Lycium barbarum polysaccharides protects retinal ganglion cells against oxidative stress injury
Lian Liu, Xiao-Yuan Sha, Yi-Ning Wu, Meng-Ting Chen, Jing-Xiang Zhong,
1 Department of Ophthalmology, Affiliated First Hospital of Jinan University, Guangzhou, Guangdong Province, China
2 Department of Ophthalmology, Guangdong Women and Children Hospital, Guangzhou, Guangdong Province, China
Abstract The accumulation of excessive reactive oxygen species can exacerbate any injury of retinal tissue because free radicals can trigger lipid peroxidation, protein damage and DNA fragmentation. Increased oxidative stress is associated with the common pathological process of many eye diseases, such as glaucoma, diabetic retinopathy and ischemic optic neuropathy. Many studies have demonstrated that Lycium barbarum polysaccharides (LBP) protects against oxidative injury in numerous cells and tissues. For the model of hypoxia we used cultured retinal ganglion cells and induced hypoxia by incubating with 200 μM cobalt chloride (CoCl2) for 24 hours. To investigate the protective effect of LBP and its mechanism of action against oxidative stress injury, the retinal tissue was pretreated with 0.5 mg/mL LBP for 24 hours. The results of flow cytometric analysis showed LBP could effectively reduce the CoCl2-induced retinal ganglion cell apoptosis, inhibited the generation of reactive oxygen species and the reduction of mitochondrial membrane potential. These findings suggested that LBP could protect retinal ganglion cells from CoCl2-induced apoptosis by reducing mitochondrial membrane potential and reactive oxygen species.
Key Words: caspase; cell apoptosis; cobalt chloride; Lycium barbarum polysaccharides; mitochondrial membrane potential; oxidative stress injury; reactive oxygen species; retinal ganglion cells
Introduction
Increased oxidative stress is associated with the common pathological process of many eye diseases, such as glaucoma, diabetic retinopathy and ischemic optic neuropathy (Gaydar et al., 2011; Tarr et al., 2013; Tanito et al., 2016). Glaucoma is considered a degenerative and progressive optic neuropathy that causes damage to the optic nerve and retinal ganglion cells (RGCs) (Zmijewski and Slominski, 2011; Agilan et al., 2016; Sharf, 2018; Adocnetto et al., 2019). A study confirmed that RGC apoptosis and optic nerve axon degeneration caused by ischemia, oxidative stress and inflammatory response are important causes of the occurrence and development of glaucoma (Chen and Zhao, 2017). The accumulation of excessive reactive oxygen species (ROS) can exacerbate the injury of retinal tissue because free radicals can cause lipid peroxidation, protein damage and DNA fragmentation (Forman, 2016).
Lycium barbarum polysaccharides (LBP), extracted from Lycium barbarum fruit, is thought to be the main component responsible for its biological activities (Amagase et al., 2009). Based on the antioxidant activity of LBP, many studies have demonstrated that LBP has protective effect against oxidative injury in various cells and tissues (Yang et al., 2017; Li et al., 2018; Niu et al., 2018; Huang et al., 2019; Yu et al., 2019). In our previous studies, we confirmed its protective effect on retinal pigment epithelial cells (Liu et al., 2015). Cobalt can cause oxidative stress by rupturing the outer cell membrane and disturbing mitochondrial respiration. Cobalt chloride acts as an hypoxia mimicking agent and is commonly used for the induction of neurodegeneration in different models (Grasselli et al., 2005; Caltana et al., 2009; del Olmo-Aguado et al., 2013; Zimmerman et al., 2018; Cheng et al., 2019).
In the present study, we assessed the ability of LBP to protect RGC-5 cells from cobalt chloride (CoCl2) damage in an in vitro oxidative stress model (Chavez and LaManna, 2002; Chowdhury et al., 2008; Ohtomo et al., 2008) and analyzed its effects on cell damage and apoptosis and their mechanism in vitro.
