One-pot synthesis of silver nanocomposites from Achyranthes aspera: An eco-friendly larvicide against Aedes aegypti L.
Aarti Sharma, Pushplata Tripathi, Sarita Kumar✉
1Department of Life Sciences (SOS), Indira Gandhi National Open University, Maidan Garhi, New Delhi 110068, India 2Department of Zoology, Acharya Narendra Dev College, University of Delhi, KalkaJi, New Delhi 110019, India
ABSTRACT Objective: To formulate silver nanocomposites from Achyranthes aspera leaf extracts and evaluate its larvicidal activity against Aedes aegypti.Methods: The silver nanocomposites were synthesized from Achyranthes aspera leaf extracts. The process was optimized and traced through UV-visible and photon correlation spectroscopy.The larvicidal potential of silver nanocomposites of Achyranthes aspera leaf extracts was assessed against the early fourth instars of Aedes aegypti and three non-target organisms. Furthermore, the most effective and eco-safe nanocomposite was characterized by different biophysical techniques including scanning electron microscopy(SEM), energy dispersive X-ray (EDX) spectroscopy, transmission electron microscopy (TEM), X-ray diffraction (XRD) and Fourier transform-infrared spectroscopy (FT-IR).Results: The formulated silver nanocomposites exhibited efficient larvicidal efficacy against Aedes aegypti. Bioassay with silver nanocomposites formulated using different AgNO3 concentrations(3, 4, and 5 mM) revealed respective LC50 values of 37.570, 6.262 and 1.041 µg/mL; 5.819, 1.412 and 0.489 µg/mL; and 5.519, 1.302 and 0.267 µg/mL after 24, 48 and 72 h. The silver nanocomposites with 4 mM AgNO3 were selected for characterization. SEM and TEM analysis revealed spherical, poly-dispersed structure with varied diameters of 1-25 nm. The XRD analysis established the crystalline and face-centred-cubic structure of silver nanocomposites with the maximum peak at a 2θ value of 37.42°. The EDX pattern showed the presence of Ag, O and C in the nanocomposites in their order of weight%. The FT-IR displayed visibly distinct peaks in different ranges demonstrating the intricacy of silver nanocomposites. In addition, the lethal concentrations of silver nanocomposites of Achyranthes aspera leaf extracts against Aedes aegypti larvae were non-toxic to non-target organisms including Gambusia affinis, Daphnia magna and Moina macrocopa.Conclusions: Silver nanocomposites synthesized with leaf extract of Achyranthes aspera provide a cost-effective and eco-safe alternative to conventional insecticides, and can be utilized as a potent mosquito nano-larvicide.
KEYWORDS: Achyranthes aspera; Aedes aegypti; Larvicidal; Silver nanocomposites; SEM-TEM; EDX; XRD; FT-IR
1. Introduction
Aedes aegypti (Ae. aegypti)-borne diseases, such as dengue,Chikungunya and Zika, are on the rise at the global level since the last few years. These diseases have become a prime concern in urban and semi-urban regions of the tropical and sub-tropical areas of the world attracting the researchers’ attention to explore novel strategies of vector control. The endemism of dengue in almost 125 countries has not only risked the health of around 40% of the world’s population but has also costed the lives of a large number of the population[1]. An alarming 30× increase in dengue incidences since last 50 years has affected approximately 3.6 billion people across the globe; while three-quarters of the global incidences have occurred in Asian Pacific countries alone, which can be attributed to the weather conditions prevalent there.
In India, dengue fever is ubiquitous in almost every region andis endemic in 34 States/Union territories, with Lakshadweep being the only exception[2]. Ministry of Family and Health Welfare, India had recorded 129 166 dengue cases with 245 fatalities in 2016 which increased alarmingly to 188 401 incidences and 325 deaths in 2017[3]. The Ministry reported the maximum outbreak in West Bengal with 37 746 cases followed by Tamil Nadu and Kerala. In the same year, 67 769 Chikungunya cases were also reported in India which was highest among the last seven years[3].
The resistance of Ae. aegypti to various groups of pesticides,including organochlorines, organophosphorous compounds,carbamates and synthetic pyrethroids is already prevalent. As the mosquito control strategies were always primarily dependent on the insecticides-based intervention measures, the development of resistance to them causes a failure of the programmes that intended to control and prevent dengue in tropical countries[4,5]. Besides,continued and indiscriminate use of toxic chemicals has raised other major concerns; for instance, lethality to the non-targeted organisms,accumulation in the environment due to non-degradable nature causing major harm and bio-magnification in the food chain[6]. In the light of the above problems, a need has arisen to develop novel, ecosafe, biodegradable and target-specific formulations. The insecticides synthesized in form of nanocomposites have been recognized as a probable and suitable alternative to conventional insecticides against mosquitoes as these are reported to impose less ecological damage and can penetrate easily in the vector, though penetration may vary depending upon the particles size[7].
Nowadays, the metallic nanoparticles formulated with noble metals, such as gold, silver, platinum and titanium are extensively used in the nano-medicine. Especially, silver nanoparticles have been observed to have potential against fungi, bacteria, Plasmodium, and mosquito larvae[8]. In the current scenario, the major focus now has been to formulate nanocomposites using biological agents because of the dire necessity to develop eco-friendly control measures. It is suggested that the formulation of plants or microorganisms-mediated nanocomposites can enhance their biocompatibility. Thus, over the past many years, various organisms, such as algae, bacteria, viruses,fungi and plants, have been utilized for the production of simple,cost-effective, and environmentally-safe metallic nanoparticles[9,10].It is apparent that the utilization of environment-friendly and costeffective approach to formulate biologically synthesized silver nanocomposites without using any toxicants, high temperature,pressure, energy and uneconomical purification techniques makes them comparatively much more beneficial than those formulated with chemical and physical methods[8]. Among these, plant-mediated synthesis of nanoparticles is considered a rapid and simplest method where plants not only act as reducing but also as capping agent[10].
