Raspberry ketone attenuates high-fat diet-induced obesity by improving metabolic homeostasis in rats
Ali Alkaladi, Haytham Ali, Aaser M. Abdelazim, Mohamed Afifi✉, Mohammed Baeshen, Ammar AL-Farga
1University of Jeddah, College of Science, Department of Biology, Jeddah, Saudi Arabia
2University of Jeddah, College of Science, Department of Biochemistry, Jeddah, Saudi Arabia
3Department of Biochemistry, Faculty of Vet. Medicine, Zagazig University, Zagazig, Egypt
4Department of Basic Medical Sciences, College of Applied Medical Sciences, University of Bisha, Bisha, Saudi Arabia
ABSTRACT Objective: To investigate the molecular mechanisms of the antiobese effect of raspberry ketone against high-fat diet fed rats. Methods: Fifty adult male rats were randomly assigned to receive a standard diet, a high fat diet, and the high-fat diet and 0.5%, 1% or 2% raspberry ketone. Body weight, biochemical parameters and gene expression of CCAAT enhancer-binding protein (C/EBP)-δ, fatty acid synthase (FAS), acetyl CoA carboxylase (ACC), peroxisome proliferator-activated receptor alpha (PPAR-α), hormone-sensitive lipase (HSL) and hepatic carnitine palmitoyltransferase 1 A (CPT1A) were investigated. Results: Body weight, blood glucose, insulin, total lipids, triacylglycerols, total cholesterol and low-density lipoprotein cholesterol were increased in high-fat diet fed rats. These high fat diet-induced changes were attenuated by treatment with raspberry ketone. High-density lipoprotein cholesterol was decreased in highfat diet fed rats but increased in rats treated with raspberry ketone. Molecular investigations showed induction of gene expression of C/EBP-δ, FAS, ACC, CPT1A and inhibition of gene expression of PPAR-α and HSL in high-fat diet fed rats as compared with control. Raspberry ketone treament reversed these changes except CPT1A. Conclusions: Raspberry ketone can prevent obesity induced by a high-fat diet in rats by induction of the expression of enzymes, controlling lipolysis and fatty acids β oxidation as well as inhibition of gene expressions of adipogenic factors.
KEYWORDS: Raspberry ketone; Obesity; Molecular mechanism
1. Introduction
Obesity is a common health problem around the world as it raises the risk factors for many metabolic diseases such as type Ⅱ diabetes mellitus, atherosclerosis, thrombosis and coronary artery diseases[1,2]. The prevalence of obesity in Saudi Arabia has been increased to be 35.5% of the population, which rings the alarm to face this great problem[3]. Obesity is a syndrome caused mainly by fat cell hyperplasia and hypertrophy due to excessive food intake[4]. The increased number of adipocytes may be related to the increased incidence of obesity and its severity[5]. Thus, overcoming adipocyte hyperplasia may be an important factor in the improvement and prevention of obesity[6]. The process of adipocyte differentiation (adipogenesis) is mainly regulated by several transcription factors including CCAAT enhancer-binding protein (C/EBP)-β and C/EBP-δ that induce peroxisome proliferator-activated receptor (PPAR)-γ and C/EBP-α expression[7,8], both regulate the main lipogenic genes including fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC)[9]. Lipolysis is the main mechanism involved in decreasing fat mass and body weight through the action of two enzymes adipose triglyceride lipase and hormone-sensitive lipase (HSL)[10]. The committed step for fatty acid oxidation depends mainly on the transport of fatty acid inside mitochondria through carnitine shuttle and controlled mainly through carnitine palmitoyltransferase Ⅰ (CPT-Ⅰ). Activation of this enzyme reflects mainly the fat burning stage of fatty acid and weight loss[11]. Raspberry fruits are an old fruit used for centuries for its several benefits. A lot of compounds are included in these fruits such as vitamins, minerals, amino acids, organic acids, and sugar, in addition to several other organic bioactive compounds including flavonoids, anthocyanin and ellagic acid which exhibit anticancer effect and protect against cardiovascular diseases[12]. Raspberry ketone [(4-hydroxyphenyl) butan-2-one], a natural phenolic compound, is present naturally in raspberries; it has been used many years ago in many industries including perfumes, cosmetics and as a flavoring agent in the food industry[13]. Raspberry ketone showed an anti-obese effect via increasing fatty acid oxidation and lipolysis[14,15]. Besides, it has anti-inflammation activity through inhibiting the nuclear kappa-β activation pathway[16] and antiandrogenic activity[17]. The trials for anti-obese effects of raspberry ketone were mainly performed on cell culture with observed few data on life experimental animals. So in the present study, we examined the possible anti-obese effect of raspberry ketone on obese rats fed with a high-fat diet.
