Beneficial effects of saffron (Crocus sativus L.) in ocular pathologies, particularly neurodegenerative retinal diseases
Jose A. Fernández-Albarral , Rosa de Hoz , Ana I. Ramírez Inés López-Cuenca Elena Salobrar-García María D. Pinazo-Durán, José M. Ramírez , Juan J. Salazar
1 Instituto de Investigaciones Oftalmológicas Ramón Castroviejo, Madrid, Spain
2 Departamento de Inmunología, Oftalmología y ORL, Facultad de Óptica y Optometría, Madrid, Spain
3 Departamento de Inmunología, Oftalmología y ORL, Facultad de Medicina, Madrid, Spain
4 Unidad de Investigación Oftalmológica Santiago Grisolia, Universidad de Valencia, Valencia, Spain
Abstract Saffron (Crocus sativus L.) has been traditionally used in food preparation and as a medicinal plant. It currently has numerous therapeutic properties attributed to it, such as protection against ischemia, as well as anticonvulsant, antidepressant, anxiolytic, hypolipidemic, anti-atherogenic, anti-hypertensive, antidiabetic, and anti-cancer properties. In addition, saffron has remarkable beneficial properties, such as anti-apoptotic, anti-inflammatory and antioxidant activities, due to its main metabolites, among which crocin and crocetin stand out. Furthermore, increasing evidence underwrites the possible neuroprotective role of the main bioactive saffron constituents in neurodegenerative diseases, such as Parkinson’s and Alzheimer’s diseases, both in experimental models and in clinical studies in patients. Currently, saffron supplementation is being tested for ocular neurodegenerative pathologies, such as diabetic retinopathy, retinitis pigmentosa, age-related macular degeneration and glaucoma, among others, and shows beneficial effects. The present article provides a comprehensive and up to date report of the investigations on the beneficial effects of saffron extracts on the main neurodegenerative ocular pathologies and other ocular diseases. This review showed that saffron extracts could be considered promising therapeutic agents to help in the treatment of ocular neurodegenerative diseases.
Key Words: AMD; crocetin; crocin; Crocus sativus L.; diabetic retinopathy; glaucoma; neuroprotection; ocular diseases; retinitis pigmentosa; saffron; safranal
Introduction
Neurodegenerative diseases such as glaucoma and age-related macular degeneration (AMD), among others (e.g., retinitis pigmentosa and diabetic retinopathy), are the main causes of irreversible blindness in the world. In these pathologies, the mechanisms that induce neuronal death are still unknown, but at present it is believed that they share some pathogenic mechanisms. The impairment in mitochondrial function (Lee et al., 2011; Lascaratos et al., 2012; Chrysostomou et al., 2013), the dysfunction in the ubiquitin—proteasome system (Campello et al., 2013), the buildup of misfolded proteins and glutamate excitotoxicity (Gazulla and Cavero-Nagore, 2006; Guimarães et al., 2009), the oxidative stress (Uttara et al., 2009), and the activation of glial cells and inflammatory processes (Verkhratsky et al., 2014; Brown and Vilalta, 2015) could be some of these pathogenic mechanisms, which could act individually or jointly (Ghiso et al., 2013). All these neurodegenerative ocular diseases have treatments that have a positive impact on the preservation of vision and slow the progression of these pathologies. However, there is still no cure. Currently, natural products derived from plants are being used to develop new therapies, due to their potential pharmacological effects and low toxicity profiles (Moshiri et al., 2014). In addition, the effectiveness of natural products to slow neurodegenerative processes through different pathways is being studied. Among these products is saffron (dried stigmas of Crocus sativus L.), a spice which is well known and used for culinary purposes. The main goal of the present review is to analyze the different studies in which saffron extract has been used for treatment of various neurodegenerative ocular diseases and other ocular pathologies.
Search Strategy and Selection Criteria
The search strategy was based on a focussed literature review. Databases used to identify the most relevant papers included PubMed and Google Scholar. Search keywords used in different forms included: “saffron”, “crocin”, “crocetin”, “safranal”, “neurodegeneration”, retinal diseases”, “ocular diseases”. Articles published in the last ten years or relevant publications were selected for this review article.