Current studies utilize a local weed, Achyranthes aspera (A. aspera)L. (Amaranthaceae) for the synthesis of novel nanoparticles in order to control the Aedes population. The plant, native to Asia and Africa, is widely naturalized all over India and can be seen growing on the roadsides, field borders and left plains. Commonly known as “devil’s horsewhip” or “prickly chaff flower” in English,it has various vernacular names, such as “Chirchita”, “Onga”,“Latjeera” or “Apamarga”, “Nayuruvi” in local languages and dialects[11]. Traditionally, the plant has been exploited for its various medicinal properties to treat asthma, cough, pneumonia and conjunctivitis and corneal abnormalities in ophthalmic cases,as well as as an antidote for the bites of poisonous snakes and reptiles. The Ayurvedic characteristics of A. aspera have been tapped to treat stomach pain, skin eruptions, piles and even boils[8,11].The presence of phytochemical constituents including betaine,ecdysterone, 6-pentatriacontanone, etc. in the plant makes it useful as an antiperiodic, diuretic, anti-asthmatic and anti-allergic agent[12].Hexane extracts prepared from A. aspera leaves and stems, and saponins isolated from the ethyl acetate extract of A. aspera leaves have been testified to exhibit larvicidal potential against the early fourth instars of Ae. aegypti and Culex quinquefasciatus[13,14]. In addition, extracts prepared from the leaves, seeds and flowers have been found to possess effective biological activity viz. anti-bacterial,anti-fungal, anti-mosquito, anti-fertility and anti-cancerous[15,16].
Keeping all the above points in view, we utilized A. aspera leaf extract to formulate silver nanocomposites (AgNCs) and assessed their toxic potential against Ae. aegypti larvae. The formation of nanocomposites was confirmed in the nano-mixture by using UVvisible and photon correlation spectroscopy (PCS) techniques and their characterization was carried out by various biophysical techniques including scanning electron microscopy (SEM), energy dispersive X-ray (EDX) spectroscopy, transmission electron microscopy (TEM), X-ray diffraction (XRD) and Fourier transforminfrared spectroscopy (FT-IR). The AgNCs were also evaluated against certain non-target organisms to assess the safe use of these compounds in the fields.
2. Materials and methods
2.1. Rearing of Ae. aegypti mosquitoes
The early fourth instars of the dengue vector, Ae. aegypti employed in the current investigations were obtained from National Institute of Malaria Research, Dwarka, New Delhi, India. The mosquitoes were reared and maintained under a controlled temperature of (28 ± 1) ℃,the relative humidity of (80 ± 5)% and photoperiod of 14 L:10 D as per the procedure adopted by Sharma et al.[16].
2.2. Plant collection
Fresh, young and disease-free leaves of A. aspera were gathered from the local areas of New Delhi, India. The plant was taxonomically identified by Professor Arun K. Pandey, Department of Botany, University of Delhi, India. The voucher sample carrying accession no. 14376 has been recorded in the Herbarium of University of Delhi, India.
2.3. Preparation of A. aspera leaf extract
The leaves of A. aspera were cleaned cautiously under running tap water followed by washing with double-distilled water. The leaves which appeared diseased and infected were discarded. Rest of the leaves were chopped into small pieces, weighed on an electrical balance (Shimadzu, BL-220H) and ground using an electric blender(Philips, HL-1606). The leaf extract was prepared taking a total of 10 g of ground leaves in a 250 mL glass beaker filled with 100 mL double distilled water. The mixed contents were heated for about 15-20 min at 60 ℃ and kept undisturbed for 2-3 h[16]. The particulates of the obtained leaf broth were cleared by sieving through a piece of muslin cloth and Whatman No. 1 filter paper. The clear aqueous extract obtained was stored at 4 ℃ in amber-coloured culture bottles for further use.
2.4. Formulation and optimization of AgNCs
AgNCs of A. aspera leaf extract were synthesized by mixing extract and silver nitrate (AgNO3) solution in different ratios. Optimization of the synthetic process was done by either varying the volume of extract or varying the concentration of silver nitrate, where other variables were kept constant.
In the first step, different volumes of leaf extract (0.8 mL to 2.0 mL) were added to 10 mL of 3 mM silver nitrate. The mixtures were incubated at 37 ℃ for 24 h and monitored intermittently to assess the bio-reduction of Ag+. On the basis of results, 1.5 mL of A. aspera extract was selected as the preferred volume for the nanocomposite synthesis.
Further optimization of silver nanocomposite synthesis process was carried out by preparing mixtures with a selected volume of A. aspera leaf extract (1.5 mL) and 10 mL of different concentrations of silver nitrate (1, 2, 3, 4 and 5 mM), and observed intermittently.
In both cases, the change in colour of nano-mixture from pale yellow to dark reddish-brown served as a primary indicator for the synthesis of nanocomposites in the solution.
2.5. Confirmatory analysis of the synthesis of AgNCs
The optimal synthesis of nanocomposites was confirmed by UVvisible spectral analysis and PCS.
2.5.1. UV-visible spectral analysis
During the process of AgNCs synthesis, completion of bioreduction of Ag+was tracked through intensity measured by UVvisible spectrophotometer (UV-1800 Shimadzu, Japan). Periodically,1 mL aliquots of samples were collected and diluted with 2 mL of double-distilled water. The mixtures were scanned at 200 to 700 nm wavelength with a resolution of 1 nm. The silver nitrate solution was used as a control.
2.5.2. PCS
The particle size and average distribution of AgNCs of A. aspera were analyzed on the basis of intensity, number and volume through dynamic light scattering (Zeta sizer, Malvern Instruments Ltd., USA)using PCS.
