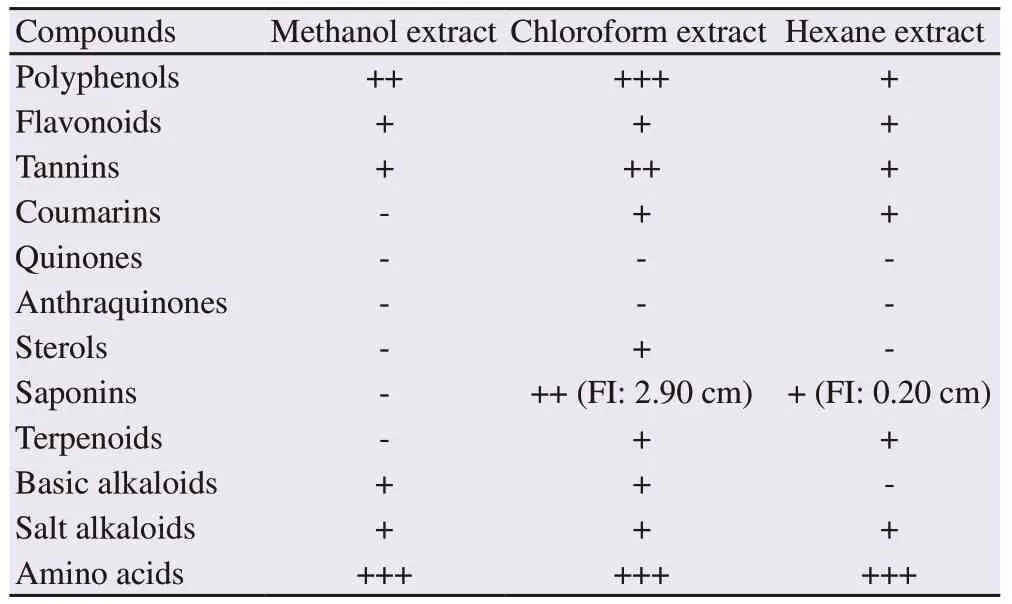
Table 1 Preliminary phytochemical screening of Caulerpa racemosa extracts.
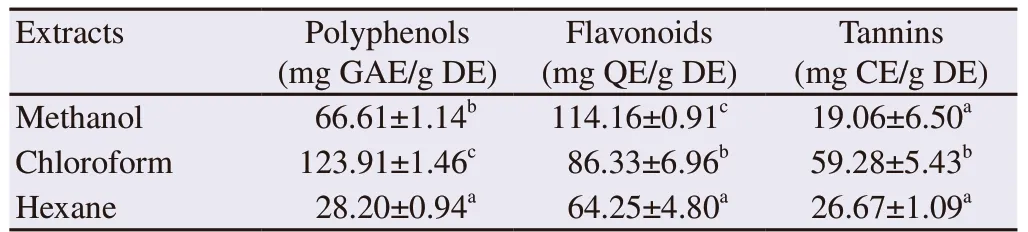
Table 2. Total phenolics, flavonoid and tannins in the three Caulerpa racemosa extracts.
3. Results
3.1. Extraction yield
The extraction yield differed significantly (P<0.001) among the three extracts. The chloroform, hexane and methanol extracts yielded(4.25 ± 0.45)%, (3.90 ± 0.10)% and (10.17 ± 0.23)%, respectively.
3.2. Phytochemical screening
The phytochemical screening revealed ten different chemical compounds (Table 1). Among the three different extracts, the chloroform one displayed the presence of a maximum number of compounds (10), followed by the hexane (8) and methanol (6) extracts.
3.3. Total phenolics, flavonoid, and condensed tannin contents
Total phenolics, flavonoid and tannin contents of the different C. racemosa extracts are represented in Table 2. Both polyphenols and tannin contents were higher in the chloroform extract than in the methanol and hexane ones (P<0.001). However, the highest flavonoid content was recorded in the methanol extract (P<0.001).