Saffron Extracts and Its Constituents
Crocus sativus L. (C. sativus) is a flowering plant belonging to the Iridaceae family (Khazdair et al., 2015). This spice has been used in food preparation since ancient times (Basker and Negbi, 1983) and is mainly cultivated in the European Mediterranean region and Asia, with Iran as the main producer country (85%) (Milajerdi and Mahmoudi, 2014; Ghaffari and Roshanravan, 2019). Saffron has been part of many pharmacological formulas used from the 16thcentury to today, being included in the Catalogues of Medicinal Plants and in European Pharmacopoeias (José Bagur et al., 2017). In the stigmas of C. sativus it is possible to find more than 100 metabolites, including isomers of carotenoids (α-crocetin, glycoside crocin (yellow color), picocrocin, and the aglycone-safranal (saffron aroma)) and antioxidants (zeaxanthin, vitamin B12 and lycopene) (Assimopoulou et al., 2005; Karimi et al., 2010; Serrano-Díaz et al., 2012; Khazdair et al., 2015), which have been demonstrated to have several pharmacological and therapeutic uses (Srivastava et al., 2010). The therapeutic activity of saffron may be due to the crocetin and crocin, its main bioactive components (Bathaie et al., 2014; Rameshrad et al., 2018; Hashemi and Hosseinzadeh, 2019). It has been observed, in both humans and animals, that crocin is hydrolyzed to crocetin during the process of intestinal absorption (Xi et al., 2007). Once in circulation, crocetin, due to its poor binding to albumin, reaches different tissues and can cross the blood-brain barrier (BBB) (Asai et al., 2005; Hosseini et al., 2018a). Crocetin can traverse the BBB by passive transcellular diffusion, as demonstrated in an in vitro study (Lautenschläger et al., 2015). In addition, a mouse in vivo study showed that trans-crocin 4, the most abundant type of crocin in saffron, can penetrate the BBB and accumulate in the brain (Karkoula et al., 2018).
It has been demonstrated that crocetin is responsible for the main therapeutic properties of saffron (Hashemi and Hosseinzadeh, 2019). Christodoulou et al. (2019) showed that after oral saffron extract administration, crocetin levels in the blood were higher than those following intravenous administration, due to the quick intestinal absorption of trans-crocetin. In addition, Zhang et al. (2017b) observed that oral administration of equal molar concentrations of crocin and crocetin showed that crocin reaches higher concentrations of crocetin in rat serum than oral crocetin administration, suggesting that oral administration of crocin has an advantage over oral crocetin administration.
Saffron’s General Therapeutic Properties
In addition to its organoleptic attributes, saffron and its main bioactive derivatives have been used routinely in traditional medicine because of their numerous therapeutic properties, such as protection against ischemia, as well as their anticonvulsant, antidepressant, anxiolytic, hypolipidemic, anti-atherogenic, anti-hypertensive, antidiabetic, and anti-cancer properties (Ríos et al., 1996; Kianbakht and Hajiaghaee, 2011; Christodoulou et al., 2015; Hosseini et al., 2018b; Ghaffari and Roshanravan, 2019). Among their pharmacological effects, a potent stimulatory effect has been reported for C. sativus extract and safranal on β2-receptors, the blocking effect of safranal on muscarinic receptors, and the inhibition of histamine (H1) receptors by C. sativus (Boskabady et al., 2010). Recently, different mechanisms of action have been attributed to crocetin, among which are the increase of oxygen transport during shock, the decrease of pro-inflammatory molecules, protection against oxidative stress, and induction of apoptosis in cancer cells (Hashemi and Hosseinzadeh, 2019).
The therapeutic properties of saffron may be due to the important anti-inflammatory, antioxidant, and anti-apoptotic properties of its bioactive components (Hosseinzadeh and Younesi, 2002; Yamauchi et al., 2011; Ohno et al., 2012; Poma et al., 2012).
There is currently a growing interest in the effects of the bioactive components of saffron on human health due to their antioxidant properties (Moallem et al., 2014). Safranal and crocin show antioxidant activities through scavenging free radicals (Assimopoulou et al., 2005; Farahmand et al., 2013; Mashmoul et al., 2013). The mode of action of these carotenoids could (Bukhari et al., 2018; Korani et al., 2019): i) modulate the detoxifying enzymes involved in counteracting oxidative stress, ii) decrease telomerase activity, iii) increase the pro-apoptotic effect in cancerous cells, iv) change the expression of genes related to the redox system of cells and inhibit the synthesis of DNA, RNA, and proteins, v) alter the stress marker genes in the endoplasmic reticulum system, vi) make changes in epigenetics, and vii) through the ability to form a strong union of crocetin with tRNA.
It has also been seen that saffron has anti-inflammatory effects. These effects could be due to its free-radical scavenging activity. Inflammatory conditions are triggered by the release of reactive oxygen species and the release of several cytokines from microglia, macrophages, and monocytes, among others (Poma et al., 2012). The anti-inflammatory effects of saffron could be due to (Soeda et al., 2001; Falsini et al., 2010; Nam et al., 2010; Natoli et al., 2010; Wang et al., 2015; Korani et al., 2019; Zeinali et al., 2019) i) the regulation of genes that control the release of pro-inflammatory cytokines from glial cells, such as interleukin (IL)-6, IL-1β, and IL-2; ii) blocking the tumor necrosis factor (TNF)-α released by microglial cells that induces DNA fragmentation, thereby suppressing cell death; iii) modulation of the expression of genes encoding adhesion molecules (vascular cell adhesion protein 1, intercellular adhesion molecule 1, and E-selectin); iv) the reduction of the mRNA expression of some pro-inflammatory enzymes (inducible nitric oxide synthase and cyclooxygenase-2); and v) modulation of the inflammatory pathways (nuclear factor-κB and mitogen-activated proteins kinase).