2.6. Assessment of larvicidal activities of AgNCs of A. aspera against Ae. aegypti
The AgNCs of A. aspera synthesized with different concentrations(3-5 mM) of silver nitrate were assessed for their larvicidal potential against the early fourth instars of Ae. aegypti based on the standard WHO protocol[17]with minor alterations. The larvicidal activities of AgNCs (2 µg/mL-50 µg/mL) were evaluated on separate groups of 20 active Ae. aegypti larvae in 199 mL of dechlorinated water.Each experiment was run in five replicates conducted concurrently.Control sets were exposed to the aqueous solution of silver nitrate alone. Larval mortality was recorded after every 24 h till 3 days of exposure.
2.7. Assessment of biotoxicity of AgNCs of A. aspera against non-target organisms
The biotoxicity of formulated AgNCs was evaluated against three non-target organisms sharing the niche with mosquito larvae:Gambusia affinis (G. affinis), Daphnia magna (D. magna) and Moina macrocopa (M. macrocopa). The bioassay was conducted as per the methodology suggested by Haldar et al.[18]. Healthy and vigorous G. affinis (0.494 ± 0.020) g were collected from freshwater ponds located at Department of Botany; while D. magna and M. macrocopa were procured from the established culture in Th!nk Laboratory,Acharya Narendra Dev College, University of Delhi, India. All the organisms were acclimatized for 3-4 d in the laboratory under controlled environmental conditions during which G. affinis was fed with commercial food pellets whereas D. magna and M. macrocopa were provided with milk drops (1 drop in 250 mL).
The bioassay was carried out in two phases and the LC50value obtained against the early fourth instars of Ae. aegypti after 24 h of exposure was selected as the exposure concentration.
2.7.1. Assessment of biotoxicity on non-target organisms
Batches of ten healthy non-target organisms were placed in separate containers filled with 500 mL dechlorinated water-AgNCs mixture (1 mL of AgNCs solution at LC50dosage against Ae. aegypti added to 499 mL of water), whereas control set-ups were exposed to dechlorinated tap water. Five replicates were run for each assay.Mortality and other abnormalities, such as sluggishness and reduced swimming activity, were observed after 24 h and 48 h of exposure.The organisms were also scrutinized for another 5 d to estimate the delayed post-treatment effects on their longevity and motility if any.
2.7.2. Assessment of biotoxicity on mosquito larvae and nontarget organisms
The non-target bioassays were also performed, in which nontarget organisms (in batches of 10) and 25 early fourth instars of Ae.aegypti were exposed together to AgNCs-water mixture as described above. Mortalities and abnormalities developed were recorded and analysed.
2.8. Characterization of AgNCs of A. aspera leaf extracts
The nanocomposites of A. aspera leaf extract exhibiting the maximum larvicidal efficacy against Ae. aegypti were characterized using different biophysical techniques.
2.8.1. SEM
The aqueous solution of AgNCs of A. aspera was centrifuged repetitively. Thin films of the solution were prepared and kept under a mercury lamp for 5 min for drying. The morphological features of the composites were noted using high-resolution scanning electron microscopy (Zeiss Model: V5.05) (Sigma) at an accelerating voltage of 20 KeV.
2.8.2. EDX spectroscopy
The qualitative and quantitative EDX analysis of the elements probably involved in the synthesis of nanocomposites was carried out at accelerating voltage of HV: 20.0 kV (Bruker instrument).
2.8.3. TEM
TEM (FEI Tecnai G230 S-TWIN) was employed to observe the structure of AgNCs at an accelerating voltage of 300 kV. A droplet of nanocomposites was placed on carbon-coated copper grid parafilm for 10-20 min and observed carefully.
2.8.4. FT-IR analysis
The nanocomposites were also analyzed by FT-IR spectroscopy(Bruker Optik, Verter 70V) to identify the functional groups present on the surface area of nanocomposites which probably play a major role in the reduction and stabilization of silver ions.
2.8.5. XRD analysis
XRD spectrum of the synthesized AgNCs was documented using Rigaku Smart Lab®X-ray Diffractometer to confirm their crystalline nature.
2.9. Data analysis
The lethal concentration value (LC50) of A. aspera AgNCs against Ae. aegypti larvae was calculated by probit analysis using SPSS,Statistical Software (Version: 19.0). Other statistical parameters including standard deviation, 95% confidential limits, chi-square and regression coefficient were also recorded to compute the significance and variation between test samples.
3. Results
3.1. Spectroscopic validation of AgNCs synthesis
The changes in the colour of extract-silver nitrate mixture indicated the synthesis of nanocomposites. The mixtures containing 3, 4 and 5 mM of silver nitrate exhibited a significant colour change from pale yellow to reddish-brown and finally dark reddish-brown, while no such changes were noticed on adding 1 mM and 2 mM of silver nitrate (Figure 1A).
The synthesis of AgNCs formulated with varying volumes of A.aspera leaf extract added to 10 mL of 3 mM silver nitrate (the lowest effective concentration) was spectroscopically monitored at 200-700 nm (Figure 1, Table 1). The highest and narrow absorbance peak was obtained at 417 nm (Figure 1B) with 1.5 A. aspera leaf extract:10 silver nitrate volume of the reaction mixture. The band of surface plasmon resonance shifted to lower wavelengths with higher volumes of extract indicating the synthesis of smaller-sized nanocomposites.
In optimization of the silver nitrate concentration (1-5 mM) in the reaction mixture, a wider and more intense absorption band was obtained with increased concentration of AgNO3revealing a slight red shift in the peak (Figure 1C). The biosynthesis of AgNCs at 3 mM silver nitrate could be traced at 418 nm, while at 4 mM and 5 mM, it was held at 403 nm (Figure 1C, Table 1). However, stable nanocomposites could not be synthesized with 1 mM and 2 mM of silver nitrate as apparent by their absorption spectra.
