Cutaneous leishmaniasis in an indigenous infant with Down's syndrome:A case report
Fernanda Fresneda Villibor, Geracina Marchesini, Ana Lúcia Roselino Ribeiro, Renata Oliveira Guaré
1Centro Universitário Tocantinense Presidente Antonio Carlos, Araguaína, Tocantins, Brasil
2Programa de Pós Graduação em Odontologia, Universidade Cruzeiro do Sul, São Paulo, São Paulo, Brasil
3Hospital de Doenças Tropicais, Araguaína, Tocantins, Brasil
4Faculdade de Ciências do Tocantins, Araguaína, Tocantins, Brasil
Keywords:Cutaneous leishmaniasis Indigenous Down’s syndrome Immune deficiencies
ABSTRACT Rationale: Leishmaniasis, caused by the protozoan Leishmania and transmitted by sandflies,is endemic to the tropical and subtropical areas of Brazil. It is one of the neglected tropical diseases in the world.Patient concerns: A 20-month-old indigenous infant with severe malnutrition and Down’s syndrome presented with a facial ulcer for 5 months.Diagnosis: Giemsa staining of scraped ulcer tissues indicated the presence of the amastigote form of Leishmania sp., and positive Montenegro’s intradermal test helped in diagnosing the condition as cutaneous leishmaniasis.Interventions: The child was hospitalized and received intravenous treatment for cutaneous leishmaniasis (1 mg/kg/day of amphotericin B).Outcomes: The condition was cured with amphotericin B (1 mg/kg/day for 14 d).Lessons: Because of infanticide practices in indigenous cultures, indigenous infants with Down’s syndrome rarely survive. Thus, no similar case has been reported in the literature.
1. Introduction
Leishmaniasis, caused by the protozoan Leishmania and transmitted by sandflies, is endemic to the tropical and subtropical areas of Brazil. It is one of the neglected tropical diseases in the world. Three major clinical forms of leishmaniasis are recognized:visceral, cutaneous, and mucocutaneous. Panamerican Health Organization reported 3 289 new cases of visceral leishmaniasis and 12 690 cases of cutaneous leishmaniasis in Brazil in 2016[1]. Brazil is one of the 10 countries that have the highest prevalence for both clinical forms[2].
Down’s syndrome presents with complex immunological alterations, such as defect in lymphocyte proliferation and reduction in the ability to produce interferon-gamma (IFN-γ), which can be associated with a considerable increase in susceptibility to infections[3-6]. This study reports a case of an indigenous infant with Down’s syndrome and cutaneous leishmaniasis. To the best of our knowledge, no similar case has been reported in the literature in indigenous population.
2. Case report
In March 2015, a 20-month-old indigenous female infant with a 5-month history of an ulcerated facial lesion, 9 days of respiratory distress and coughing, was hospitalized at the Hospital for Tropical Diseases (Araguaína, Tocantins, Brazil). The patient also had Down’s syndrome and congenital heart disease (perimembranous interventricular communication).
This case report was conducted after Ethics Committee approval(CE-UCS-037/2014). A written informed consent to report this case with accompanying images was obtained from the parents of the patient. During the physical examination, she presented with the following signs and symptoms: severe malnutrition (4.65 kg),tachy dyspnea, under activity, whining, crying, feverless, anicteric,cyanosis, pallor, and characteristics of Down’s syndrome[7].In addition, she presented with an ulcerated facial lesion in the zygomatic region, with raised edges, without secretion, extending to the upper lip and nasal congestion, with a yellowish green nasal discharge (Figure 1). Regarding the circulatory system, her heart rate, checked in two phases, showed the presence of holosystolic grade 4 heart murmur. She had a scaphoid abdomen with no visceromegaly; her upper and lower limbs showed no morphological changes.
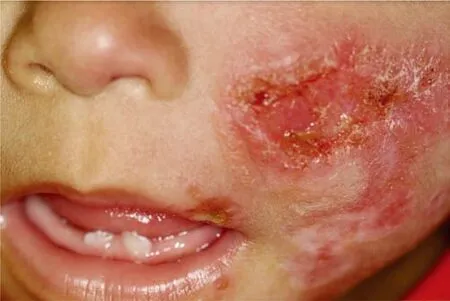
Figure 1. A 20-month-old indigenous female infant with a 5-month history of an ulcerated facial lesion, 9 days of respiratory distress and coughing.The patient also had Down’s syndrome and congenital heart disease(perimembranous interventricular communication). Ulcerated facial lesion in the zygomatic region, which was without secretion and extended to the upper lip, compatible with cutaneous leishmaniasis.

Figure 2. Ulcerated facial lesion in the zygomatic region, after the first week,which showed a decrease in diameter and depth and major improvement in the appearance of the lesion.
Laboratory tests were performed at the time of hospitalization and the following results were observed: positive Montenegro’s intradermal test and presence of Leishmania after direct examination to determine the strain of the facial lesion by Giemsa staining.
Intraoral physical examination, performed by a dental surgeon,revealed the eruption of teeth 71 and 81 and appearance of a normal gum; however, she had a leishmaniasis lesion on the left upper lip.