Crocin administration prevents BBB disruption, inhibiting, in part, the alterations of tight junctions due to ischemia in a middle cerebral artery occlusion model. BBB integrity is important to protect the neuronal environment from harmful elements, such as infiltrated inflammatory cells and cytokines, which can induce neuronal damage (Zhang et al., 2017a).
In addition, saffron shows an anti-apoptotic effect. In neuronal damage, an inhibitory effect on Bcl-2, Bax, and caspase-3 expression has been observed after crocin supplementation (Yamauchi et al., 2011).
Finally, saffron has been related to the cannabinoid system. In an experimental model of retinal damage associated with continuous exposure to bright light, it was observed that the administration of saffron counteracted the harmful effects on the retina mediated by the cannabinoid system, thereby suggesting that saffron and cannabinoid receptors could share the same mechanism of action in neuroprotection (Maccarone et al., 2016).
Toxicity and Adverse Side Effects of Saffron
Some studies have focused on assessing the toxicity of saffron mainly due to dose-dependent adverse effects. These toxic effects of saffron have been documented for daily doses of more than 5 g, becoming lethal for doses of 20 g (Schmidt et al., 2007). Low doses of saffron between potentially toxic ranges can cause dizziness, nausea, vomiting and diarrhea, but high doses can exacerbate these toxic effects causing numbness, tingling of the extremities, cutaneous and conjunctival yellow pigmentation and spontaneous bleeding on the lips, eyelids, nose, and uterus (Schmidt et al., 2007; WHO, 2007; Modaghegh et al., 2008). Oral administration of more than 5 g can cause thrombocytopenia, localized skin hemorrhages, stimulating effects of the uterus, emmenagogue and abortive effects, so it should be used with caution in patients with bleeding disorders, anticoagulant treatment and pregnancy (Hepner et al., 2002; WHO, 2007; Moshiri et al., 2014; Heitmar et al., 2019). Therefore, the main clinical and experimental studies performed use milligram doses, which have been shown to have no adverse side effects. Clinical studies with saffron tables in doses of 200 and 400 mg showed no clinically important alterations. The patients treated in this study exhibited changes of normal range in some hematological and biochemical parameters (Modaghegh et al., 2008).
Saffron and Neurodegeneration
Recently, the neuroprotective role of dietary saffron has generated great interest. Extracts of C. sativus and its components can exert neuroprotection in neurodegenerative diseases, such as Alzheimer’s and Parkinson’s diseases, because they can interact with the glutamatergic, cholinergic and dopaminergic systems (Khazdair et al., 2015). Neuroprotective effects have been observed in both experimental models (Ahmad et al., 2005; Geromichalos et al., 2012; Purushothuman et al., 2013; Khazdair et al., 2015) and clinical studies in patients (Akhondzadeh et al., 2010; Khazdair et al., 2015). Clinical studies have demonstrated that saffron oral administration in mild-to-moderate Alzheimer’s disease patients ameliorated cognitive function (Akhondzadeh et al., 2010). Safranal prevented neuronal loss due to amyloid β (Aβ) in CA1’s hippocampal area, via decrease of apoptosis, oxidative stress, inflammation, cholinesterase activity, and neutrophil infiltration, and also through the preservation of mitochondrial integrity in an Alzheimer’s disease rat model (Baluchnejadmojarad et al., 2019). Trans-crocin-4 inhibited Aβ fibrillogenesis in vitro (Papandreou et al., 2006). In a sporadic Alzheimer’s disease model in rats (induced by intracerebroventricular injection of streptozotocin), treatment with saffron extract improved cognitive impairment (Khalili and Hamzeh, 2010; Khalili et al., 2010).
In a Parkinson’s disease model, it was shown that exercise and crocin supplementation, due to their anti-inflammatory and antioxidant properties, could ameliorate motor and memory deficits (Shahidani et al., 2019). In addition, C. sativus hydroethanolic extract has a neuroprotective effect on the nervous system of the meriones shawi rodent, suggesting that saffron could be a possible therapeutic agent in neurodegenerative disorders, including dopaminergic and noradrenergic injuries trigged by heavy metals like in Parkinson’s disease (Tamegart et al., 2019). Crocetin administration in an induced animal model of Parkinson’s disease 6-hydroxydopamine reduced dopamine utilization, thereby exerting a neuroprotective effect (Ahmad et al., 2005). In the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinsonism in mice, saffron pre-treatment reduced dopaminergic cell loss in the nigra pars compacta and retina (Purushothuman et al., 2013).
Saffron and Ocular Neurodegenerative Diseases
It has been postulated that saffron supplementation can exert beneficial effects in ocular neurodegenerative diseases, such as glaucoma, AMD, diabetic retinopathy, and retinitis pigmentosa, among others (Bonyadi et al., 2014; Sepahi et al., 2018; Broadhead et al., 2019) (Table 1 and Figure 1).