3.2. Validation of AgNCs synthesis via PCS analysis
PCS analysis of nanocomposites synthesized with 3, 4 and 5 mM silver nitrate showed the average particle size distribution in the range of 30.41-61.02 nm (Table 2, Figure 2); whereas, the polydispersity index was found to be under the desired range of index i.e. 0.05 to 0.70 indicating the optimal size distribution of nanocomposites. In addition, the high photon count rates in each nanocomposite solution indicated the good sample quality with optimum signal for analysis. The synthesis of nanocomposites under ideal conditions was further confirmed by ideal signal to noise ratio through the intercept output of dynamic light scattering analysis (0.9 to 1.0) and high % intensity of 98.0%, 98.5% and 100.0%.
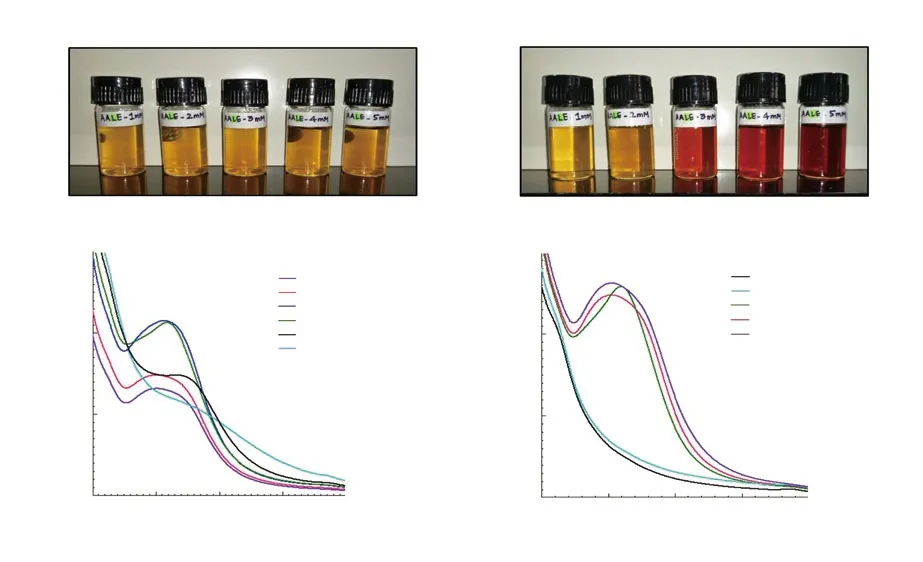
Figure 1. A) Colour change observed during synthesis of silver nanocomposites at constant volume of Achyranthes aspera leaf extracts (1.5 mL) with different concentrations of silver nitrate (1-5 mM); B) UV-Vis spectra of silver nanocomposites formed with different volumes of Achyranthes aspera leaf extract added to 10 mL of silver nitrate (3 mM); C) UV-Vis spectra of silver nanocomposites formed with selected volume of leaf extract (1.5 mL) added to different concentrations of silver nitrate (1-5 mM).
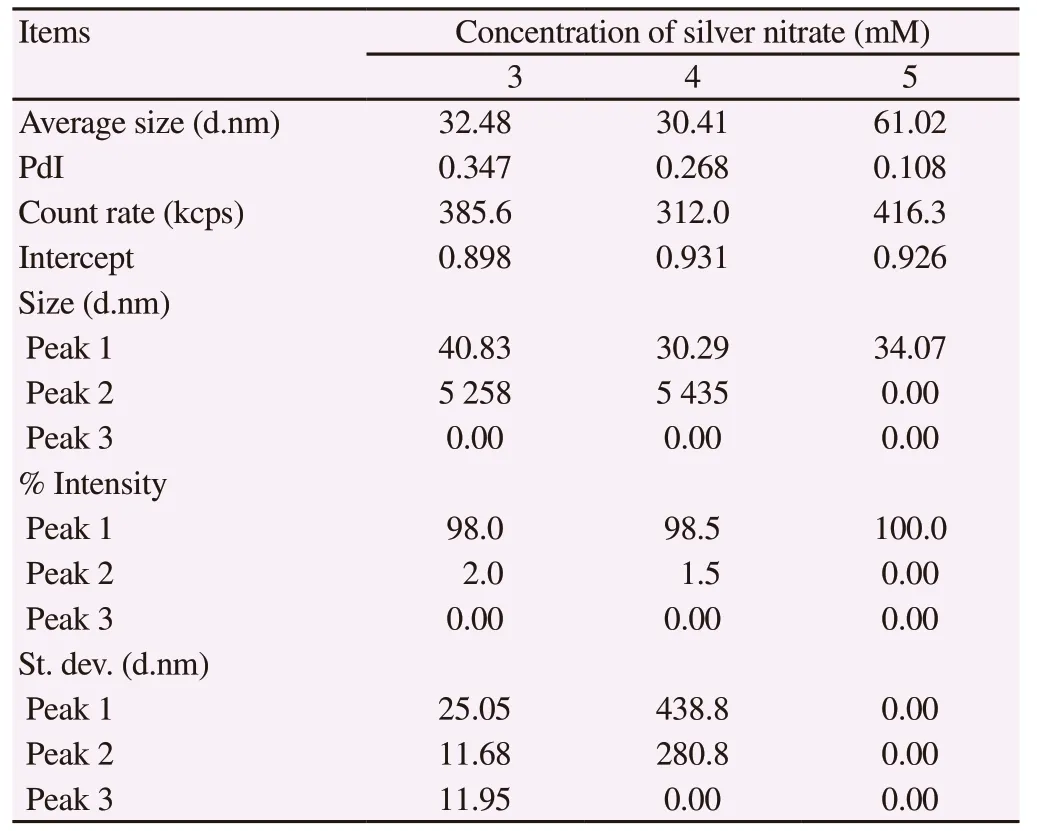
Table 2. Photon correlation spectroscopy analysis of average particle size distribution of Achyranthes aspera leaf extract-mediated silver nanocomposites at different concentrations of silver nitrate.
