Alpinia officinarum Hance extract alleviates particulate matter-induced lung injury in mice
Yao-xin Zhao, Wen-jing Ruan, Wen-lai Xue, Ling Zhao
School of Biology and Pharmaceutical Engineering, Wuhan polytechnic University, Wuhan, China
Keywords:Alpinia officinarum Hance extract Particulate matter Lung injury
ABSTRACT Objective: To evaluate the effect of Alpinia officinarum Hance (A. officinarum) extract on lung injury caused by particulate matter (PM).Methods: The Kunming mice were intranasally instilled with PM and treated with A.officinarum extract for 3 weeks. Bronchoalveolar lavage fluid, blood and lung samples were collected for biochemical, serological and histopathological studies.Results: Serological analysis showed that albumin levels, lactate dehydrogenase and alkaline phosphatase activities in bronchoalveolar lavage fluid were significantly reduced after administrations of 50, 100 and 200 mg/kg of A. officinarum extracts to the PM injured mice.Markers of oxidative stress, nitric oxide, malondialdehyde levels and nitric oxide synthase activities, were significantly decreased. Correspondingly, total superoxide dismutase activity was improved dramatically. The expressions of interleukin-6 and tumor necrosis factor alpha were also down-regulated obviously. In addition, pathological sections of lung tissue showed that A. officinarum could reduce the infiltration of inflammatory cells, pulmonary edema and pulmonary fibrosis. These results showed that A. officinarum extract could alleviate PMinduced lung injury via reducing the permeability of cell membranes in lung tissue, eliminating oxidative stress and relieving inflammatory response.Conclusions: A. officinarum extract was an efficient treatment for PM-induced lung injury in mice, and it may be a promising therapeutic agent in future.
1. Introduction
Exposure to air pollution causes a variety of diseases, such as ischaemic heart disease, cerebrovascular disease, chronic obstructive pulmonary disease, and lower respiratory infections, leading to increased burdens in health care globally[1]. One of the most concerned factors in air pollution is particulate matter (PM), which is the main cause of premature death[2]. Epidemiological studies have shown that PM, especially PM2.5in atmosphere is implicated to the occurrence of respiratory system abnormalities, including decreased lung function, asthma attacks, bronchial inflammation and lung cancer[3-5]. Currently, researches have shown that PMinduced respiratory system diseases are mainly caused by increased pulmonary vascular permeability and haematological changes,including tissue edema, inflammatory damage and oxidative stress[6-8], resulted in histopathological and biochemical alterations of lungs and increased release of inflammatory factors. Severe and persistent inflammatory response damages alveolar epithelial cells and causes excessive repair, which in turn leads to pulmonary fibrosis or other related lung injury diseases[9,10]. Until now, many chemical candidates have been studied pre-clinically[11], however,only a few are permitted to be tested in clinical trails[12]and no drug has been approved. Therefore, the ongoing search of novel therapeutic agents is needed urgently.
Alpinia officinarum Hance (AO), belonging to the Zingiberaceae family, is a medicinal and food plant distributed widely in the tropical and subtropical regions of Southeast Asia[13]. It is commonly used as a flavouring and antioxidant reagent to regulate the freshness and extend the shelf life of foods. It is also used alone or in combination with other herbs to treat common cold, inflammation,digestive disorders and so on[14]. Recent researches showed that AO is pharmacologically effective in anti-inflammation, antioxidation[15], antitumor[16], anti-bacteria[17], anti-osteoporosis[18],and lipid metabolism regulation[19], which prompted us to investigate whether it is also effective on PM-induced lung injury. Therefore,the objective of this study was to investigate the therapeutic effects of AO extract on PM-induced lung injury in mice.
