Renoprotective effect of umbelliferone in high-fat diet/streptozotocin-induced type 2 diabetic rats
Jarinyaporn Naowaboot, Nuntiya Somparn, Suphaket Saenthaweesuk2Division of Pharmacology, Department of Preclinical Science, Faculty of Medicine, Thammasat University (Rangsit Campus), Pathum Thani 220, Thailand
2Division of Anatomy, Department of Preclinical Science, Faculty of Medicine, Thammasat University (Rangsit Campus), Pathum Thani 12120, Thailand
ABSTRACT Objective: To evaluate the renoprotective effect of umbelliferone in high-fat diet/streptozotocin-induced type 2 diabetic rats.Methods: We established a streptozotocin-induced type 2 diabetic model in male Wistar rats. The rats were fed with high-fat diet (45 kcal% lard fat) and injected with 35 mg/kg streptozotocin. Diabetic rats were treated with umbelliferone for 8 weeks. At the end of the experimental period, the serum and kidney were used for measuring biochemical parameters, protein expression and histological analysis. Results: After 8-week treatment, umbelliferone decreased fasting plasma glucose, concentrations of malondialdehyde and monocyte chemoattractant protein-1 in the plasma and tissues. It also significantly reduced serum creatinine, blood urea nitrogen, serum advanced glycation end products, as well as kidney weight in type 2 diabetic rats (P<0.05). Moreover, umbelliferone reduced the 24-h urine albumin, but increased 24-h urine creatinine excretion (P<0.05). In renal protein expression, umbelliferone decreased the levels of transforming growth factor-β1 and fibronectin while increasing the levels of superoxide dismutase and catalase (P<0.05). Renal histological examination revealed an enlarged glomerular size in diabetic rats, which was smaller in umbelliferone-treated diabetic rats.Conclusions: Umbelliferone alleviates renal dysfunction in diabetes via decreasing hyperglycemia, oxidative stress, inflammation and glycation.
KEYWORDS: Umbelliferone; Type 2 diabetes mellitus; Oxidative stress; Inflammation; Glycation
1. Introduction
Diabetic nephropathy is one of the main complications in type 1 and type 2 diabetes mellitus[1]. The major causes of pathogenesis in diabetes and hypertension are associated with oxidative stress and inflammation[2]. Higher levels of oxidative stress and inflammation are significantly correlated to the pathogenesis of diabetic nephropathy[3]. Oxidative stress condition is caused by an imbalance of reactive oxygen species (ROS) production and antioxidant defense system[4]. The oxidative stress induced by hyperglycemia has been reported to damage the kidney[5]. Lipid peroxidation is regularly indicative of stimulation of cellular oxidative stress. Malondialdehyde (MDA) production is considered as an indicator for lipid peroxidation[6]. There are many sources of ROS generation under chronic hyperglycemia in the diabetic condition, of which advanced glycation end products (AGEs) are the major ones which are involved in the development of type 2 diabetes and diabetic complications[7].
The markers of diabetic nephropathy progression are associated with increased circulating creatinine and blood urea nitrogen (BUN), together with reduced urinary creatinine excretion[8]. The stimulation of inflammatory process demonstrated a robust correlation with the development and progression of diabetic nephropathy[9]. A monocyte chemoattractant protein-1 (MCP-1) is a proinflammatory chemokine mainly expressed in kidney of diabetic animal models[10]. The conditions of oxidative stress, inflammation and fibrosis are mostly expressed in the progression of diabetic nephropathy[11]. The elevation of fibronectin and type Ⅳ collagen results in fibrosis in the mesangium and renal tubulointerstitium, which is an indicator of diabetic nephropathy[12]. Transforming growth factor-β1 (TGFβ1), as a profibrotic protein, is importantly related to the diabetic nephropathy[13]. The diabetic condition can stimulate oxidative stress and induce TGF-β1 expression[13].
Umbelliferone, a simple coumarin, is commonly found in plant families of Asteraceae, Rutacea and Umbelliferae[14]. Umbelliferone has been reported to show many pharmacological properties such as antioxidant, antibacterial, anti-inflamamtory, anticancer and antidiabetic effects[14]. On diabetes and its complications, umbelliferone exerts an antihyperglycemic effect in streptozotocin (STZ)-induced type 1 diabetic rats[15]. Reportedly, umbelliferone can restore the impaired level of glucose and lipid metabolism in high-fat diet/STZ-induced type 2 diabetic rats[16]. A recent study reported that umbelliferone exerts a protective effect on renal damage in type 1 diabetic rats[17]. It might also play a role in reducing diabetic complications. Therefore, the objective of the present study was to evaluate the effect of umbelliferone on diabetic nephropathy in high-fat diet/STZ-induced type 2 diabetes in a rat model.