Glaucoma
Glaucoma is a neurodegenerative disease related to retinal ganglion cell (RGC) loss and microglial activation. At present, treatment mainly aims to control intraocular pressure but, on occasion, this does not prevent the disease’s progression. Thus, today, neuroprotection could constitute one of the main objectives of recent studies.
The over-stimulation of a type of ionotropic glutamate receptor, such as N-methyl-D-aspartate, is related to several retinal pathologies, including glaucoma. In a retinal damage experimental mouse model induced by intravitreal N-methyl-D-aspartate injection, oral crocetin administration reduced retinal impairment partly by inhibiting caspases and therefore decreasing apoptosis of retinal ganglion cells (Ohno et al., 2012).
Microglial activation is directly related to neurodegeneration in glaucoma (Gallego et al., 2012; De Hoz et al., 2013; Rojas et al., 2014; de Hoz et al., 2018). Stimulation with lipopolysaccharide, which is used as an inflammatory model that triggers microglial activation, causes the release of factors such as TNF-α, cyclooxygenase-2, inducible nitric oxide synthase and IL-1β, among others, which are mediators of the inflammatory process (Nam et al., 2010). Moreover, membrane-bound fractalkine (CX3CR1) deficiency increases microglial neurotoxicity and RGC loss (Wang et al., 2014). Pretreatment of BV2 microglia cells cultured with crocin reversed the morphological changes, including down-regulation of CX3CR1 expression, and up-regulation of TNF-α, cyclooxygenase-2, inducible nitric oxide synthase, and IL-1β in a lipopolysaccharide in vitro model (Lv et al., 2016).
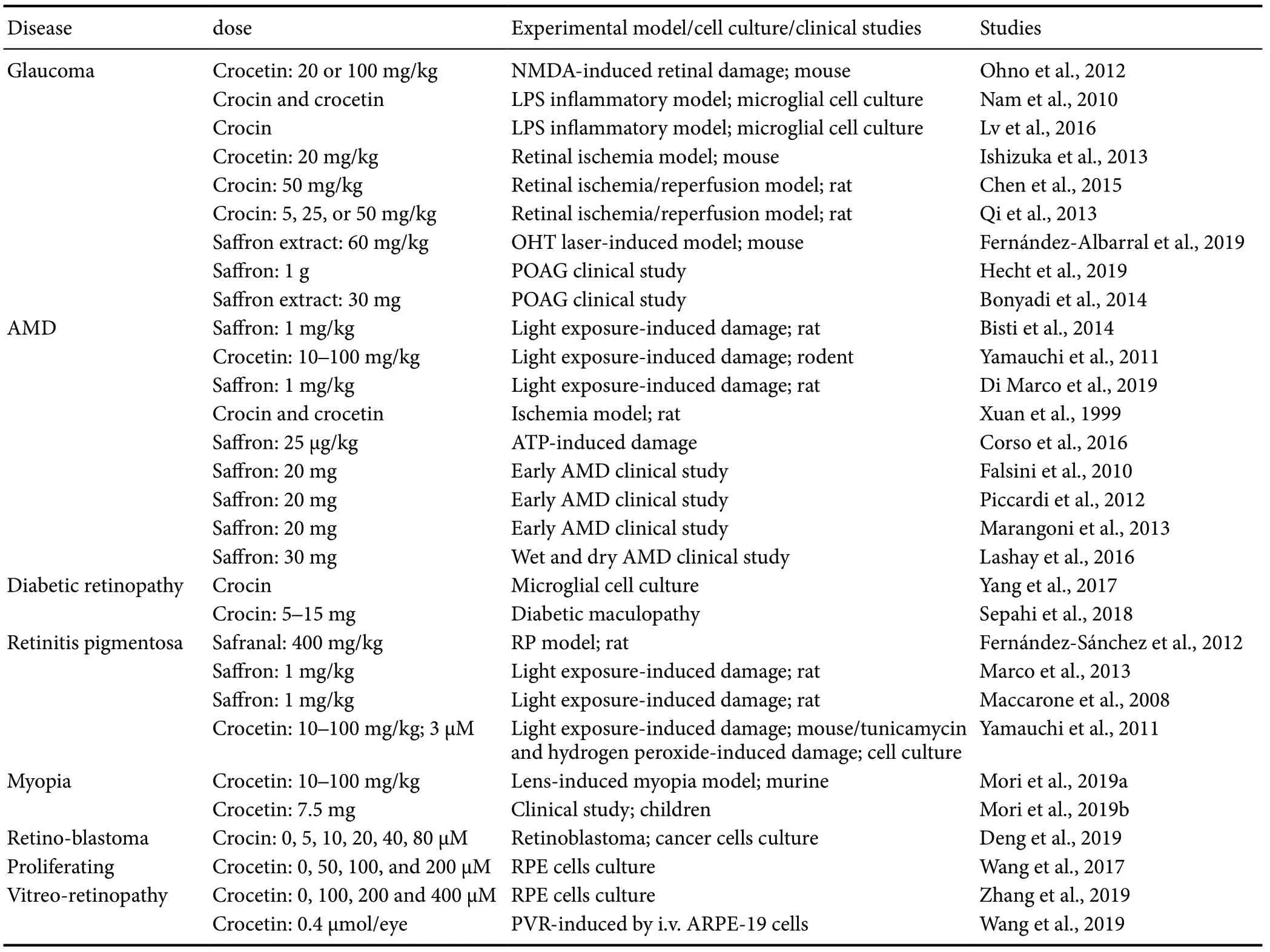
Table 1 Studies of the beneficial effects of saffron, crocin, crocetin, or safranal supplementation in ocular diseases
In many ocular diseases, such as glaucoma, retinal ischaemia plays an important role by inducing neuronal cell death (Ishizuka et al., 2013). Crocin and crocetin inhibit RGC death in mouse and rat models of ischemia/reperfusion (Ishizuka et al., 2013; Qi et al., 2013; Chen et al., 2015). The possible neuroprotective effects against ischemic damage could be due to oxidative stress inhibition through different mechanisms. These mechanisms include a decrease in the number of positive TUNEL cells and 8-hydroxy-2-deoxyguanosine positive cells (a good biomarker of oxidative damage), and the phosphorylation levels of c-Jun, JNK, p38, and NF-κB, which are elevated after retinal damage (Ishizuka et al., 2013).