2. Materials and methods
2.1. Reagents
The rhizomes of AO were collected in Xuwen County, Guangdong Province. The voucher specimen (No: WHPU-1813) was authenticated by Prof. Ping Chen and preserved in the Herbarium of Wuhan Polytechnic University, Wuhan, China. Fine particulate matter (PM2.5) was collected onto microfiber filter membranes at Hubei Environmental Monitoring Centre Station (114.37° E, 30.54° N) by Hubei Academy of Environmental Sciences from November 2017 to March 2018. Single-walled carbon nanotubes (SWNTs, length 0.5 to 2.0 μm) were purchased from Chengdu Organic Chemistry Co., Ltd., Chinese Academy of Sciences. Albumin (ALB), lactate dehydrogenase (LDH), alkaline phosphatase (AKP), nitric oxide(NO), nitric oxide synthase (NOS), total superoxide dismutase(T-SOD), malondialdehyde (MDA) kits were purchased from Nanjing Jiancheng Biological Engineering Research Institute.Enzyme-linked immunosorbent assay kits for mice tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) were purchased from Elabscience Biotechnology Co. Ltd (Wuhan, China).
2.2. Preparation of PM suspension
The PM2.5-adsorbing filter membranes were cut into 1 cm×3 cm slices and immersed in ultra-pure water. After ultrasonic vibration 30 min for three times and filtration, the PM2.5was obtained. PM2.5and SWNTs of the same quality were mixed in physiological saline to prepare the PM suspension with a concentration of 80 mg/mL. The PM suspension was sterilized by autoclaving for 30 min and stored at 4 ℃.
2.3. Preparation of AO extract
The rhizomes of AO were extracted twice with 75% ethanol. After recovering ethanol to 40% concentration, precipitation was obtained.Then the precipitation was dissolved in 50% ethanol, separated by D101 macroporous resin with ethanol-water gradient as solvent. The AO extract was prepared by recovering and drying the fraction eluted by 75% ethanol. A certain weight of dry AO extract was suspended in 0.4% sodium carboxymethyl cellulose (CMC-Na) to prepare a dose of 200 mg/kg for experiment. The suspension of 100 mg/kg and 50 mg/kg dosage were obtained by gradient dilution.
AO extract was diluted and subjected to analyse by Shimadzu LC-20AT series HPLC with cosmosil column C18, 250.0 mm×4.6 mm,5 μm. Analysis was carried out at a flow rate of 1 mL/min with the detection wavelength set at 326 nm and the injection volume was set at 10 μL with a runtime of 100 min. Mobile phase composition selected was mixture of methanol and 0.2% phosphoric acid(55: 45 v/v). Column temperature was maintained at 40 ℃. The chromatogram of AO extract was shown in Figure 1.
2.4. Animals
Kunming mice (weight, 18-22 g) were obtained from Laboratory Animal Centre, Huazhong University of Science and Technology(Certificate No. SCXK: 2016-0009, Wuhan, China). All mice were kept at 22-25 ℃, 40%-60% relative humidity, 12 hours of light a day, and free accessed to food and distilled water.
2.5. Ethical approval
The experimental procedures and animal welfare were in accordance with Wuhan Polytechnic University’s experimental animal ethics regulations, and all animal experiments were approved by the Wuhan Polytechnic University Animal Experimental Ethics Committee (No: 20180807017) on 7 August 2018.