Materials and Methods
Cell culture
The cell line RGC-5 was provided by Jinan University, Guangzhou, China. The rat RGC-5 cells were maintained in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Invitrogen) 1 g/L glucose, 1% antibiotic solution (100 U/mL penicillin and 100 μg/mL streptomycin; Invitrogen) in the presence of 95% air and 5% CO2at 37°C. When grown to 80-85% confluence, the cells were collected for different assays.
CoCl2-induced oxidative damage model
Cell counting kit-8 (CCK-8; Transgen, Beijing, China) was used in to determine the cell viability of RGCs. The RGC-5 cells were seeded into 96-well plates with six replicates for each group. When grown to 80-85% confluence, cells were treated with different concentrations of CoCl2(St. Louis, MO, USA) (0, 50, 100, 200, 400, and 800 μM, respectively). After 24 hours of incubation, the cells were incubated with 10 μL of CCK-8 solution for 2 hours at 37°C and measured by Microplate reader (BioTek, Winooski, VT, USA) at 450 nm optical density (Liu et al., 2015).
LBP intervention
LBPs are a group of water-soluble polysaccharides, including rhamnose, fructose, arabinose, galactose, and galacturonic acid, with a molecular weight of 10-2300 kDa. LBPs were provided and identified by Professor Kwok-fai So. The preparation for LBP was the same as reported previously (Liu et al., 2015). To determine the optimal and safe concentration of LBP, RGC-5 cells were seeded into 96-well plates with six replicates for each group. When grown to 80-85% confluence, cells were treated with different concentrations of LBP (0, 0.01, 0.05, 0.1, 0.5, 1.5 or 10 mg/mL, respectively) for 24 hours. Then the cells were exposed to 200 μM CoCl2and incubated for 24 hours. After that, the cells were incubated with 10 μL of CCK-8 solution for 2 hours at 37°C and their optical density was measured at 450 nm.
Cell apoptosis analysis
To quantify apoptosis cells, Annexin V and propidium iodide staining were used. Briefly, RGC-5 cells were grown on a six-well plate at 2 × 105cells per plate and incubated with or without 0.5 mg/mL LBP for 24 hours before 200 μM CoCl2treatment. Thereafter, cells were harvested and stained with Annexin V-Fluorescein and propidine iodide in a binding buffer for 20 minutes. The fluorescence was measured by flow cytometry (BD Biosciences, Becton, NJ, USA). The percentage of early apoptosis (cells in Q3 area) and total apoptosis cell was calculated by flow cytometry.
Detection of intracellular ROS
To investigate the intracellular ROS generation in RGC-5 cells, RGC-5 cells were treated with CoCl2(200 μM) for 24 hours, the experimental group was preincubated with LBP (0.5 mg/mL) for 24 hours. Cells were resuspended with 200 μL diluted 2′-7′-dichlorodihydrofluorescein diacetate (DCFHDA) (10 μM) and incubated for 20 minutes. After washing, cells were harvested and the average fluorescence intensity of dichlorofluorescein (DCF) was measured by flow cytometry (BD Biosciences). The excitation wavelength was set to 488 nm and the emission wavelength was set to 525 nm.
Measurement of mitochondria transmembrane potential
In this assay, cells were incubated with 0.5 mg/mL LBP for 24 hours, then exposed to 200 μM CoCl2for 24 hours (except the control without CoCl2). Cells were incubated with 200 μL 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1) (BD Biosciences Pharmingen, San Diego, CA, USA) for 15 minutes at 37°C away from the light, then rapidly washed twice with buffer solution. The fluorescence intensity of JC-1 monomer and JC-1 polymer was measured by flow cytometry. JC-1 is an ideal fluorescent probe widely used for mitochondrial membrane potential (MMP) detection. When MMP is higher, JC-1 forms aggregates in the mitochondrial matrix and emits red fluorescence; while when MMP is lower, JC-1 exists as a monomer and produces a green fluorescence. Therefore, the change of fluorescence color can be used to reflect the change of MMP, and the decline of MMP is a marker event in the early stages of apoptosis, so the conversion of JC-1 from red fluorescence to green fluorescence can also be used as an indicator for detection of early apoptosis (Chinopoulos et al., 1999).