3.3. Larvicidal activities of AgNCs of A. aspera against Ae.aegypti
The AgNCs synthesized from A. aspera leaf extract exhibited effective toxic potential against early fourth instars of Ae. aegypti. The efficacy augmented with increasing AgNO3concentration (P < 0.05). The nanocomposites formed with 4 and 5 mM silver nitrate caused almost similar larval mortality (P > 0.05). The respective LC50values obtained were 37.570 µg/mL (3 mM AgNCs), 5.819 µg/mL (4 mM AgNCs)and 5.519 µg/mL (5 mM AgNCs) after 24 h of exposure (Table 3). No larval mortality was observed in the control assays.
Nanocomposites showed prolonged effect and resulted in higher larval mortality with increased duration of exposure. The toxicity assays with 4 mM AgNCs reduced the LC50value by 75.73% on prolonging the exposure for another 24 h and by 91.59% when the larval exposure was prolonged for 48 h. Based on the optimization results and non-significantly different larvicidal activity of 4 mM AgNCs and 5 mM AgNCs, further investigations were held with silver nanocomposites synthesized at 4 mM of silver nitrate to keep the silver content at the minimal possible concentration.
3.4. Biotoxicity of AgNCs against non-target organisms
The evaluation of biotoxicity of 4 mM AgNCs against three nontarget organisms, G. affinis, D. magna and M. macrocopa alone showed their safety against non-target organisms at concentrations toxic to the mosquito larvae. Similarly, the toxicity assessment of AgNCs against non-targets together with early fourth instar larvae of Ae. aegypti showed that nanocomposites did not cause any significant toxicity to non-targets. However, the exposure caused appreciable mortality of Ae. aegypti larvae, 48% to 51% on exposure at LC50value (5.819 µg/mL). In addition, prolonged exposure of nontargets for next 5 d did not inflict any toxicity or alterations in their longevity and swimming activity.
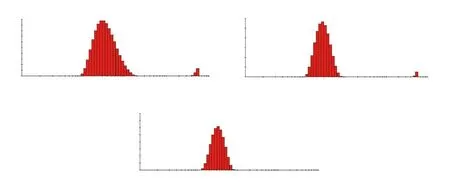
Figure 2. Photon correlation spectroscopy of the size distribution of Achyranthes aspera leaf extract-mediated silver nanocomposites at (A) 3 mM, (B) 4 mM and (C) 5 mM silver nitrate.
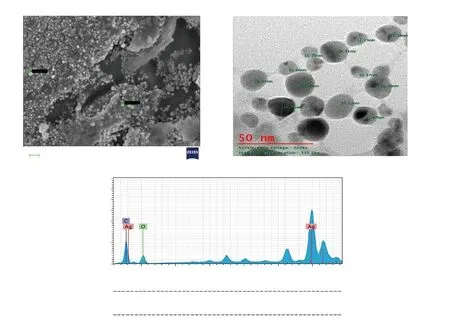
Figure 3. Characterization of silver nanocomposites synthesized from Achyranthes aspera leaf extract and 4 mM AgNO3 by A) scanning electron microscopy,B) transmission electron microscopy and C) energy-dispersive X-ray spectroscopy. EL = Element; AN = Atomic number; O: oxygen; C: carbon; Series =characteristic X-ray lines; unn. C [wt. %] = un-normalised concentration in percent weight of the element; norm. C [wt. %] = normalised concentration in percent weight of the element; Atom. C [at. %] = the atomic weight percent; Error, (1 Sigma) [wt. %] = error in the weight percent concentration at 1 sigma level.
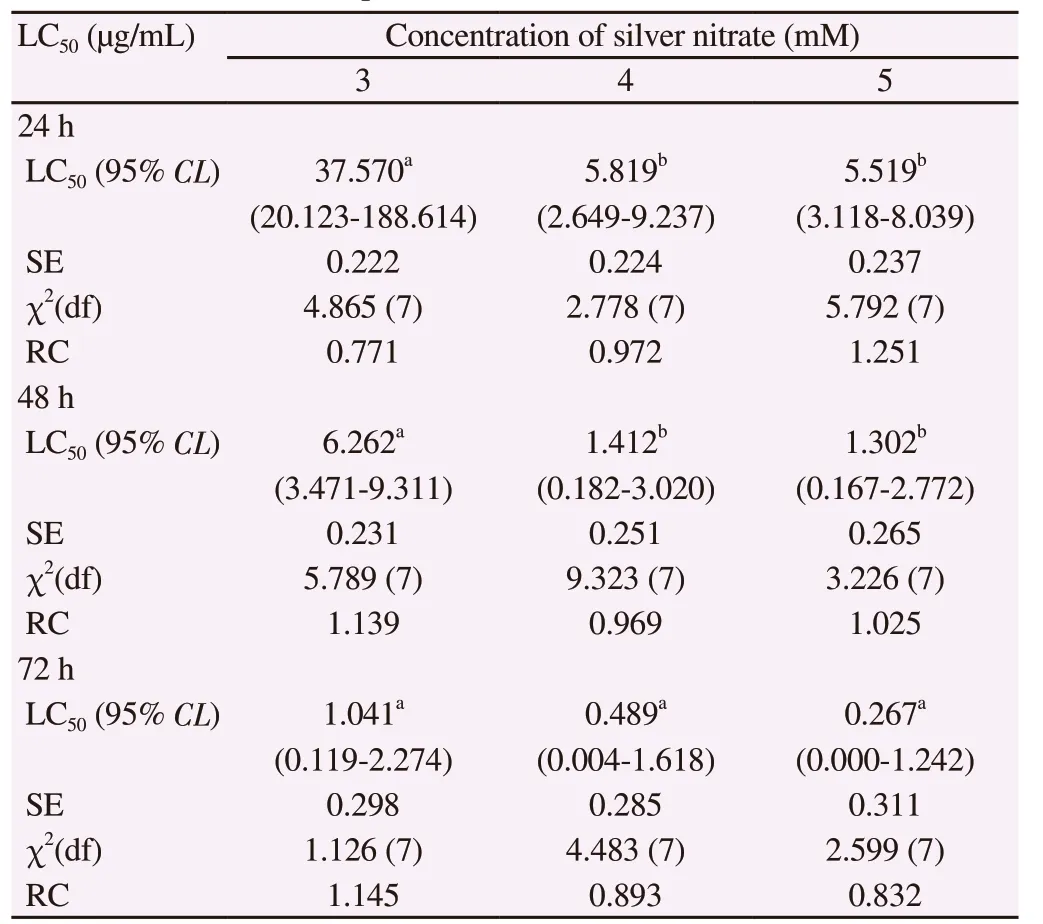
Table 3. Larvicidal activity of the silver nanocomposites synthesized from leaf extract of Achyranthes aspera against early fourth instars of Aedes aegypti after 24, 48 and 72 h of exposure.