2. Materials and methods
2.1. Chemicals and reagents
All chemicals including umbelliferone were purchased from Sigma-Aldrich (St. Louis, MO, USA). Low-fat diet (LFD; D12450H) and high-fat diet (D12451) were obtained from Research diets (New Brunswick, NJ, USA). Primary antibodies (β-actin, catalase, manganese superoxide dismutase, TGF-β1 and fibronectin) and secondary antibody were obtained from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). Polyvinylidene difluoride (PVDF) membrane was obtained from EMD Millipore (Billerica, MA, USA). TPER®and Halt®protease inhibitor cocktail was obtained from Thermo Scientific (Rockford, IL, USA).
2.2. Ethical statement
All animal experiments were endorsed by the Animal Ethics Committee of Thammasat University, Pathum Thani, Thailand (Rec. No. AE 007/2014). Animal experiments were conducted according to the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC accreditation).
2.3. Animals, induction of diabetes and experimental design
Twenty-four male Wistar rats (National Laboratory Animal Center of Mahidol University, Nakhon Pathom, Thailand), weighing about 120-150 g, were fed low-fat diet or high-fat diet for 12 weeks. The animals were housed at Thammasat University Laboratory Animal Center, in a standard room at (25±2) ℃ and a 12-h dark/light cycle. A type 2 diabetic rat model was induced, as described previously[16]. After 3-week high-fat diet feeding, rats were intraperitoneally injected with STZ (35 mg/kg). One week later, the rats with fasting blood glucose (FBG) >180 mg/dL were chosen for diabetic investigation. Rats were divided into three groups (n=8 per group) as follows: groupⅠ: low-fat diet-fed rats (normal control rats) were orally administered with 5% gum arabic; group Ⅱ: diabetic control rats were orally administered with 5% gum arabic; and group Ⅲ: diabetic rats were orally administered with umbelliferone 30 mg/kg/day. The dose of umbelliferone was based on our previous study[16]. All treatments were continued for 8 weeks.
Since gum arabic has been considered as a nontoxic substance and used in drug preparations[18], the concentration of 5% gum arabic was selected for dissolving umbelliferone[19].
At week 7, the amounts of 24-h urine were collected for determination of albumin and creatinine levels. We only collected the 24-h urine after 7-week treatment because we noticed that the metabolic cages could promote stress response in our Wistar rats. In our preliminary experiment, we found that after the rats were transferred back into the standard cages, food and water intake as well as the body weight did not seem to stabilize for a few days. Therefore, we decided to investigate the 24-h urine albumin and creatinine in only week 7. FBG and the body weight were measured before and after 8-week treatment. After 8-week treatment, the overnight-fasted rats were sacrificed using isoflurane anesthesia. Blood samples were collected from the heart, and the kidneys were immediately removed for biochemical, histological, and protein expression analysis. The kidney weight of mice was measured by the ratio of kidney weight to final body weight.
2.4. Determination of serum creatinine, BUN, AGEs, MDA and MCP-1
Concentrations of serum creatinine and BUN were evaluated using the colorimetric kit (Wako, Osaka, Japan). Serum AGEs content was determined using ELISA kit (Abcam, Cambridge, MA, USA). Serum MDA was estimated using thiobarbituric acid reactive substances assay kit (TBARS, Cayman Chemical, MI, USA) and serum MCP-1 using ELISA kit (Thermo Scientific).
2.5. Determination of 24-h urine albumin and creatinine
Urine creatinine concentration was estimated using the colorimetric kit (Wako, Osaka, Japan) and urine albumin was measured using ELISA kit (Abcam, Cambridge, MA, USA).
2.6. Kidney MCP-1 and MDA determination
The kidney MDA was extracted as previously described[20], and the level was evaluated using TBARS assay kit (Cayman Chemical). Kidney MCP-1 was extracted similar to the protocol described previously for pancreas insulin[21], and the concentration was estimated using ELISA kit (Thermo Scientific).
2.7. Western blot analysis
The homogenization and extraction of kidney were prepared in TPER®mixed with Halt®protease inhibitor cocktail. Protein samples (20 µg) were used for Western blot analysis. The protocol was determined as described in our previous study[20]. All intensity of the target proteins (catalase, manganese superoxide dismutase, TGF-β1 and fibronectin) was normalized to that of β-actin.