Statistical analysis
Data were analyzed using the SPSS 19.0 (IBM, Armonk, NY, USA). Data were presented as the mean ± SD. For comparison of the different groups, statistical comparisons were performed by one-way analysis of variance and Student-Newman-Keuls test. Differences were considered significant at P < 0.05.
Results
Effect of CoCl2 on cell viability in RGC-5 cells
We assessed the effect of CoCl2on RGC-5 cell viability using the CCK-8 assay. Cell viability was reduced with increasing concentrations of CoCl2, when incubated for 24 hours (Figure 1). There was an approximate 50% decrease in cell viability using 200 μM CoCl2. We chose this concentration for all subsequent treatments in this study.
The optimal concentration of LBP for apoptosis induction
Cells were treated with seven different concentrations of LBP for 24 hours and then incubated with 200 μM CoCl2for 24 hours. The viability of cells was assessed by CCK-8. As shown in Figure 2, the cells treated with more than 0.1 mg/mL LBP improved their viability and 0.5 mg/mL of LBP was determined to give the optimal protection against induced apoptosis.
Protective effect of LBP on CoCl2-induced cell apoptosis
We also evaluated the effect of LBP on CoCl2induced cell apoptosis using flow cytometry (Figure 3A). As shown in Figure 3C, CoCl2increased the number of apoptotic cells from 12.45 ± 0.18% to 55.72 ± 4.39% (P < 0.01). In contrast, the LBP group significantly decreased the CoCl2induced apoptotic proportion (P < 0.01). The early apoptotic rate of RGC-5 pretreated with LBP for 24 hours was significantly lower than that in the CoCl2group (Figure 3B).
LBP pretreatment prevents CoCl2-induced oxidative stress
We investigated whether the protective effects of LBP were related to its ability to inhibit CoCl2-induced oxidative stress in vitro. The degree of oxidative stress generated by CoCl2was determined by measuring the intracellular content of ROS. As shown in Figure 4, CoCl2increased intracellular ROS production 2-fold over that of the control, which was in turn lessened by LBP pretreatment (P < 0.01).
CoCl2 and LBP alter the mitochondrial transmembrane potential of RGC-5 cells
Mitochondrial transmembrane potential, as measured by the ratio of JC-1 red/green fluorescence intensity, in the CoCl2injury group was significantly lower than that in the control group (Figure 5). The ratio of JC-1 red/green fluorescence intensity in LBP pretreated group was twice as high as the CoCl2group, which meant that LBP can inhibit the decrease of MMP induced by CoCl2in RGC-5 cells, thereby reducing the early apoptosis of cells (P < 0.01).
Discussion
The main constituents of wolヅerries include LBP, scopoletin, and 2-O-β-D-glucopyranosyl-L-ascorbic acid (Chen et al., 2014). The polysaccharide LBP, the main effective ingredient of Lycium barbarum, isolated from the aqueous extracts of Lycium barbarum, consists of six monosaccharides; glucose, arabinose, galactose, mannose, rhamnose, and xylose (Cheng et al., 2015). Published studies have associated LBP intake with a number of therapeutic effects, including antiaging (Deng et al., 2003), metabolic effects (Luo et al., 2004), neuroprotective effects in neurodegeneration (Ho et al., 2007) and neurotoxicity (Ho et al., 2009), including ocular neuroprotective effects (Chiu et al., 2009). The neuroprotective effects of LBP are some of the most widely studied fields regarding LBP’s therapeutic effects. Yu and coworkers (Yu et al., 2018) used an in vitro model to study the effects of LBP in oxygen glucose deprived/reperfused primary hip-pocampal mice neuron cells and found that LBP significantly increased cell viability, reduced lactate dehydrogenase levels and reduced ROS in a dose-dependent manner. Others demonstrated LBP’s ability to protect retinal neurons and could be used to prevent or slow down the progression of diseases such as diabetic retinopathy, glaucoma, and retinopathy of prematurity (Yang et al., 2017). The latest LBP chemical component analysis demon-strated that all glycopeptides in LBP act to eliminate lipid peroxidation (Varoni et al., 2017; Chen et al., 2018; Schoppet et al., 2018). Thus, it was speculated that as an antioxidant, LBP may decrease cell injury induced by oxygen free radicals and protect cell development and differentiation.