3.4. In vitro antioxidant activities
The chloroform extract exhibited the greatest DPPH scavenging power, with the lowest IC50[(1.98±0.08) mg/mL] compared to the hexane and methanol extracts [(11.07±1.83) mg/mL vs. (42.06±1.04)mg/mL, respectively] (P<0.001). Its IC50was statistically comparable(P>0.05) to IC50of ascorbic acid [(0.23±0.03) mg/mL].
Similarly, the chloroform extract possessed the highest antioxidant capacity in terms of ABTS radical cation scavenging ability[(1.66±0.05) mg/mL] (P<0.05), whereas a higher concentration of hexane and methanol extracts were required to scavenge this radical[(3.54±0.04) mg/mL].
Antioxidant potential of C. racemosa extract was also determined by the β-carotene bleaching method based on the oxidation of linoleic acid. Among the three extracts, the hexane and chloroform ones showed interesting antioxidant activity in terms of β-carotene bleaching [IC50= (0.39±0.02) mg/mL and (0.43±0.00) mg/mL, respectively] (P>0.05) followed by the methanolic extract[(0.86±0.02) mg/mL] (P<0.05).
3.5. Antibacterial activity
The antibacterial activity of C. racemosa extracts against the tested human pathogen bacteria is shown in Table 3. The three strains S. aureus, B. cereus, and P. aeruginosa were sensitive to the tested extracts, whereas Enterococcus faecalis, Escherichia coli, and Proteus mirabilis were resistant. Overall, the hexane extract had the highest antibacterial activity against the three sensitive strains with a higher antibacterial effect on S. aureus. The three extracts were more effective on B. cereus and P. aeruginosa than penicillin. The antibiotic effect of cefazolin on B. cereus was comparable to that exerted bymethanol and chloroform extracts (P>0.05) but was slightly inferior than the antibacterial effect of the hexane extract (P<0.05).
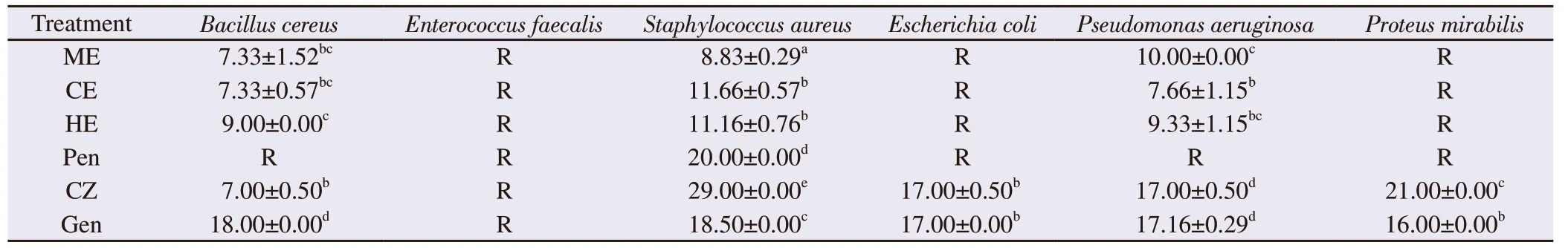
Table 3. Antibacterial activity of Caulerpa racemosa extracts against standard human pathogen bacteria.
3.6. GC-MS
The GC-MS analysis of the crude extract of C. racemosa revealed the presence of 14 fatty acids, three phytosterols and 7 terpenes/terpenoids(Table 4). The chloroform extract contained more monounsaturated fatty acids and polyunsaturated fatty acids. All the identified terpenes and terpenoids were present in the hexane extract (Table 4). In addition to the former compounds, some identified volatile components possessing biological activities are reported in Table 5.