2.8. Histological analysis
A portion of the kidney was fixed with 10% formalin and embedded in paraffin. The tissue sections (3 µM) were stained with hematoxylin and eosin (H&E) for microscopic observation (Olympus, Tokyo, Japan). The software of Image J (National Institute of Health, MD, USA) was used for calculating glomerular areas.
2.9. Statistical analyses
The data are presented as mean±SEM of each group. Significant differences among groups were analyzed by one-way analysis of variance (ANOVA) and Tukey’s post-hoc test. Statistical analyses were performed using computer-based software SigmaStat (Systat Software, CA, USA). P<0.05 was regarded to be significant.
3. Results
3.1. Biochemical parameters
There were no significant differences in the initial body weight of all groups. After 8-week treatment, no significant differences were observed in the body weight among the three groups (Figure 1A). Increased kidney weight was observed in diabetic control group (Figure 1B). Compared to the diabetic control group, umbelliferone treatment significantly reduced kidney weight. FBG of the diabetic control group was still high after 8 weeks. FBG was significantly decreased in UMB-treated group as compared to the diabetic control group. However, the diabetic rats treated with umbelliferone significantly decreased the FBG by 31% as compared to before umbelliferone treatment (Figure 1C).
In the diabetic control group, serum and kidney MDA were increased (Figure 2A and B). In addition, the diabetic control group showed the elevated levels of serum and kidney MCP-1 (Figure 2C and D). However, these parameters were significantly decreased in umbelliferone-treated diabetic group. Moreover, the diabetic rats treated with umbelliferone significantly decreased the serum AGEs concentration (Figure 2E).
In comparison with the diabetic control group, umbelliferonetreated diabetic group exhibited a significant reduction in serum creatinine and BUN levels (Figure 3A and B). Umbelliferone treatment also showed a significant increase in urine creatinine excretion and a decline in the level of urine albumin as compared to the diabetic control rats (Figure 3C and D).