2. Materials and methods
2.1. Chemicals
Raspberry ketone (purity ≥ 99%) was purchased from Wuhan Hezhong Chemical Manufacture Co. Ltd. China. The product was diluted in salad oil to reach required concentrations of 0.5%, 1%, and 2% for use.
2.2. Ethical statement
All the experimental procedures were conducted in line with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023) with the approval of Zagazig University ethics committee under the approval number ZU-IACUC/4/F/29/2018.
2.3. Animals
Fifty adult male albino rats weighing (120 ± 10) g were used in this study. Animals were housed in groups of 10 in cages at (25±0.5) ℃, under a 12:12 light/dark cycle, with free access to diets and water.
2.4. Animal grouping and treatment
After 2-week baseline period, rats were divided into five groups: normal control group fed a standard diet (25% maize, 15% barley meal, 15% soybean flour, 10% cabbage, 12% bran, 5% bone meal, 10% fish protein concentrate, 2% salt, and 1% yeast). Diabetic group received a high-fat diet (82% standard diet, 9% lard, 0.5% cholesterol, 8.3% yolk powder, and 0.2% sodium taurocholate)[12]. The other three groups received raspberry ketone in a concentration of 0.5%, 1%, and 2%, respectively for 12 weeks, along with a highfat diet[18].
2.5. Sampling protocol
All animals were weighed weekly for 12 weeks, fasted for 12 h, and then sacrificed for collection of blood and tissue samples (liver and adipose tissue) for biochemical and molecular investigations. Blood samples were collected by cervical decapitation of sacrificed rats for separation of serum that was used in estimation of biochemical parameters. Hepatic and adipose tissue samples were collected in liquid nitrogen and preserved at -80 ℃ till further use for biochemical and molecular analysis.
2.6. Biochemical determination
Serum glucose concentration was measured by Vitro Scient kits[19], serum insulin concentration by IMMULITE as described by Chevenne et al.[20], and total lipids by ABC diagnostics kits[21]. Moreover, total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triacylglycerols (TAG) were measured by Spinreact kits[22]. All steps were performed following the manufacturer’s instructions.
2.7. Molecular biological determination
Total RNA of hepatic and adipose tissue was extracted from all groups using the GeneJET RNA Purification Kit from ferments (St. Leon-Rot, Germany) following the manufacturer’s instructions. The quality and quantity of total RNA were examined using NanoDrop®(ND-1000 Spectrophotometer, NanoDrop Technologies, Wilmington Delaware, USA). Only pure samples were used for subsequent steps. One gram of total RNA was used for the synthesis of firststrand cDNA using reverse-transcriptase enzyme from a Quantitect RT Reverse Transcription kit (Qiagen, Hilden, Germany). One µL of total cDNA was mixed with 12.5 µL of 2×SYBR®Green PCR kit (Qiagen), 5.5 µL of RNase free water, and 0.5 µL (10 pmol/µL) of each forward and reverse primer for the measured genes. Primer3 software was used for primer design (The Whitehead Institute, http://bioinfo.ut.ee/primer3-0.4.0/ ) as per the published Rattus norvegicus, C/EBP-δ, FAS, ACC, PPAR-α, HSL, CPT1A and β-actin sequences (NM_013154.2, NM_017332.1, AB004329.1, NM_013196.1, NM_012859, FQ210484.1, and NM_031144.2), respectively, of NCBI database; all primers provided by Sigma-Aldrich (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) are shown in Table 1. PCR reactions were carried out in a thermal cycler (AbiPrism 7300) (Applied Biosystems, USA). The contamination of non-specific PCR products was excluded using a melting curve analysis to all final PCR products after the cycling protocol. The relative fold changes were calculated relative to the β-actin gene via the comparative 2-ΔΔCtmethod[23].