3.5. Characterization of AgNCs
The high-resolution SEM revealed that nanocomposites were spherical with an average diameter of 25 nm and mostly in an aggregated form (Figure 3A). TEM further established the spherical shape of A. aspera-mediated AgNCs with an average size of 16 nm and uniformity in distribution, though the composites were observed as non-agglomeration in the solution (Figure 3B).
The elemental composition of AgNCs documented by EDX patternevidenced the presence of Ag, O and C in their order of weight%.The highest weight% (62.14%) indicated the strong signal of the silver element in AgNCs of A. aspera and the reduction of silver ions to the element (Figure 3C). The Bragg reflection peaks at 2θ degree: 27.22°, 31.74°, 37.42°, 45.75°, 54.23°, 56.89° and 76.69°corresponding to the respective lattice planes (111), (200), (211),(220), (311), (222) and (421) were noticed by XRD analysis; the most intense peak was detected at (211) (Figure 4A). These results indicated the crystalline and face-centered-cubic structure of composites.
The FT-IR spectrum of synthesized AgNCs (Figure 4B) showed 10 main peaks at 3 019.41, 2 412.27, 2 316.77, 1 608.70, 1 501.55,1 305.12, 1 203.42, 1 144.41, 935.56 and 620.34 cm-1. The peak at 3 019.41 cm-1was probably due to the average intensity of alkene=C-H with a weak and broad stretch of O-H group. The peaks at 2 412.27 cm-1could be assigned to weak vibrations of S-H thiols and the sharp peak of P-H phosphine stretching, while the peak at 2 316.77 cm-1probably signified strong absorption band of Si-H silane functional group. Furthermore, peak at 1 608.70 cm-1possibly corresponded to strong vibrations of NH2while the peak at 1 501.55 cm-1could be linked to the presence of medium N-H amide band.The spectrum also revealed a peak at 1 305.12 cm-1, which may be due to strong C-F thiol and sulfone S=O stretching, whereas a peak at 1 203.42 cm-1could be attributed to strong P=O and aromatic N-O stretching. The peak obtained at 1 144.41 cm-1may possibly be accredited to a strong vibration of P=O and S=O stretching. In addition, the peak observed at 935.56 cm-1could be attributed to strong bending vibrations of P-OR esters and N-O, whereas the peak identified at 620.34 cm-1could correspond to alkyl halides like C-Cl and C-Br.
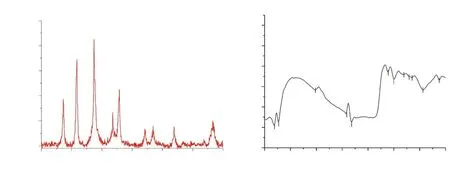
Figure 4. Spectrum pattern of silver nanocomposites synthesized from Achyranthes aspera leaf extract by (A) X-ray diffraction and (B) Fourier transforminfrared spectroscopy.
4. Discussion
Nanoparticles synthesis has attracted much attention due to an increasing need to develop environment-friendly approaches for mosquito control. Employment of chemical insecticides as control measures of mosquitoes has led to the development of insecticide resistance in mosquitoes, making their control difficult. Because of the toxicity of conventional insecticides to our environment and nontarget population, the researchers are exploring their replacement.Consequently, plant-mediated AgNCs are being explored as mosquito larvicides due to their cost-efficacy, eco-friendliness and easy synthesis without using any toxicant, and maintaining difficult physical conditions during synthesis[8].
The choice of the botanical agent for the synthesis of nanocomposites is the major and determinant factor for the kind of formulations,biophysical characteristics and toxicity potential[19]. The synthesis of AgNPs has been attempted with various plants’ extracts which showed that nanocomposites formulated with bioactive phytochemical constituents impart enhanced toxicity[20]. These bioactive phytoconstituents are often observed to be effective against Aedes, Culex and Anopheles larvae at quite low levels of concentration and thus, can be utilized for the mosquitocidal nano-formulations with low mammalian toxicity[21].