3.2. Kidney histology
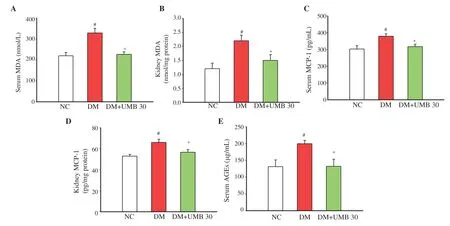
Figure 2. Serum MDA (A), kidney MDA (B), serum MCP-1 (C) and kidney MCP-1 (D) and AGEs (E) in normal control rats (NC), diabetic control rats (DM), and diabetic rats treated with UMB. Values are represented as mean ± SEM (n=8). #P<0.05 compared to the normal control group. *P<0.05 compared to the diabetic control group. UMB: umbelliferone; MDA: malondialdehyde; MCP-1: monocyte chemoattractant protein-1; AGEs: advanced glycation end products.
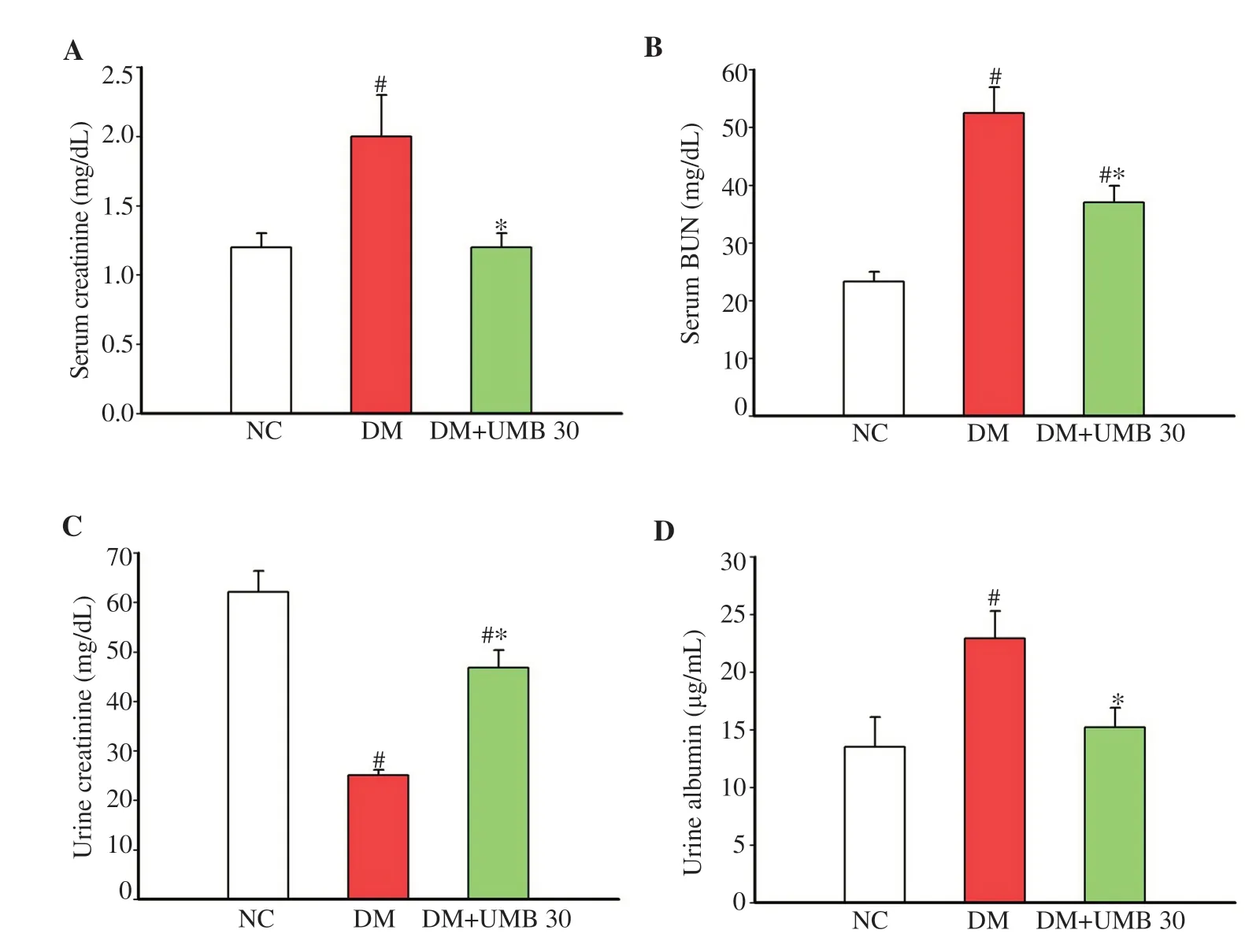
Figure 3. Serum creatinine (A), serum BUN (B), 24-h urine creatinine (C) and 24-h urine albumin (D) in normal control rats (NC), diabetic control rats (DM), and diabetic rats treated with UMB. Values are represented as mean ± SEM (n=8). #P<0.05 compared to the normal control group. *P<0.05 compared to the diabetic control group. UMB: umbelliferone; BUN: blood urea nitrogen.
Staining of the kidney tissues demonstrated that the glomeruli of normal control group were morphologically normal, whereas that of the diabetic control group was expanded after the 12-week experimental period (Figure 4A). Umbelliferone reversed these changes and the treated group showed a regular pattern of glomeruli similar to that in normal control rats. Moreover, the glomerular size was clearly increased in the diabetic control group. However, umbelliferone treatment significantly reduced the glomerular size as compared to the diabetic control group (Figure 4B).
3.3. Kidney protein expression
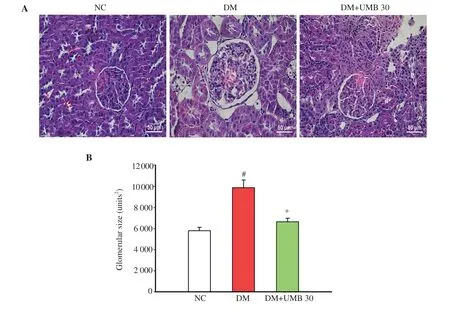
Figure 4. Representative photomicrographs of glomeruli at 400× magnification (A) and glomerular size (B) in normal control rats (NC), diabetic control rats (DM), and diabetic rats treated with UMB. Values are represented as mean ± SEM (n=8). #P<0.05 compared to the normal control group. *P<0.05 compared to the diabetic control group. UMB: umbelliferone.