2.6. Animals model
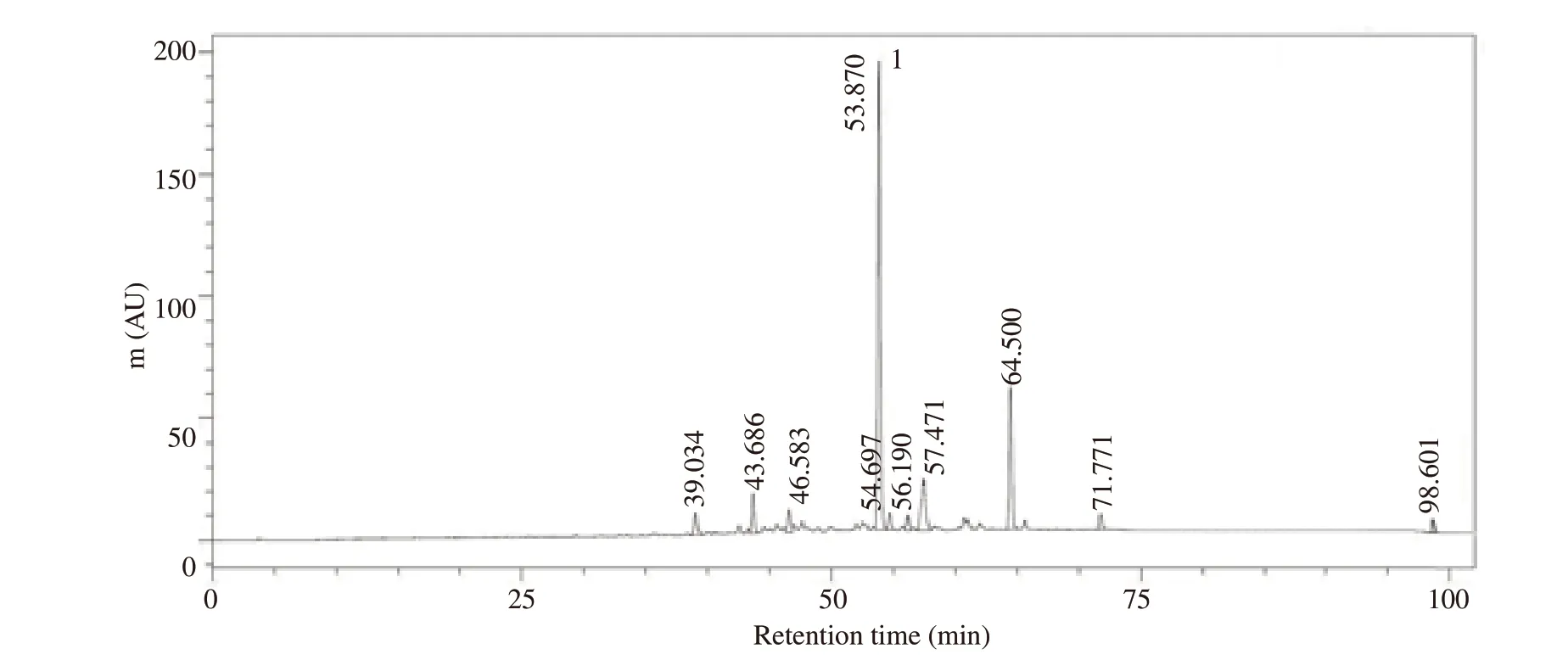
Figure 1. HPLC chromatogram (326 nm) of Alpinia officinarum extract. Mobile phase: Methanol and 0.2% phosphoric acid (55: 45, v/v); flow rate: 1 mL/min; column temperature: 40 ℃. Peak 1 was galangin.
Fifty Kunming mice were randomly divided into five groups:control group (CON, intranasally instilled saline and oral 0.4%CMC-Na), model group (PM, intranasally instilled PM suspension and oral 0.4% CMC-Na), low dose of AO group (PM+LD-AO,intranasally instilled PM suspension and oral AO, 50 mg/kg),medium dose of AO group (PM+MD-AO, intranasally instilled PM suspension and oral AO, 100 mg/kg), and high dose of AO group (PM+HD-AO, intranasally instilled PM suspension and oral AO, 200 mg/kg). The mice of PM and AO treatment groups were intranasally instilled with PM suspension (40 mg/kg) in the afternoon on the day 1, 5, 9, 13, 17 and 21, while the control group were intranasally instilled with equal saline. From the 2nd to 22nd day, AO solutions (50, 100 and 200 mg/kg suspended in CMC-Na,oral) were administered to the treatment groups every morning,respectively, the same dose of CMC-Na solutions were administered to the PM and the control groups. The physiological status of the mice was monitored weekly, and all mice were sacrificed in the afternoon on the 22nd day. Blood samples were collected into heparin tubes from retro-orbital sinus and centrifuged at 1 500×g for 10 min at 4 ℃. The serum was obtained and stored at -80 ℃ before assay. Immediately after the mice were sacrificed, the right lung lobe was rinsed three times with 0.8 mL phosphate buffered saline through trachea, and the bronchoalveolar lavage fluid (BALF) was recovered. The left upper lobe was removed and fixed with 4% paraformaldehyde. The remaining lung tissue was rapidly frozen in liquid nitrogen and stored at -80 ℃[19].