Following the diagnosis of leishmaniasis, the child was hospitalized for commencement of intravenous treatment for cutaneous leishmaniasis (1 mg/kg/day of amphotericin B). Additionally,antibiotic therapy was prescribed for symptomatic treatment of pneumonia with ceftriaxone (100 mg/kg/day), oxacillin(200 mg/ kg/ day), and methylprednisolone (2 mg/kg/day for 2 d).Laboratory tests were performed including blood count and liver,kidney, and heart function tests. In addition, nutritional support,respiratory, physiotherapy, and general care were included in treatment procedures. After the first week, we observed major improvement in the appearance of the lesion, which showed a decrease in diameter and depth, improved respiratory status, and weight gain following nutritional counseling (Figure 2).
During hospitalization, results of kidney (urea, creatinine,and electrolytes) and liver function tests (glutamic oxaloacetic transaminase, glutamic pyruvic transaminase, gamma glutamyl transferase, alkaline phosphatase, and serum albumin), and electrocardiographic and echocardiographic assessments were specifically recorded. These examinations were performed before,during, and after treatment, except for electrocardiography, which was hindered by operational difficulties.
After 14 d, significant improvement in the lesion and complete resolution of the respiratory infection were observed but evolved with moderate hypokalemia. Due to the favorable developments and other difficulties in monitoring of cardiac function, amphotericin B treatment was interrupted after 14 d; the child was referred to outpatient follow-ups every 3 d for reassessment of the lesion. After 15 d of follow-up, the child showed complete resolution of the skin and lesion, and such that follow-ups (medical and dental) were conducted biweekly. After 6 weeks, the child’s facial and lip lesions had healed completely, with excellent general health and weight gain of 1 kg (Figure 3). Eight months after treatment, the child presented with no new signs or symptoms of leishmaniasis.

Figure 3. Lesion after 45 days. The child’s facial and lip lesions had healed completely.
3. Discussion
In some Brazilian indigenous tribes, infanticide practices remain owing to cultural particularities, regarding whether or not the infant can survive and develop within the tribe they were born into[5]. Given this fact, reports of indigenous children with Down’s syndrome and leishmaniasis are absent from the literature.
This population has probably been affected due to the adaptation capacity of Leishmania vectors[7]and poor sanitary and housing conditions among certain tribes. It is also possible that many cases are not reported because indigenous people follow their own medical practices, and do not always seek medical care from hospitals.
The etiologic diagnosis of cutaneous leishmaniasis, in all its forms,is a difficult task. The parasitological tests that aim to identify the parasite primarily consist of direct microscopy, in vitro culture, and histopathological examination[8].
The therapeutic strategies against leishmaniasis is restricted because one of the most recommended drugs are the pentavalent antimonials[9]. However, due to the patient’s congenital heart disease, we opted for amphotericin B, even though this drug is not risk-free for individuals with acute heart diseases. Liposomal amphotericin B, although less toxic, was ruled out because it is still an off-label drug, according to the treatment protocol proposed by the Brazilian Ministry of Health[10]. It is worth highlighting that we were treating a patient with trisomy 21 (Down’s syndrome), who was indigenous, severely malnourished (age: 20 months; weight: 4.65 kg), pneumonic, and sinusopathic. Several authors have reported changes in all aspects of immune response, regardless of the clinical expression of these changes in children with Down’s syndrome[4].Individuals with Down’s syndrome may be highly prone to infections, usually of the upper respiratory tract, characterized by increased severity and prolonged course of disease, which are partially attributed to defects of the immune system[4].
Delayed tooth eruption is common in patients with Down’s syndrome[6]and this fact was discussed with the parents, together with guidance on the care required to maintain oral health of children, to prevent problems that could have a negative impact on the systemic health of the child.
Multidisciplinary follow-up for children with Down’s syndrome is essential and even more important when they present with comorbidities, such as leishmaniasis and malnutrition. In this case, after being discharged from hospital, follow-up on the child continued in outpatient services by a pediatrician, an infectious disease specialist, a pediatric dentist, and a nutritionist for 6 months.Children with Down’s syndrome present with immune deficiencies that may predispose them to leishmaniasis in endemic locations.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Authors’ contributions
FFV developed the literature search, definition of intellectual content, manuscript preparation and manuscript review. Both GM and ALRR contributed to the manuscript preparation and review.ROG contributed to manuscript preparation, manuscript editing and manuscript review.
 Asian Pacific Journal of Tropical Medicine2019年12期
Asian Pacific Journal of Tropical Medicine2019年12期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Multi-targeting cytotoxic drug leads from mushrooms
- Spectrum of thalassemia mutations in fetuses of Han and Li ethinicities in Hainan province, China
- Seroprevalence of brucellosis among exposed agro-pastoral communities in southern Saudi Arabia
- Effect of El Niño Southern Oscillations on the incidence of enteric fever in Ahmedabad, India from 1985 to 2017
- Prevalence and risk factors of avian influenza H9N2 among backyard birds in Iran in 2015
- Alpinia officinarum Hance extract alleviates particulate matter-induced lung injury in mice