Recently, in a mouse model of glaucoma, the anti-inflammatory and neuroprotective effect of the saffron extract (standardized to 3% crocin content) (Almodóvar et al., 2018), has been demonstrated (Fernández-Albarral et al., 2019). In this model, an increase of ocular hypertension (OHT) was induced by unilateral laser photocoagulation of the limbal and episcleral veins. Treatment with saffron extract reduced microglial cell number and the signs of microglial activation, including process retraction and soma size, both in OHT eye and the normotensive contralateral eye. Furthermore, down-regulation of P2RY12 induced by the OHT increase was reversed in part by saffron extract supplementation (Fernández-Albarral et al., 2019). Up-regulation of P2RY12 occurs immediately after damage, and a few hours later the P2RY12 expression is down-regulated, finally becoming undetectable in highly activated microglial cells (Haynes et al., 2006). Thus, this result indicates that saffron extract could be exerting an anti-inflammatory effect through the regulation of P2RY12 expression (Fernández-Albarral et al., 2019). Finally, in this model, saffron’s neuroprotective role has also been demonstrated; saffron prevents the significant loss of Brn3a+RGCs in OHT eyes, at 7 days after OHT laser induction (Fernández-Albarral et al., 2019). This neuroprotective effect of the saffron extract could be due to its anti-inflammatory effects and its antioxidant capacity (Fernández-Albarral et al., 2019).
The only studies on using saffron in patients with glaucoma are focused on analyzing its possible ocular hypotensive effect, and have obtained contradictory results. In a randomized controlled pilot study on patients with primary ocular angle glaucoma, saffron supplementation (1 g twice a week) did not appear to affect intraocular pressure in the short term (1 month) (Hecht et al., 2019). However, in another pilot study, a dosage of 30 mg/day aqueous saffron extract orally for three weeks produced a decrease of the intraocular pressure in patients with primary ocular angle glaucoma (Jabbarpoor Bonyadi et al., 2014).
Age-related macular degeneration
AMD is a neurodegenerative ocular disorder, in which pathogenesis involves chronic inflammation (Buschini et al., 2011) and oxidative stress (Beatty et al., 2000). Changes in the photoreceptor cells and the retinal pigment epithelial cells (RPE) are early events (Johnson et al., 2003) that cause loss of visual function. Subtle visual losses, caused by the alteration in the function of the different subpopulations of photoreceptors and/or by their post-receptoral neurons can be recorded by electrophysiological and psychophysical techniques (Mayer et al., 1992a, b; Phipps et al., 2003).
Long-term light exposure has been related to the pathogenesis of AMD. The experimental model of light exposure is the main model used in experimental studies of AMD. In animal models and in AMD patients, saffron treatment has been shown to have beneficial effects on the degenerative progression of AMD (Bisti et al., 2014). In an albino rat light exposure-induce damage model, saffron-treated healthy retinas showed no saffron metabolites, however, in damaged retinas treated with saffron, crocin was detected in the retinal tissue due to the blood retinal barrier breakdown (Bisti et al., 2014). In a rodent model of light-induced damage, crocetin oral administration prevented decreases both the a-wave and b-wave amplitudes of the flash electroretinogram; it also reduced the number of TUNEL+cells in the retinal outer nuclear layer and inhibited a decrease in the thickness of this layer. Crocetin could reduce photoreceptor apoptosis by suppressing the increase in caspase 3 and caspase 9 activities after retinal damage (Yamauchi et al., 2011). It has been shown that saffron treatment could be very effective in maintaining retinal morphology by decreasing metalloproteinase activation in an AMD albino rat experimental model. This suggests a new way of setting crocin apart from the other known mechanisms, such as free radical scavenging (Di Marco et al., 2019). In addition, crocus analogs isolated from saffron produce a notable increase in retinal and choroidal blood flow and produced an amplitude recovery of the b-wave, thereby facilitating the recovery of retinal function after acute retinal ischemia (Xuan et al., 1999).