When I was superintendent1 of schools in Palo Alto, California, Polly Tyner, the president of our board of trustees(), wrote a letter that was printed in the Palo Alto Times. Polly s son, Jim, had great difficulty in school. He was classified as educationally handicapped and required a great deal of patience on the part of his parents and teachers. But Jim was a happy kid with a great smile that lit up the room. His parents acknowledged his academic difficulties, but always tried to help him see his strengths so that he could walk with pride. Shortly after Jim finished high school, he was killed in a motorcycle accident. After his death, his mother submitted this letter to the newspaper.
2.7. BALF biochemical analysis
The BALF was centrifuged at 250×g for 10 min at 4 ℃. The contents of ALB and the activities of LDH and AKP in BALF were determined by commercial kits following the manufacturer’s instructions.
2.8. Biochemical analysis of lung tissue homogenate
The lung tissue was homogenized by ultrasonic and then centrifuged. The supernatant centrifuged at 250×g for 10 min was determined for the contents of T-SOD and MDA. The supernatant centrifuged at 2 500×g for 5 min was analysed for IL-6 and TNF-α.All assays were carried out by kits according to the manufacturers’protocols.
2.9. The serum biochemical analysis
The levels of NO and the activities of NOS in serum were determined by commercial kits according to the manufacturer’s protocols.
2.10. Histopathological examination
The fixed specimens were embedded in paraffin and cut into 5 μm sections. After decolorization for 15 min, sections were hydrated in the fractionated alcohol series, rinsed 3 times with 1% phosphate buffered saline and then dyed with hematoxylin and eosin (H&E)and Masson[20]. The samples were documented by Nikon ECLIPSE 80i (Nikon, Tokyo, Japan) at ×200 magnification for further analysis.
2.11. Statistical analysis
The results were expressed as mean±SD of ten animals used in each group. Mean of various parameters were compared by oneway analysis of variance followed by Tukey post hoc tests for intergroup comparisons. A value of P<0.05 as considered statistically significant. All data were analyzed using SPSS 22.0.
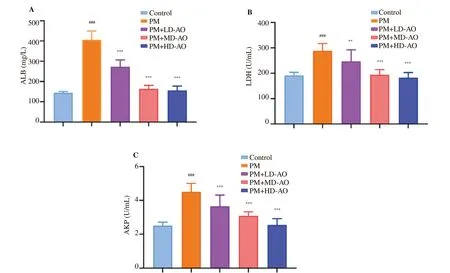
Figure 2. Effects of Alpinia offi cinarum on the levels of albumin (A), the activities of lactate dehydrogenase (B) and alkaline phosphatase (C) in the bronchoalveolar lavage fluid of particulate matter-induced mice. AO: Alpinia officinarum; PM: particulate matter; LD: low dose; MD: medium dose; HD: high dose. All values are expressed as mean±SD (n=10). ###P<0.001 compared with control group. **P<0.01, ***P<0.001 compared with PM group.