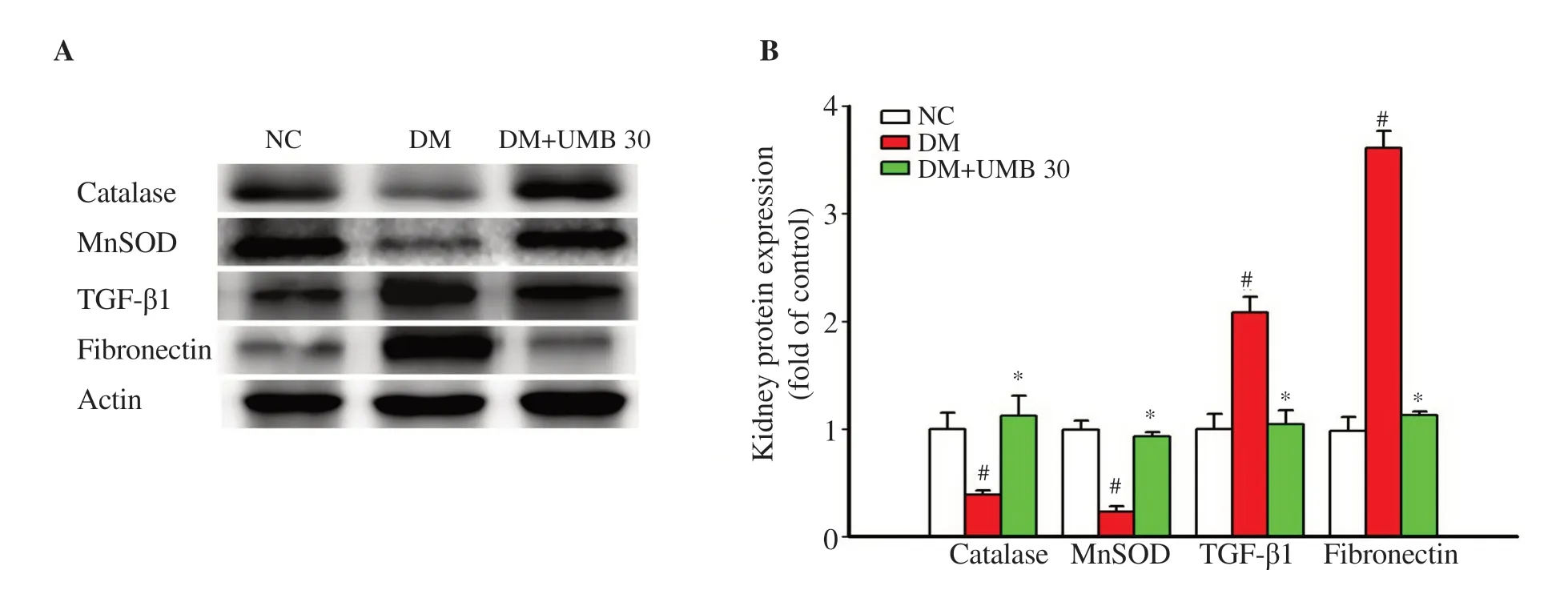
Figure 5. Kidney protein expressions of catalase, MnSOD, TGF-β1 and fibronectin by Western blot assay (A) and quantitatively shown in bar graphs (B) in normal control rats (NC), diabetic control rats (DM), and diabetic rats treated with UMB. Values are represented as mean ± SEM (n=8). #P<0.05 compared to the normal control group. *P<0.05 compared to the diabetic control group. UMB: umbelliferone; MnSOD: manganese superoxide dismutase; TGF-β1: transforming growth factor-β1.
Compared with the diabetic control rats, the protein expressions of catalase and manganese superoxide dismutase were markedly increased in umbelliferone-treated diabetic rats (Figure 5A and B). In addition, the diabetic rats treated with umbelliferone showed a significant reduction of the protein expressions of TGF-β1 and fibronectin (Figure 5A and B).
4. Discussion
This study revealed that umbelliferone treatment significantly decreased hyperglycemia, inflammation, oxidative stress and glycation in high-fat diet/STZ-induced type 2 diabetic rat model. These results indicated a renoprotective effect of umbelliferone, which was dependent on glucose-lowering effect, including anti-inflammatory, antioxidant and antiglycation effects. The current study also demonstrated that umbelliferone treatment can delay the hyperfiltration via reducing kidney weight and urinary protein excretion in diabetic rats.