A plant extract used in the green synthesis of nanoparticles can act both as reducing as well as capping agent and biophysical parameters of nanoparticles can be controlled depending upon its selection of botanical agent[19]. The leaf extracts of various plants including A. aspera, Azadirachta indica, Alternanthera sessilis,Ocimum sanctum, Argemone mexicana, Annona squamosa, Allmania nadiflora, Bryophyllum pinnatum, Bocopa monnieri, Breynia retusa,Eruca sativa, Cochlospermum religiosum, Cajanus cajan, Catharanthus rosesus, Ocimum basilicum, Impatiens balsamina, Spinacia oleracea,Ricinus communis, and Datura metel have been investigated for the synthesis of AgNPs[22]. The AgNPs synthesized from A. aspera leaves[8,12], Achyranthes bidentata[23], Carissa carandas and Malva sylvestris[24,25], Chomelia asiatica[26]have been assayed against Ae. aegypti, Anopheles stephensi, and Culex quinquefasciatus. The reports showed appreciable larvicidal effects of these formulations and suggested their use as low-cost, eco-safe and promising agents against dengue, malarial and filariasis vectors. Apart from their mosquitocidal properties, AgNPs derived from the leaves of A.aspera have also been reported to impose cytotoxic effects on different bacterial strains (Pseudomonas aeruginosa, Escherichia coli,Staphylococcus aureus) and fungus (Candida albicans)[11].
The current study explores the possible use of A. aspera-mediated nanocomposites as a control agent of Ae. aegypti. Our earlier studies have revealed the larvicidal activity of hexane leaf extracts of A.aspera with LC50value of 83 µg/mL[14]. The formulation of A. aspera leaf extract in the form of nanocomposites can form a bioinsecticide with higher efficacy against dengue vector at lower dosages but safe to humans, environment, and non-targets.
It has been reported that utilization of aqueous plant extracts for the nanocomposite synthesis instead of plants extracted in organic solvents is an eco-friendlier and cost-effective biosynthetic process[27]. Hence, the current study synthesized nanocomposites from aqueous leaf extracts of A. aspera. The synthesis process was optimized and the toxicity assessment of nanocomposites was conducted against Ae. aegypti larvae and non-target organisms.Effective nanocomposites were characterized by different biophysical techniques.
The synthesis of nanocomposites in our study was indicated by a conspicuous change in the colour of reaction mixture, the colour transforming from pale yellow to yellowish-brown and then to dark brown within 30-45 min. The appearance of similar colour during the formulation of silver nanoparticles has been reported by Mathur[28]. The change in colour of the colloidal solution of AgNCs can be attributed to the excitation of surface plasmon vibration, a characteristic property of metallic nanocomposites directly related to the AgNO3concentration[8]. The appearance of a narrow, most conspicuous and the highest peak of surface plasmon resonance at 417 nm in the UV-visible absorption spectra of AgNCs of A. aspera leaf extract signified the maximum reduction of the silver nitrate at this wavelength. In accordance with our results, synthesis of AgNCs from A. aspera leaf extract at similar wavelengths of 417, 419 and 424 nm has been reported by Amaladhas et al.[11], Gude et al.[12]and Hariprasad et al.[22], respectively. In contrast, Elumalai et al.[8]obtained the absorption spectrum of nanocomposites synthesized from A. aspera leaf extract at a much higher wavelength of 452 nm. Nevertheless, the synthesis of AgNCs from the leaf extract of another species of Achyranthes, Achyranthes bidentata was reported at a comparable wavelength of 420 nm[23].
Our results also depicted that the intensity and breadth of absorption bands increased with increased concentration of AgNO3resulting in a slight red shift. Reports have shown that the bands of surface plasmon resonance are influenced by size,shape, composition, morphology and dielectric environment of the synthesized AgNCs[24]. It seems that widening peak may be because of slow reduction rates causing the synthesis of large-sized and polydispersed nanoparticles[29]. Earlier, Gude et al.[12]had advocated a positive correlation between percent formation of AgNCs and the intensity and breadth of absorption bands.
The larvicidal assay of early fourth instars of Ae. aegypti with AgNCs from A. aspera leaf extract showed significant efficacy of nanocomposites which increased with AgNO3concentration and duration of exposure. Our results are in accordance with that of Elumalai et al.[8]who evaluated silver nanocomposites of A. aspera against Ae. aegypti larvae and found them effective; though the LC50dosage (3.68 mg/mL) obtained by them was approximately 600 times higher than that observed in the present study. It may be attributed to the geographical variation in plants leading to a difference in composition of phyto-components of leaves which might be responsible for causing larval mortality. Earlier, Usha et al.[23]exposed Ae. aegypti larval population to silver nanoparticles synthesized from leaves of Achyranthes bidentata and observed 100%mortality on exposure to 40 mg/L dosage and above; while obtained only 20% larval mortality on exposure to 30 mg/L AgNPs.
AgNPs synthesized from different plant extracts have been assessed against different mosquito species with varied results. The AgNPs synthesized from Leucas aspera leaf extracts showed 8.563 mg/L LC50against fourth instar larvae of Ae. aegypti[30]. The toxicity of Nicandra physalodes-mediated AgNPs was most effective against Anopheles stephensi with LC50of 12.39 µg/mL, followed by Ae.aegypti with LC50of 13.61 µg/mL and Culex quinquefasciatus with LC50of 14.79 µg/mL[31]. Likewise, Hymenodictyon orixense silver nanocrystals possessed efficient larvicidal potential against Anopheles subpictus, Aedes albopictus and Culex tritaeniorhynchus with respective LC50values of 17.10 mg/mL, 18.74 mg/mL and 20.08 mg/mL[32].
The AgNCs synthesized from A. aspera leaf extract did not inflict any lethal effects on the non-target organisms at the concentration found fatal to the early fourth instars of Ae. aegypti. These results are in agreement with findings of Govindarajan and Benelli[33]who revealed the negligible toxicity of AgNCs formulated from Barleria cristata extract against G. affinis, Diplonychus indicus and Anisops bouvieri with LC50values ranging from 633.26 µg/mL to 8 595.89µg/mL. Likewise, Carissa carandas-mediated AgNPs exhibited little toxicity against these non-targets with LC50values ranging from 1 097.87 µg/mL to 17 249.89 µg/mL[24]. Similarly, Malva sylvestrismediated AgNPs were also found safe against G. affinis[25].