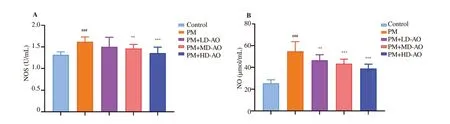
Figure 3. Effects of Alpinia officinarum on the activity of nitric oxide synthase (A) and the content of nitric oxide (B) in serum of particulate matter-induced mice. AO: Alpinia officinarum; PM: particulate matter; LD: low dose; MD: medium dose; HD: high dose. All values are expressed as mean±SD (n=10).###P<0.001 compared with control group. ***P<0.001 compared with PM group.
3. Results
3.1. AO mitigates lung injury by reducing cell permeability
ALB, LDH and AKP in BALF reflect the changes of cell permeability and inflammatory response. In order to investigate the effects of AO on cell permeability and inflammatory response after PM-induced lung injury in mice, ALB contents, LDH and AKP activities were determined. As shown in Figure 2, significant(P<0.001) increases of the ALB contents and the LDH and AKP activities were observed in BALF in PM group compared to the control group. AO intervention inhibited the increase of ALB contents, LDH and AKP activities induced by PM, indicating that AO intervention could reduce PM-induced cell permeability.
3.2. AO reduces the NO levels and decreases NOS activities in PM-induced lung injury in mice
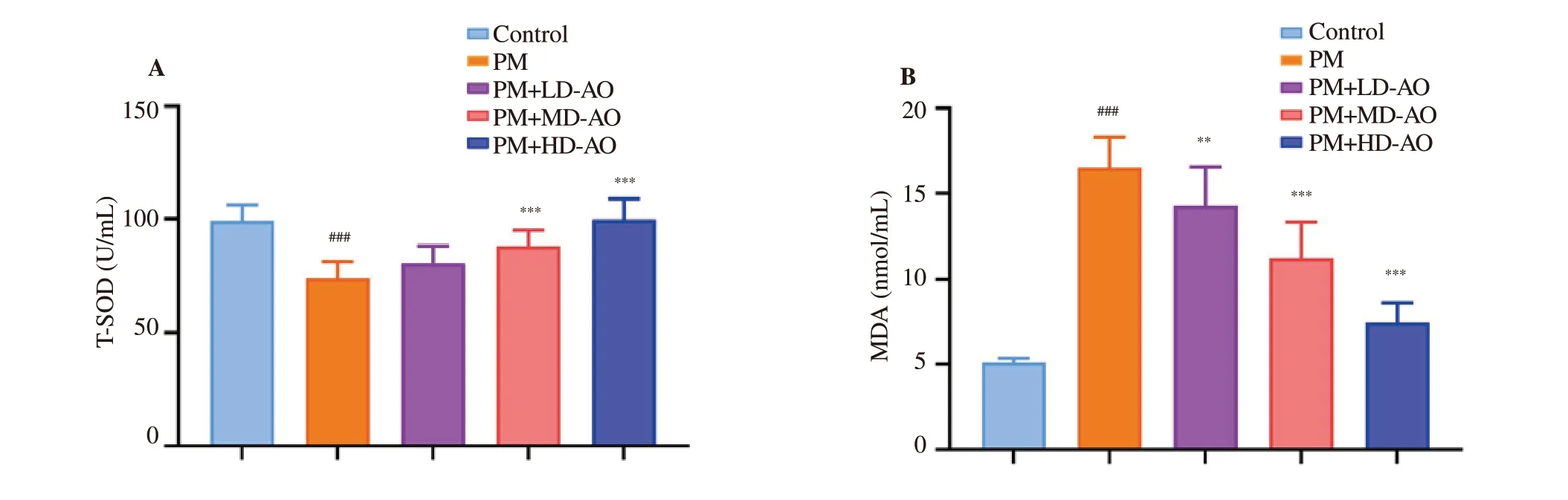
Figure 4. Effects of Alpinia officinarum on total superoxide dismutase (A) and malondialdehyde (B) in lung tissue of particulate matter-induced mice. AO:Alpinia officinarum; PM: particulate matter; LD: low dose; MD: medium dose; HD: high dose. All values are expressed as mean±SD (n=10). ###P<0.001 compared with control group. **P<0.01, ***P<0.001 compared with the PM group.