The P2X7 receptor (P2X7R) is related to retinal neurodegenerative diseases, such as AMD and retinitis pigmentosa. P2X7R is found in cells of different layers of retinal tissue, including microglial cells, and is responsible for the modulation of the health and signaling of the cells (Vessey et al., 2012). This receptor is distinguish by two statuses of permeability that lead a intracellular Ca2+concentration ([Ca2+]i), increase, which could be essential for cell function (Surprenant et al., 1996). Studies in vitro have shown that saffron decreases the [Ca2+]iresponse provoked by purinergic P2X7R stimulation, thereby revealing a new mechanism by which saffron can achieve protective activity in neurodegenerative diseases (Corso et al., 2016).
Saffron has also been used in clinical studies to test its possible neuroprotective effects on AMD patients. In early AMD, short-term treatment with saffron enhances retinal flicker sensitivity generated in the electroretinogram by photoreceptors and bipolar cells, improving visual function. Saffron could reverse the damage of these cells caused by oxidative stress (Falsini et al., 2010). In addition, in early AMD, saffron treatment induces macular function ameliorations from baseline that are widespread over long-term follow-up (Piccardi et al., 2012), though this improved independently of the two major risk genotypes (CFH and ARMS2) related to AMD (Marangoni et al., 2013). In addition, longterm daily supplementation with saffron was related with a statistically significant shift in macular optical coherence tomoqraphy and electroretinogram parameters in AMD patients, both in the dry and in the wet type, which ameliorated retinal functions (Lashay et al., 2016). Saffron treatment additionally can lead to a mid-term, significant amelioration in retinal function in mild/moderate AMD patients, including those using the age-related eye disease study (AREDS)supplements. Saffron supplementation was both efficacious and safe in preserving retinal function. However, clinically, the degree of response obtained with this study was small (Broadhead et al., 2019). Recently, in a comparative study on AREDS treatment and saffron treatment in AMD patients, visual function remained stable in saffron treated AMD patients and deteriorated in the AREDS group. The difference between both studies was that in AREDS, the components are only antioxidants, while saffron has other effects, such as its anti-inflammatory properties, in addition to its antioxidant capacity. This study supports the benefit of saffron treatment compared to pure antioxidant supplementation (Di Marco et al., 2019).
Diabetic retinopathy
Diabetic retinopathy (DR) is one of the main complications of diabetes mellitus. DR is related to different etiopathogenic mechanisms, such as oxidative stress, apoptosis, and inflammation (Domingueti et al., 2016; Yaribeygi et al., 2019a), which leads to RGC degeneration and loss (Barber, 2003). Several studies show the hypoglycemic effects of saffron, due to its capacity to inhibit the pathophysiologic pathways related to insulin resistance, such as oxidative stress and inflammation. It has been postulated that saffron is able to ameliorate insulin resistance in pre-diabetic patients through different mechanisms: i) inhibition of β-cell failure, ii) increased glucose uptake by insulin-dependent tissues, iii) improvement of glycemic control (Yaribeygi et al., 2019b).
In a BV-2 and N9 microglial cell culture, following hyperglycemic and hyperlipidemic treatment in order to induce an experimental diabetes, crocin decreased microglial activation through its anti-oxidant and anti-inflammatory properties. These neuroprotective effects could be due to the activation of the PI3K/Akt signaling pathway. Thus, the authors concluded that crocin could be used to control microglial activation in DR patients (Yang et al., 2017). These neuroprotective and anti-oxidant effects have also been observed in diabetic macular edema patients previously treated with photocoagulation and anti-vascular endothelial growth factor, in combination with crocin oral supplementation. Crocin decreased the central macular thickness and improved the best-corrected visual acuity in these patients (Sepahi et al., 2018).
Retinitis pigmentosa
Retinitis pigmentosa (RP) constitutes a neurodegenerative dystrophy group characterized by peripheral visual loss and hemeralopia that can be accompanied by central vision impairment. The oxidative stress leading to photoreceptor apoptosis is implicated in the pathogenic mechanism (Remé et al., 1998). Most cases of RP are caused by mutations in the rhodopsin-encoding gene, with P23H being the main mutation of this gene (Dryja et al., 1990). In a model of autosomal dominant RP (P23H rats), safranal treatments ameliorated the loss of photoreceptors and protected their electroretinographic response, showing higher amplitudes for both photopic and scotopic responses in animals treated with safranal. Furthermore, these treatments preserved the photoreceptors’ synaptic contact with other postsynaptic neurons, thus improving retinal function and ameliorating vascular network disruption. These authors postulated that safranal treatment could be useful in patients with retinitis pigmentosa to help delay retinal degeneration (Fernández-Sánchez et al., 2012, 2015). Saffron protected retinal photoreceptors in a light damage model of photoreceptor degeneration in rats (Maccarone et al., 2008; Marco et al., 2013). Saffron supplementation, in combination with photobiomodulation (low-level infrared radiation), produced a decrease in photoreceptor death, thereby improving their viability and reducing the Müller cell glial fibrillary acidic portein up-regulation (Marco et al., 2013). In addition to the model mentioned above, saffron extract not only improved the photoreceptors’ morphological features, but also their functional characteristics, as revealed by their flash electroretinogram responses (Maccarone et al., 2008).