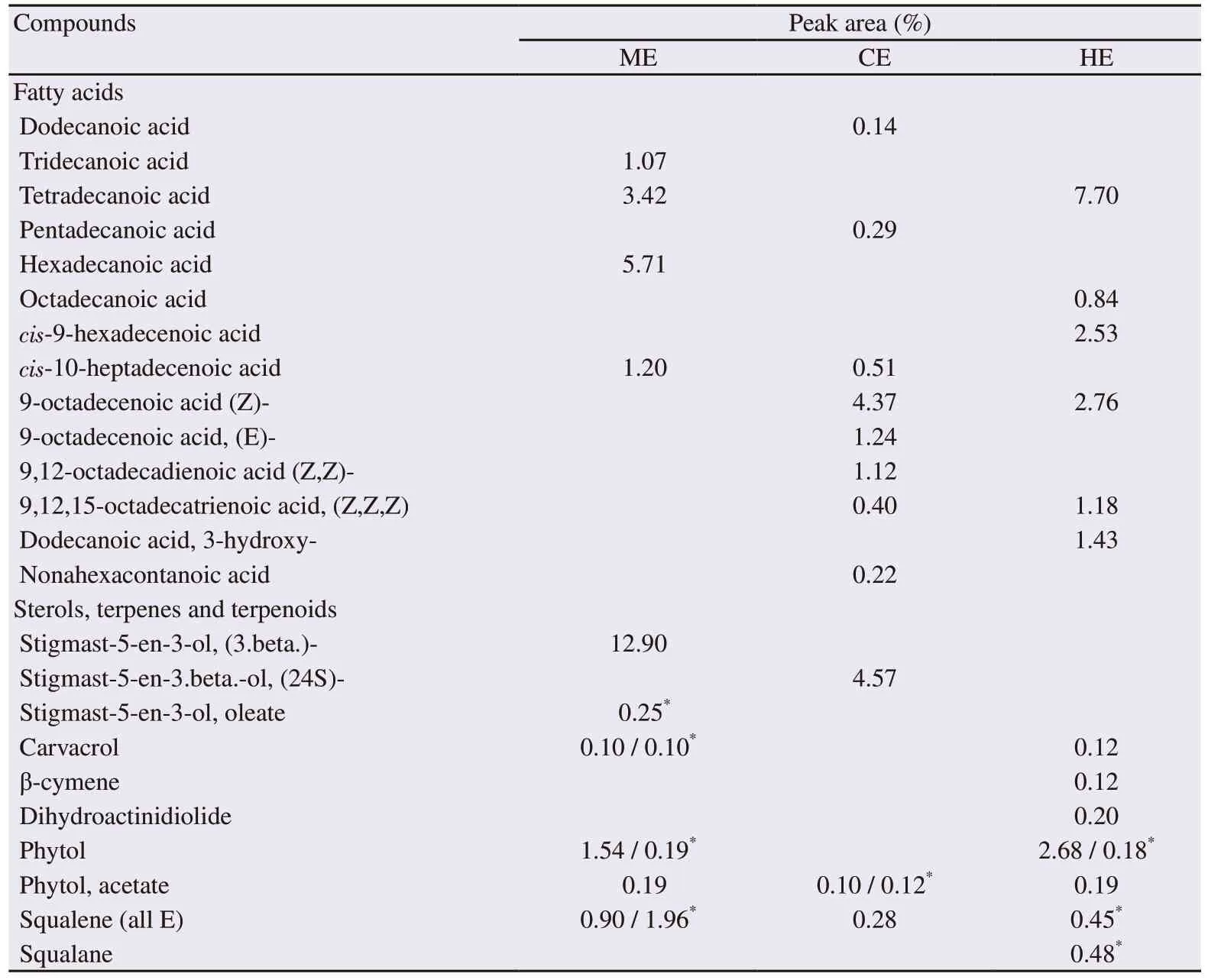
Table 4. Fatty acids, sterols, and terpenes identified in methanol, chloroform and hexane extracts of Caulerpa racemosa by GC-MS.