Table 1. The primer sequences and PCR conditions of the studied genes.
2.8. Statistical analysis
All data were analyzed using a one-way analysis of variance (ANOVA) using SPSS statistical version 22 software package (SPSS, Inc, USA). The inter-grouping homogeneity was determined by Duncan’s test. Data were presented as mean ± SD and P<0.05 was considered statistically significant.
3. Results
3.1. Effect of raspberry ketone on body weight of adult male rats
In Table 2, high-fat diet group showed a significant increase in body weight which started from the fourth week when compared with control group. Treatment with raspberry ketone in all doses decreased the weight in a dose-dependent manner, with 2% dose group showing the most significant result.
3.2. Effect of raspberry ketone on biochemical parameters of rats
In the group fed a high-fat diet, a significant increase was observed in blood glucose and insulin, total lipids, TAG, total cholesterol, LDL-C whereas a significant decrease was found in HDL-C. Treatment with raspberry ketone in all doses improved the glucose and insulin concentration with a decrease in lipid profile and an increase in HDL-C (Table 3).
3.3. Effect of raspberry ketone on gene expression in rats
Molecular investigations showed induction of gene expression of C/EBP-δ, FAS, ACC, CPT1A and inhibition of gene expression of PPAR-α and HSL in high-fat diet fed rats compared with control. Raspberry ketone treatment downregulated the gene expressions of C/EBP-δ, FAS, ACC, and upregulated the expressions of PPAR-α, HSL and CPT1A (Figure 1).

Table 2. Effect of high-fat diet (HFD) and raspberry ketone (RK) on body weight of rats (g).
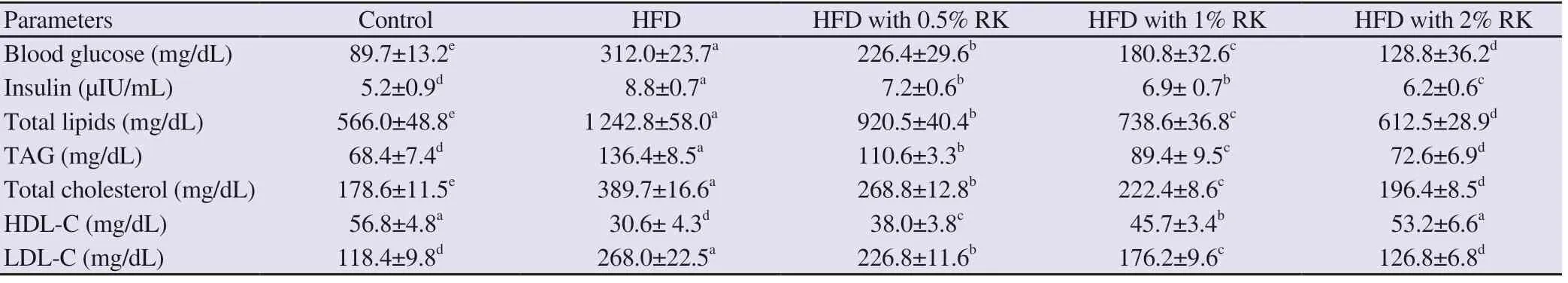
Table 3. Effect of high-fat diet (HFD) and raspberry ketone (RK) on biochemical parameters of rats.

Figure 1. Relative mRNA expression levels of (A) CCAAT enhancer binding protein delta [(C/EBP)-δ], (B) fatty acid synthase (FAS), (C) acetyl CoA carboxylase (ACC), (D) peroxisome proliferator activated receptor alpha (PPAR-α), (E) hormone sensitive lipase (HSL), (F) carnitine palmitoyltransferase 1A (CPT1A) genes relative to β-actin gene. Columns carry different letters (a, b, c, d and e) are statistically significant at P < 0.05.