It has also been shown in an experimental mouse model of light-induced retinal damage that oral crocetin supplementation significantly diminished photoreceptor degeneration, thereby preventing decreases in both a-wave and b-wave flash electroretinogram response amplitudes and improving inner retinal function. It also inhibited a decrease in the outer nuclear layer thickness and halved the expression of TUNEL-positive cells in this layer (Yamauchi et al., 2011). In this same work, the effects of crocetin administration in an RGC-5 cell culture (a mouse ganglion cell-line transformed using the E1A virus) were also analyzed. After the damage induced by exposure to tunicamycin and hydrogen peroxide, it was demonstrated that crocetin inhibited RGC-5 cell death by 50-60%, decreasing the activity of caspase 3 and 9 (Yamauchi et al., 2011).
Other Ocular Diseases
Saffron supplementation has also been used in non-neurodegenerative ocular diseases, showing the beneficial effects of this natural compound (Table 1 and Figure 1).
Myopia
The notable increase in the number of myopia cases in recent years shows the need to find novel treatments that can decrease myopia progression. In a murine model of lens-induced myopia, it was shown that crocetin oral treatment can improve the refraction shift and decrease axial length, together with an increase in choroidal thickness (Mori et al., 2019a). A clinical study also showed crocetin’s effect in the control of myopia progression in children aged between 6 and 12 years old. Two parameters were evaluated in order to test the effi-cacy of crocetin supplementation. Both spherical equivalent refractions and axial length were significantly larger in the untreated group (-0.41 ± 0.05 D; 0.21 ± 0.02 mm, respectively) than in the crocetin treated group (-0.33 ± 0.05 D; 0.18 ± 0.02 mm, respectively) (Mori et al., 2019b).
Retinoblastoma
Retinoblastoma is the most common primary malignant eye tumor in children. Among the genes involved in its development, MYCN is a transcription factor proto-oncogene, whose overexpression or amplification occurs in retinoblastoma. Crocin has anti-tumorigenic effects on human retinoblastoma cell lines in a MYCN-dependent manner, which could contribute to the prevention and treatment of retinoblastoma (Deng et al., 2019).
Proliferating vitreoretinopathy
Proliferative vitreoretinopathy (PVR) constitutes the most important complication after rhegmatogenous retinal detachment. PVR is characterized by the formation of fibrotic membranes that can contract and lead to tractional retinal (re-)detachment (Ryan, 1985). The main type of cell found in fibrotic membranes is RPE. These cells undergo an epithelial-mesenchymal transition and transform into myofibroblast-like cells, and acquire migratory abilities, become resistant to apoptosis and generate extracellular matrix constituents. The epithelial-mesenchymal transition process of RPE is important in PVR development (Tamiya et al., 2010). In a study conducted by Wang et al. (2017), the effect of crocetin on proliferation, migration, and epithelial-mesenchymal transition in ARP-19 cells (a human retinal pigment epithelial cell line) was investigated. In this work, it was found that crocetin causes the inhibition of the mechanisms mentioned above in RPE cells, mediated by transforming growth factor-β2. In another study, it was demonstrated that the administration of crocetin could inhibit the migration and proliferation of the cell line ARPE-19 by modulating the regulators of the Bcl-2 family in a concentration- and time-dependent manner. The authors postulated the possible role of crocetin in the treatment of PVR (Zhang et al., 2019). In addition, other work evaluated the toxicity, pharmacokinetics and possible inhibitor effect of PVR in rabbit eyes of intraocular crocetin in an experimental PVR model induced with an intravitreal injection of ARPE-19 cells. After crocetin treatment, the authors did not observe toxicity and found that crocetin significantly inhibited PVR progression, together with the reduction in the expression of α-SMA (a mesenchymal marker), Ki67 (a marker of cellular proliferation), and collagen fibers (Wang et al., 2019).