The biophysical characterization of AgNCs formulated in the present study revealed their spherical shape, polydisperse nature and average diameter of 1-25 nm. In agreement with our findings,AgNCs from A. aspera leaf extracts were found spherical with size ranging from 7 nm to 14 nm[8]and 12.82 nm[11]. Similar spherical shape, polydisperse nature and an average diameter ranging from 22.4 nm to 42.9 nm of AgNCs synthesized from A. aspera leaf extract have been recorded by Gude et al.[12]. Likewise, SEM analysis of AgNCs formulated using Emblica officinalis evidenced their spherical shape with a diameter ranging from 7.5 nm to 25 nm, averaging 16.8 nm[34]. Comparable spherical-shaped, stable and polydispersed AgNPs with an average size of 20-100 nm were formulated using Annona squamosa leaf extract[35]. Vignesh et al.[36]suggested that the stability of nanocomposites is attributed by the surface coating by a thin layer of the biomolecules.
It has also been suggested that the shape of the metallic nanoparticles markedly varies according to their optical properties and elemental composition which can be examined by EDX technique[37,38]. The EDX pattern of AgNCs synthesized in the present study showed the presence of Ag, O and C in their order of %weight. Similar findings were reported by Elumalai et al.[8]who also recorded high %weight of silver and weak signals of carbon and oxygen in the EDX spectrum of nanoparticles synthesized from A. aspera leaf extract.They proposed that these signals might have arisen from the surface biomolecules of the nanocomposites. However, Govindarajan et al.[19]suggested that the presence of carbon in the EDX pattern of nanoparticles may be possibly due to the carbon tape used in mounting the nanomaterial.
The XRD analysis of the AgNCs of A. aspera leaf extract in our investigations revealed seven main peaks of Bragg reflections at different lattice planes, suggesting crystalline nature and facecentred-cubic structure of nanocomposites; while FT-IR analysis showed a total of 10 peaks, stretches and vibrations. The similar crystalline structure of AgNCs formed with A. aspera leaf extract was also shown by Hariprasad et al.[22]and Elumalai et al.[8]. The diverse peaks in FT-IR spectrum might have originated due to the existence of different phytochemicals in the leaf extract of A. aspera which may play a significant role as a reducing and stabilizing agent in the fabrication of AgNCs[39,40]. This is supported by the presence of diverse organic molecules: alkenes, alkanes, amines, aromatic amines, thiol, phenols, and halo compounds, largely donated by flavonoids, carbohydrates and proteins in the FT-IR spectrum.Among these, the carbonyl group might have been contributed from amino acid residues and proteins which may have “capped”the AgNCs enhancing their ability to bind metal[41]. It was also advocated that proteins present in the plant extract could cover the metal nanocomposites to prevent their aggregation, thereby stabilizing the medium[41].
Our investigations showed the appreciable larvicidal efficacy of AgNCs synthesized from aqueous leaf extract of A. aspera against early fourth instars of Ae. aegypti, much higher in comparison to the lethality caused by crude hexane leaf extract[14]. The possible mechanism(s) behind the higher potential of AgNCs of A. aspera leaf extract than the extract alone may be credited to the interaction between extracellular lipoprotein matrix and nanocomposites which probably attracted the positive silver ions and augmented the penetrability of the cell membrane[24]. Probable binding of AgNPs and sulphur from proteins or any other compounds or phosphorous from DNA has also been suggested[42]. It may decrease adenosine triphosphate synthesis and reduce ion exchange which can adversely affect membrane penetrability in due course causing denaturation of the cellular enzymes and disruptive function of organelles and eventually leading to cell death. However, the mode of entry of AgNCs within the larval body of Ae. aegypti, the target site, the path of NCs to reach the target site and mechanism of larvicidal action require a more comprehensive study.
In conclusion, Ae. aegypti is the prime vector responsible for the spread of several globally concerned diseases. Use of harmful chemical for mosquito control has raised alarm to look for alternate eco-friendly strategy. The present study explores an effective and alternative strategy for dengue vector control employing AgNCs synthesized biologically from A. aspera leaf extract. The A. aspera leaf extract-mediated AgNCs showed excellent larvicidal potential against Ae. aegypti larvae and was found safe against non-target organisms. Characterization of nanocomposites revealed their spherical shape, crystalline nature, poly-dispersity, stability over time, face-centred cubic geometry and an average size ranging from 1 to 25 nm. The research highlighted that A. aspera could be used as an excellent bio-reductant for the large-scale green synthesis of AgNCs. A. aspera-mediated AgNCs can be employed as an eco-safe and novel mosquito nano-larvicide.
Conflict of interest statement
We declare that there is no conflict of interest.
Funding
This research was partly supported by the research grant from University Grant Commission, New Delhi (Award No.53583) and partly from research contingency from Acharya Narendra Dev College, New Delhi.
Authors’ contributions
AS conceived the idea, conducted the experiments and wrote the manuscript. AS and SK designed the experiments and sourced for funds. PT and SK supervised and guided the experiments. AS analysed the results and SK helped in the statistical analysis. All authors were involved in the finalization of manuscript.
 Asian Pacific Journal of Tropical Biomedicine2020年2期
Asian Pacific Journal of Tropical Biomedicine2020年2期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- In vitro anti-inflammatory, anti-oxidant and in vivo anti-arthritic properties of stem bark extracts from Nauclea pobeguinii (Rubiaceae) in rats
- Micro RNA deregulation and cancer and medicinal plants as microRNA regulator
- Ethanol extracts of Hizikia fusiforme induce apoptosis in human prostate cancer PC3 cells via modulating a ROS-dependent pathway
- Antioxidant and antibacterial activities and identification of bioactive compounds of various extracts of Caulerpa racemosa from Algerian coast