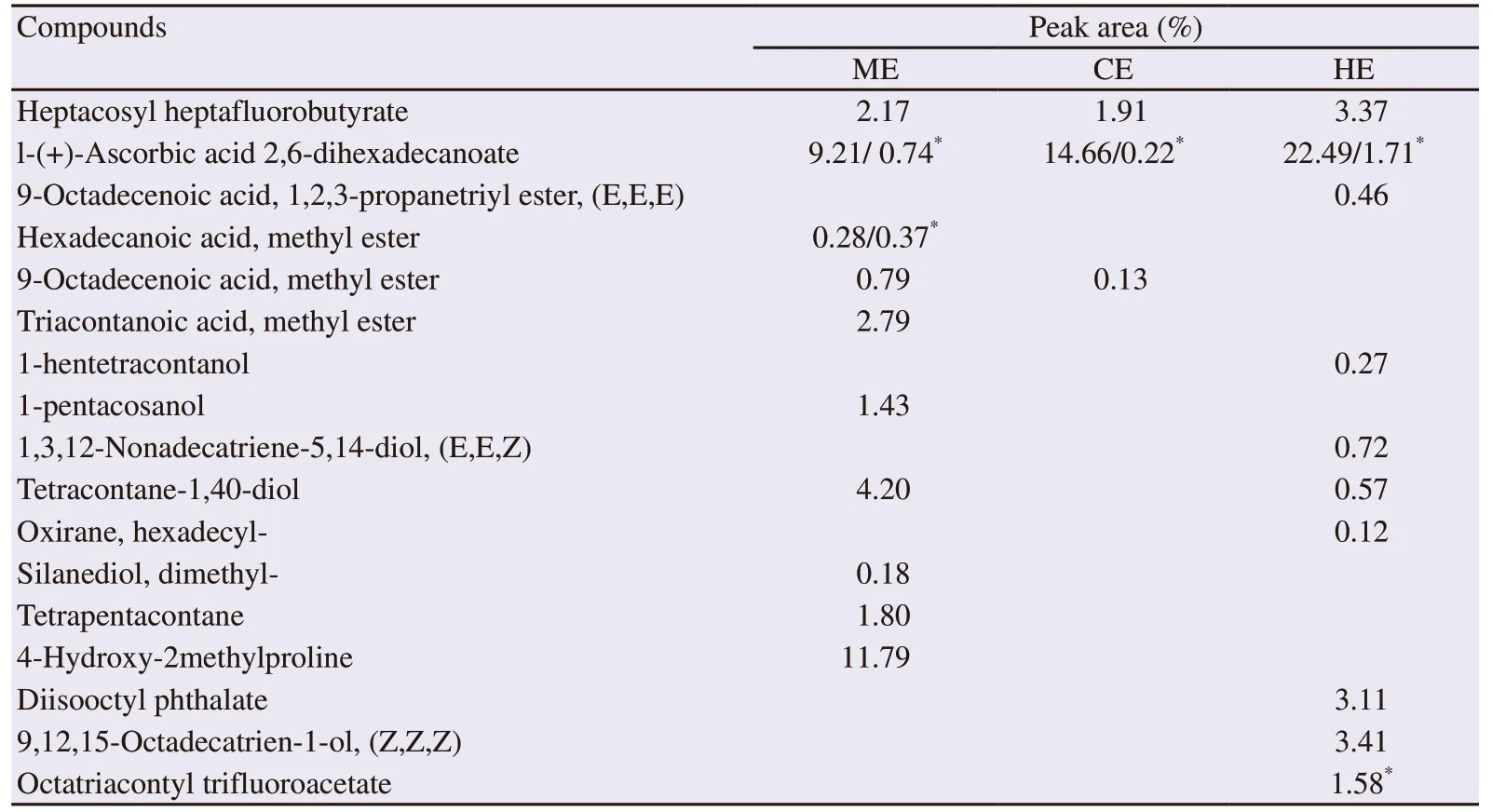
Table 5. Volatile bioactive phytocomponents identified in methanol, chloroform and hexane extracts of Caulerpa racemosa by GC-MS.