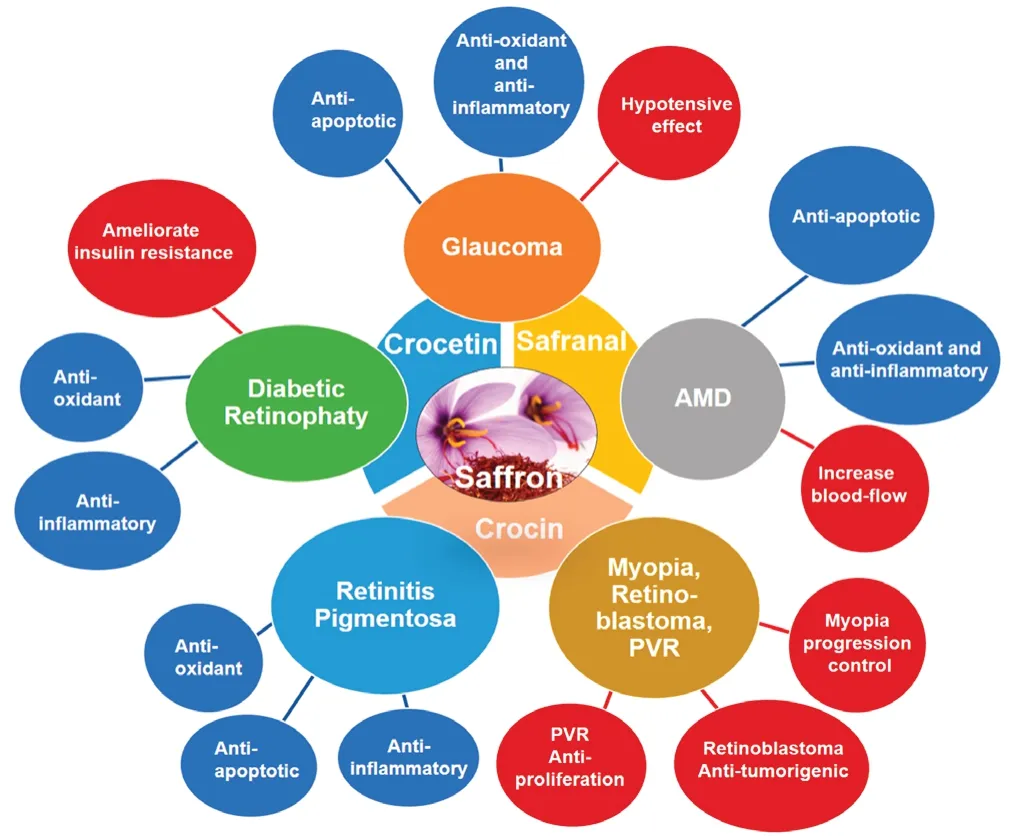
Figure 1 Schematic representation of the different properties of saffron and/or its constituents that could be responsible for their therapeutic beneficial effects in retinal neurodegenerative diseases such as glaucoma, AMD, diabetic retinopathy, retinitis pigmentosa and in others ocular pathologies.
Conclusion
Studies focused on saffron’s properties show the efficiency of saffron supplementation and of its compounds on ocular diseases, especially in neurodegenerative diseases. Several ethiopatogenic mechanisms are implicated in neurodegenerative impairment, including oxidative stress, neuroinflammation, and neural cell apoptosis. The main effective compound of C. sativus is crocetin, which has antioxidant, anti-inflammatory, and anti-apoptotic properties and is able to act against the ethiopatogenic mechanisms mentioned above. Both experimental and clinical studies support the possible therapeutic role of saffron in ocular diseases.
Author contributions:Conceptualization: AIR, RdH, JMR and JJS; data curation: JAFA, AIR and RdH; formal analysis: JAFA, ESG and ILC; funding acquisition: AIR, RdH, JMR and JJS; investigation: JAFA, AIR, RdH, ESG, ILC and JJS; methodology: JAFA, AIR, RdH and JMR; project administration: AIR, RdH, JMR and JJS; resources: AIR, RdH, JMR and JJS; supervision: AIR, RdH and JMR; validation: JAFA, AIR, ESG, ILC, JMR and JJS; visualization: JAFA, AIR, RdH, ESG, ILC and JMR; writing original draft: JAFA, AIR, RdH, JMR and JJS; writing review and editing: JAFA, AIR, RdH, ESG, JMR and JJS. All authors approved the final manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:This work was supported by the Ophthalmological Network OFTARED (RD16/0008/0005, RD16/0008/0022‚ of the Institute of Health of Carlos III of the Spanish Ministry of Economy; by the PN I+D+i 2008-2011, by the ISCIII-Subdirección General de Redes y Centros de Investigación Cooperativa, by the European program FEDER. SAF-2014-53779-R: from the Spanish Ministry of Economy and Competitiveness and by Articulo 83 118-2017 (UCM-Pharmactive Biotech). José A. Fernández-Albarral is currently supported by a Predoctoral Fellowship (FPU17/01023) from the Spanish Ministry of Science, Innovation, and Universities. Inés López-Cuenca is currently supported by a Predoctoral Fellowship (CT42/18-CT43/18) from the Complutense University of Madrid.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Jiaxing Wang, Emory University, USA.
- 中国神经再生研究(英文版)的其它文章
- Exercise promotes recovery after motoneuron injury via hormonal mechanisms
- Large animal ischemic stroke models: replicating human stroke pathophysiology
- Autophagy and inflammation in ischemic stroke
- Electrical stimulation and denervated muscles after spinal cord injury
- Toxic tau: structural origins of tau aggregation in Alzheimer’s disease
- Modification of tubular chitosan-based peripheral nerve implants: applications for simple or more complex approaches