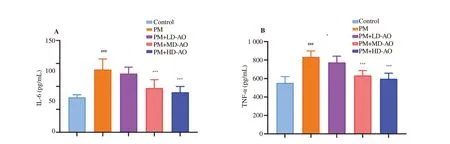
Figure 5. Effects of Alpinia officinarum on the level of transforming growth IL-6 (A) and TNF-α (B) in lung tissue of particulate matter-induced mice. AO:Alpinia officinarum; PM: particulate matter; LD: low dose; MD: medium dose; HD: high dose. All values are expressed as mean±SD (n=10). ###P<0.001 compared with control group. *P<0.05, **P<0.01 and ***P<0.001 compared with the PM group.
To determine the positive influence of AO on free radical accumulations and protein repair after PM-induced lung injury, NO levels and NOS activities were measured. As shown in Figure 3, 22 days after PM induction, the NO levels and the NOS activities in PM group were significantly (P<0.001) increased compared with control group. After AO intervention, NO levels were decreased significantly(P<0.01 or P<0.001) with the increase of AO doses compared with PM group. Similarly, NOS activities showed dose-dependent gradual inhibitions by AO, in which 100 mg/kg and 200 mg/kg AO administration led to significant (P<0.001) inhibitions. These data suggested that AO could alleviate free radical accumulations and induce protein repair in PM-induced lung injury in mice.
3.3. AO attenuates the levels of MDA and increases T-SOD activities in PM-induced lung injury in mice
PM-induced oxidative damages were determined by measuring the key enzyme (T-SOD) and lipid peroxide (MDA) in the lung tissue.In this study (Figure 4), compared with the control group, the T-SOD activities in PM group were significantly (P<0.001) decreased, while the MDA levels were significantly (P<0.001) increased. In PM+MDAO group and PM+HD-AO group, the T-SOD activities were significantly (P<0.001) higher than that in PM group, but slightly higher in PM+LD-AO group. MDA levels showed a dose-dependent decrease after AO intervention (P<0.01 or P<0.001). The results indicated that the administration of AO alleviates oxidative damage of lung injury induced by PM in mice.
3.4. AO down-regulates IL-6 and TNF-α expressions in PM-induced lung injury in mice
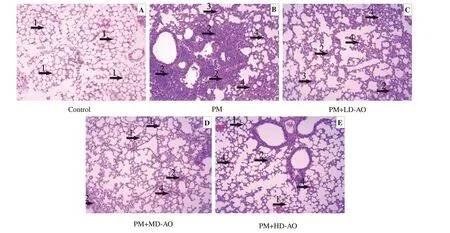
Figure 6. Alpinia officinarum ameliorated particulate matter-induced histological changes in the lung. AO: Alpinia officinarum; PM: particulate matter; LD:low dose; MD: medium dose; HD: high dose. Lung sections were obtained from vehicle-treated mice (A), PM treated mice (B), PM+LD-AO treated mice(C), PM+MD-AO treated mice (D) and PM+HD-AO treated mice (E). After H & E staining, histological examination was performed by light microscopy(magnification×200). 1 indicates normal lung tissue, arrow 2 indicates inflammatory cell infiltration, arrow 3 indicates edema and arrow 4 indicates thickening of alveolar septum.

Figure 7. Alpinia officinarum ameliorated particulate matter-induced histological changes in the lung. AO: Alpinia officinarum; PM: particulate matter; LD:low dose; MD: medium dose; HD: high dose. Lung sections were obtained from vehicle-treated mice (A), PM treated mice (B), PM+LD-AO treated mice(C), PM+MD-AO treated mice (D) and PM+HD-AO treated mice (E). After Masson staining, histological examination was performed by light microscopy(magnification × 200). 1 indicates normal lung tissue, 2 indicates inflammatory cell infiltration, 3 indicates edema and 4 indicates pulmonary fibrosis.