4. Discussion
Seaweed adaptative chemical response to the marine environment is subjected to spatial and temporal variations. In fact, the same species could vary the biosynthesis level of its metabolites and/or produce new ones. The present results demonstrated that the invasive green seaweed C. racemosa has relatively modified its metabolic profile as an adaptative response to “the Mediterranean biotope”.
Overall, compared to the native C. racemosa, the one collected from Mostaganem coast displayed some variations in metabolite concentrations. The total phenolics and flavonoid contents were higher than those found in the C. racemosa harvested from Veraval Coast in India[18], Malaysia[1]and Sumenep and Madura in Indonesia[27],but lower than the one collected from Takalar regency in Indonesia[28].Moreover, the preliminary phytochemical screening in the present study revealed the presence of several compounds such as flavonoids,tannins, coumarins, carbohydrates, steroids and alkaloids that were not reported in the chloroform extract of C. racemosa from Southeast coast of India[29]. This difference can be attributed to the climate and geographical conditions from where the alga was harvested.
The concentrations of total phenolic compounds, flavonoids, and tannins differed between the three extracts, which is related to the solvent polarity. Chloroform, whose polarity is intermediate between methanol and hexane, was the most effective solvent in total phenols extraction in the present study. In fact, the chloroform extract displayed the highest total phenolics and tannins content which suggests the non-polar properties of these components. On the other hand, the preliminary phytochemical analysis revealed the presence of more metabolites in chloroform extract than in hexane and methanol ones.As a result, the chloroform extract showed the highest efficiency at both scavenging DPPH and ABTS radicals and protecting against β-carotene bleaching. In general, higher total phenolic content results in a higher antioxidant capacity[21]; however, the phenol compounds are not the only ones responsible for antioxidant activity. Non-polar compounds like glycolipids, phospholipids, steroids, terpenes, fatty acids, carotenoids, and tocopherols contribute to DPPH scavenging ability[30]. Likewise, non-polar compounds present in both hexane and chloroform extracts are involved in the lowering β-carotene degradation rate. According to Chew et al.[31], β-carotene bleaching assay gives an indication of lipophilic compound level. On the other hand, it seems that tannins took part in radical scavenging ability. Indeed, an inverse correlation was found between tannin contents and IC50values for scavenging DPPH (r=-0.78, P<0.05) and ABTS (r=-0.96, P<0.001)radicals; whereas, no correlation was found for total polyphenol contents.
In addition to their relative antioxidant capacity, the different tested extracts displayed a differential antibacterial effect on the two Grampositive strains S. aureus (ATCC 25923) and B. cereus (ATCC 10876),and on the Gram-negative P. aeruginosa (ATCC 27853), with relatively better efficiency from the hexane extract. Non-polar extracts usually comprised non-polar compounds such as fatty acids and terpenoids,known for their antimicrobial properties[32]. The most effective saturated, mono-unsaturated, and polyunsaturated fatty acids against bacteria growth are those with chain lengths of C12, C16:1, and C18:2,respectively[32]. In addition to the presence of conventional fatty acids,dodecanoic acid, 3-hydroxy- (3-hydroxy-lauric acid) was identified by GC-MS in hexane extract. This hydroxy fatty acid, known for its antifungal activity[33], could contribute to the antibacterial activity of hexane extract.
The identified terpenoids, such as squalene, squalane, carvacrol,and β-cymene, are other secondary metabolites involved in inhibiting bacteria growth. Besides, triacontanoic acid, methyl ester[6],1-pentacosanol, and tetracontane-1,40-diol[33], were also reported to have antibacterial activity. Finally, several chemical functional groups in algae, such as polyphenols, peptides, polysaccharides, sterols,aromatic organic acids, alcohols, aldehydes, ketones, and halogenated furanones have been reported as bacterial inhibitors[13].