Oxidative stress damage is a major cause of nerve damage, and the retina is very sensitive to oxidative stress (Chen and Zhao, 2017; Zhao et al., 2019). When damage occurs, such as ischemia-reperfusion or glaucoma, ROS will exceed the maximum capacity of the endogenous antioxidant system and result in its accumulation and destructive action on macromolecules, cells and tissues. (Osborne et al., 2004; Tezel, 2006; Kupsco and Schlenk, 2015). Mitochondria are the main subcellular structures for oxidative respiration and the most important source of endogenous ROS production. Hypoxia can inhibit the mitochondrial inner membrane electron transport chain, reduce the MMP and cause an increase in mitochondrial membrane permeability, leading to the release of pro-apoptotic factors and ROS into the cytoplasm (Marques et al., 2017). At the same time, ROS can cause an increase of mitochondrial membrane permeability, so ROS and mitochondrial membrane permeability affect each other. In our experiments, CoCl2caused a significant decrease of MMP in RGC-5 cells. After pretreating with LBP, cells significantly increased the level of MMP. The level of intracellular ROS was significantly inhibited when LBP was administered beforehand.

Figure 3 Lycium barbarum polysaccharides (LBP) inhibits apoptosis of RGC-5 cells induced by cobalt chloride (CoCl2).

Figure 4 Inhibition by Lycium barbarum polysaccharides (LBP) on the increase of reactive oxygen species (ROS) in RGC-5 cells induced by cobalt chloride.
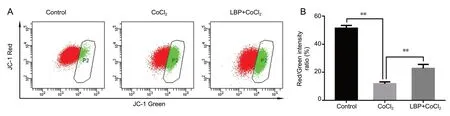
Figure 5 Inhibitory effect of Lycium barbarum polysaccharides (LBP) on mitochondrial membrane potential reduction induced by cobalt chloride (CoCl2) in RGC-5 cells.
In summary, we constructed an CoCl2-injured RGC-5 cell model to simulate the oxidative stress damage of RGCs in glaucoma and found LBP can prevent RGC-5 cells apoptosis induced by CoCl2. Regulation of MMP and lowering ROS levels may be the underlying mechanism by which LBP protects RGC-5 cells from oxidative injury. This study used hypoxia induced in cultured retinal cells as the model. Further research in vitro might use isolated retinas. In later stages of research, the therapeutic effect should be studied in an animal model to provide the basis of a new treatment for glaucoma patients in the clinic.
Acknowledgments:The authors expressed their thanks to Professor Kwok-fai So and Da-Xiang Lu (Jinan University, Guangzhou, China) for providing LBP and cell line RGC-5.
Author contributions:All authors participated in the study design, conduction, and evaluation. LL and XYS drafted the manuscript. All authors contributed to the preparation of the manuscript and approved the final version of the manuscript.
Conflicts of interest:The authors declare that they have no conflict of interest.
Financial support:This work was supported by grants from Project of Administration of Traditional Chinese Medicine of Guangdong Province of China, No. 20161071 (to LL), and Medical Scientific Research Foundation of Guangdong Province of China, No. A2019098 (to LL). All authors declare that the financial supports did not affect the paper’s views and statistical analysis of the objective results of the research data and their reports.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Exercise promotes recovery after motoneuron injury via hormonal mechanisms
- Large animal ischemic stroke models: replicating human stroke pathophysiology
- Autophagy and inflammation in ischemic stroke
- Electrical stimulation and denervated muscles after spinal cord injury
- Beneficial effects of saffron (Crocus sativus L.) in ocular pathologies, particularly neurodegenerative retinal diseases
- Toxic tau: structural origins of tau aggregation in Alzheimer’s disease