4. Discussion
Raspberry ketone as the aromatic compound present mainly in raspberry fruits was reported to produce many beneficial effects including anti-inflammatory, antioxidant, anticancer and anti-obese effect[24]. The exact mechanisms of its anti-obese effect are not fully investigated and understood as many reports referred to many different mechanisms of its action. Therefore, our study was focused on this point. Obesity was induced by a high-fat diet[12] and the obesity was confirmed through body weight measurement and blood parameters. It has been known that feeding with high-fat diet induced obesity (increase in body weight) and insulin resistance that were detected by an increase in serum glucose with an increase of insulin level at the same time[18]. Total lipids, TAG, total cholesterol and LDL-C were also significantly increased with a significant decrease in HDL-C levels which reflects an increased risk of coronary heart disease associated with obesity. Raspberry ketone clearly showed its anti-obese effects through improvement on serum parameters in a dose-dependent manner; the results obtained showed that treatment with raspberry ketone in all doses showed a significant decrease in serum glucose, insulin, total lipids, TAG, total cholesterol, and LDL-C with a significant increase in HDL-C levels when compared with high-fat diet treated group.
The anti-obese effect of raspberry ketone examined in this experiment runs in two ways, the first way involved the detection of expression levels of some adipogenic genes and genes involved in fat synthesis. The mRNA expression level of C/EBP-δ, the transcriptional factor enhancing protein involved in adipogenesis through activation of PPAR-γ and C/EBP-α genes which affect the main enzymes in lipogenesis was significantly increased by feeding a high-fat diet, which reflects an increase in the number of fat cells (hyperplasia); in the same way, there was also a significant increase in the expression levels of ACC and FAS genes, the two main enzymes involved in fat synthesis which reflects the increase in the size of fat cell (hypertrophy) due to excessive fat intake. Treatment with raspberry ketone alleviates hyperplasia and hypertrophy of fat cells by decreasing the expression levels of both C/EBP-δ, ACC and FAS genes, therefore decreasing the body weight and producing anti-obese effects[6]. This mechanism demonstrates the suppressive effect of raspberry ketone on adipogenesis and fat accumulation by inhibiting the genes involved in this process. On another way, the mechanism of body weight loss depends mainly on increasing the fat burning via enhancing the oxidation level of fatty acids and lipolysis. However, this mechanism was significantly disabled by feeding a high-fat diet. Treatment with raspberry ketone significantly turns this mechanism on through increasing the expression level of PPAR-α, the main gene involved in activation of lipid catabolism in the peroxisome. In addition, raspberry ketone increases the expression levels of HSL and CPT1A which play important roles in lipolysis and fatty acid oxidation, respectively. This effect was demonstrated by body weight loss[25]. Inactivation of ACC in the first mechanism was related mainly to its phosphorylation through AMPK pathway[26], which resulted in decrease in malonyl CoA as the main allosteric inhibitor of CPT-Ⅰ; activation of CPT-Ⅰ which was important for fatty acids transport to mitochondria led to activation of fatty acids β-oxidation and subsequent body weight loss[27].
In summary, raspberry ketone can prevent obesity induced by high-fat diets in rats by induction of the expression of enzymes, controlling lipolysis and fatty acids β oxidation and inhibition of gene expressions of adipogenic factors.
Conflict of interest statement
The authors declare no conflict of interest.
Acknowledgments
This work was funded by the University of Jeddah, Saudi Arabia, under grant No (UJ-18-18-DR). The authors, therefore, acknowledge with thanks the University technical and financial support.
Funding
This work was funded by the University of Jeddah, Saudi Arabia, under Grant No (UJ-18-18-DR).
Authors’ contributions
AK and MA participated in the design of the study and helped to draft the manuscript. HA and MA carried out the molecular genetic studies. AMA analysed and interpreted data. MB and AA helped to draft the manuscript.
 Asian Pacific Journal of Tropical Biomedicine2020年1期
Asian Pacific Journal of Tropical Biomedicine2020年1期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Hepatoprotection by dandelion (Taraxacum officinale) and mechanisms
- Renoprotective effect of umbelliferone in high-fat diet/streptozotocin-induced type 2 diabetic rats
- Evolution of specific RNA aptamers via SELEX targeting recombinant human CD36 protein: A candidate therapeutic target in severe malaria
- Antioxidant and anti-melanogenic activities of ultrasonic extract from Stichopus japonicus
- Anti-microsporidial effect of thymoquinone on Encephalitozoon intestinalis infection in vitro