The present extracts seemed to be more effective than those from the red seaweeds Pterocladiella capillacea and Osmundaria obtusiloba which showed no antibacterial activity against the same used standard strains S. aureus and P. aeruginosa[30]. In the study of Nor Afifah et al.[34], no antibacterial activity was displayed by the chloroform,hexane or methanol extracts (100 mg/mL) of the green alga Halimeda discoidea on P. aeruginaosa (ATCC 27853); however, its chloroform extract was more effective on B. cereus (ATCC 10876). These same strains were also resistant to the methanolic extract of Ruta angustifolia at the 100 mg/mL in the study of Shuib et al.[35].However, the essential oils of Rhus coriaria[16]and Dysphania ambrosioides[36]were effective in inhibiting S. aureus (ATCC 25923) growth. Thymus kotschyanus water extract also inhibited the S. aureus (ATCC 25923) and even Escherichia coli (ATCC 25922) growth[37].
It appeared also that the present three extracts are more effective on the standard strains B. cereus, and P. aeruginosa than penicillin.The antibacterial efficiency variation of the seaweed extracts depends on the chemical composition of the extracts[10], seaweed species,environmental aspects and the tested strains[14].
Besides the antibacterial property, anti-inflammatory activity was attributed to some identified compounds such as 4-hydroxy-2-methylproline[38], 9-octadecenoic acid, methyl ester and hexadecanoic acid, methyl ester[39]. These two later compounds are also known for their cardiovascular protective property as well as l-(+)-ascorbic acid 2,6-dihexadecanoate[40] and some identified fatty acids and sterols.
Some volatile compounds were for the first time identified in C.racemosa. Among them, the phytosterol 24β epimer, the γ-sitosterol commonly called the clionasterol, which was identified in Caulerpa taxifolia and Caulerpa prolifera[41]and not in C. racemosa.Antioxidant[42], antidiabetic and anticancer activities[43]were attributed to this phytosterol. Squalane, 4-hydroxy-2methylproline,nonahexacontanoic acid and dihydroactinidiolide were among other metabolites that have not been mentioned before in the C. racemosa.
Other compounds, with interesting pharmacological properties, such as thymol, cis-13,16-docosadienoic acid (in hexane extract), DHA and arachidonic acid, methyl ester (in methanol extract), were identified but not cited because of their very weak peak areas (less than 0.1%).
C. racemosa harvested from the West Algerian coast has been identified as a rich source of bioactive compounds with nutritional and pharmacological interest. Some of the identified components are common to C. racemosa from the other geographical areas and some of them are new bioactive compounds not reported in previous studies about this species. This reflects the metabolic adaptation of this seaweed to their environmental conditions. Further work is underway which is aimed at investigation of the possible nutraceutical application of this green alga, especially on metabolic disorders.
Conflict of interest statement
We declare that there is no conflict of interest.
Acknowledgments
The authors are grateful to Dr Bouhidjra, B.B. and Dr Belbachir,N., from aquaculture and marine sciences department (Mostaganem University) for Caulerpa racemosa identification. We are also thankful to Dr Bouzouina M. from Agronomy department (Mostaganem University) for his support.
Authors’ contributions
LB designed the experiment and supervised all parts of the study.Both LB and MB contributed to the sample collection and extraction,phytochemical analysis, and antioxidant activity assessment. LB performed the antibacterial activity study. ACD and YB carried out the GC-MS analysis. Data analysis and manuscript drafting were performed by LB. All authors approved to the final version of the manuscript.
 Asian Pacific Journal of Tropical Biomedicine2020年2期
Asian Pacific Journal of Tropical Biomedicine2020年2期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- One-pot synthesis of silver nanocomposites from Achyranthes aspera: An eco-friendly larvicide against Aedes aegypti L.
- In vitro anti-inflammatory, anti-oxidant and in vivo anti-arthritic properties of stem bark extracts from Nauclea pobeguinii (Rubiaceae) in rats
- Micro RNA deregulation and cancer and medicinal plants as microRNA regulator
- Ethanol extracts of Hizikia fusiforme induce apoptosis in human prostate cancer PC3 cells via modulating a ROS-dependent pathway