Prolonged hyperglycemia contributes to the progression of diabetic complications. The inflammation, cytokine release and cellular death are caused by hyperglycemia condition, leading to the development of diabetic complications such as nephropathy[22]. Oxidative stress, inflammation, and fibrosis are involved in the progression of diabetic nephropathy[11,23]. There has been reported that the inhibition of renal inflammatory cell recruitment protects against renal dysfunction in diabetic nephropathy[24]. Reportedly, the development of early diabetic nephropathy is related to the activation of MCP-1[25]. Therefore, MCP-1 plays a major role in the incidence and progression of diabetic nephropathy, and levels of kidney MCP-1 show a correlation with markers of diabetic nephropathy. Interestingly, umbelliferone treatment diminished the serum and kidney MCP-1 concentration in diabetic rats.
Oxidative stress is a major cause in the development of diabetic vascular complications, especially type 2 diabetes[26]. The elevation of ROS level in diabetes might occur due to declined antioxidant enzyme productions such as catalase, superoxide dismutase, and glutathione peroxidase[26]. The various amounts of antioxidant enzymes make the tissues sensitive to oxidative stress[26]. In addition, we observed increased ROS generation in the diabetic rats as assessed by elevated MDA production and elevated antioxidant enzyme consumption such as superoxide dismutase and catalase. Umbelliferone administration decreased the MDA level and increased the superoxide dismutase and catalase protein expression in kidney. The production of AGEs was connected with the pathogenesis of type 2 diabetes and diabetic complications, including diabetic nephropathy[7]. This phenomenon was related to the alterations in the redox state caused by constant hyperglycemia and enhanced AGEs[27]. The current results also showed the antiglycation effect of umbelliferone by reducing the level of serum AGEs, thereby indicating that umbelliferone may alleviate ROS generation and restore antioxidant status.
TGF-β1 formation is relevant to the development of kidney fibrosis[28]. The stimulation of TGF-β1 led to the augmentation of collagen Ⅰ, fibronectin, and connective tissue growth factor[28]. Another research demonstrated that the formation of TGF-β1 was associated with enhanced ROS production and suppressed antioxidant enzymes, which in turn, caused a redox imbalance[29]. Then, ROS induced/activated TGF-β1 and mediated several fibrogenic effects of TGFβ[29]. Moreover, this study found that the protein expressions of kidney fibronectin and TGF-β1 were suppressed by umbelliferone treatment. Development of diabetic nephropathy is related to increased circulating creatinine and BUN, along with decreased urine creatinine excretion[8]. In our present results, the diabetic nephropathy was significantly alleviated by umbelliferone treatment. Umbelliferone could decrease the serum creatinine and BUN, increase the urine creatinine excretion, and reduce the 24-h urinary microalbumin concentrations.
Based on pharmacological reports of umbelliferone, we assumed that the possible renoprotective mechanism of umbelliferone in the rat model with diabetic nephropathy may be due to suppression of oxidative stress and inflammation by reducing kidney MCP-1 and TGF-β1 protein expression, and stimulating the protein expressions of catalase and superoxide dismutase in the kidney. However, the precise molecular mechanism underlying these effects of umbelliferone was not explored completely. Therefore, further study on umbelliferone should be performed to clarify how the umbelliferone suppresses the oxidative stress and inflammation pathways.
In conclusion, umbelliferone improves the diabetic kidney function, which might be connected with anti-hyperglycemia, antioxidant, antiinflammation, and antiglycation effects. Thus, the current findings indicated that umbelliferone is effective in improving diabetic nephropathy in high-fat diet/STZ-induced diabetic condition.
Conflict of interest statement
We declare that there is no conflict of interest.
Acknowledgments
The authors gratefully acknowledge the financial support provided by Thammasat University Research Fund under the TU Research Scholar, Contract No.GEN2/33/ 2018.
Funding
This work was financially supported by Thammasat University Research Fund under the TU Research Scholar, Contract No.GEN2/33/ 2018.
Authors’ contributions
JN designed the experiments, collected the samples, analyzed the data and wrote the manuscript. NS and SS collected the samples. All authors contributed to the final version of the manuscript.
 Asian Pacific Journal of Tropical Biomedicine2020年1期
Asian Pacific Journal of Tropical Biomedicine2020年1期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Hepatoprotection by dandelion (Taraxacum officinale) and mechanisms
- Raspberry ketone attenuates high-fat diet-induced obesity by improving metabolic homeostasis in rats
- Evolution of specific RNA aptamers via SELEX targeting recombinant human CD36 protein: A candidate therapeutic target in severe malaria
- Antioxidant and anti-melanogenic activities of ultrasonic extract from Stichopus japonicus
- Anti-microsporidial effect of thymoquinone on Encephalitozoon intestinalis infection in vitro