3.5. AO Alleviates pathological changes
The pathological changes of lung tissue stained with H & E in each group were observed under optical microscope (Figure 6). The alveolar structure of mice in control group was clear and intact, with no alveolar edema or inflammatory cell infiltration. In tissue of mice from the PM group, the alveolar septum was thickened, and the alveolar cavity was infiltrated by inflammatory cells with the edema of epithelial cells. In AO treatment groups, the alveolar septum was narrowed and inflammatory cell infiltration was relieved, both of which exhibited in dose-dependent manners.
The pathological changes of lung tissue in each group of Masson staining were observed under optical microscope (Figure 7): alveoli were arranged in an orderly manner in control group, and the structures of alveoli were clear and complete with almost no tissue fibrosis. Disordered alveolar arrangement and severe deformation were found in lung tissue of mice from PM group, with thickened interval, narrowed alveolar space, and pulmonary fibrosis. These changes were gradually reduced in dose-dependent manners in AO treatment groups.
4. Discussion
Generally, in the early stages of economic growth, air pollution increases, including PM2.5emissions[21], which leads to the obvious increased morbidity of lung diseases[22]. Unfortunately, there is no effective prevention measures by now[12]. Pharmacological studies have demonstrated that PM is prone to deposit in the alveoli after entering the lungs, and then it destroys the alveolar structure,increases the cell membrane permeability of the lung tissue, and causes nutrient efflux. At the same time, it causes oxidative stress damage accompanied by inflammatory reactions, and leads to further aggravation of tissue injury[23,24]. AO has been recorded as affinal drug and diet[13], with anti-inflammatory, antioxidant and other pharmacological activities[14]. In this study, we investigated the possible beneficial effects of AO on PM-induced lung injury which included the following four aspects: leakage of intracellular proteins,oxidative stress, inflammatory response and histomorphological changes.
When PM reaches the lungs and deposits inside the alveoli, it causes oxidative stress and generates excessive free radicals, and then reduces the cell membrane fluidity and increase the membrane permeability of alveolar typeⅡ epithelial cells[25]. These processes usually lead to the leakage of the intracellular proteins, including ALB, LDH and AKP, and the significant increase of extracellular detectable proteins. ALB and LDH are used to assess the loss of cellular nutrients and indirectly assess the extent of cell membrane damages[26,27]. AKP is produced by alveolar typeⅡ epithelial cells and neutrophils, which indicates the degree of inflammatory cell infiltration in lung tissue[28,29]. In this study, elevated ALB, LDH and AKP were observed after exposure to PM, which illustrated the increased permeability, loss of nutrients and inflammatory damage happened in alveolar typeⅡ epithelial cells. These were also confirmed with the pulmonary edema observed in H&E staining,and was consistent with previous report[30]. The intervention of AO reduced the levels of extracellular ALB, LDH and AKP, indicating that AO protected alveolar cells by reversing the permeability.
Oxidative stress (OS) refers to the imbalance between oxidation and anti-oxidation in the body, leading to inflammatory infiltration of neutrophils and production of excessive oxidative intermediates.oxidative stress is a negative effect produced by free radicals,which plays an important role in aging and diseases[31]. MDA,T-SOD, NO, NOS are well known markers of oxidative stress in reactive organisms[32,33]. In this study, after administration of PM in mice, we detected the decreased activity of T-SOD, the increased accumulations of MDA and NO, as well as the increased activity of NOS. These results indicated that administration of PM could iduce oxidative stress reactions. Markers of oxidative stress were significantly attenuated after the mice were treated with AO extract,indicating the antioxidant effect of AO extract. For example, it has been reported that MDA, the final product of lipid peroxidation, is at least partly responsible for the loss of cell membrane fluidity[34,35];the fact that AO extract significantly decreased MDA, which suggested that restoration of membrane permeability was related to the reduction of oxidative stress.
TNF-α and IL-6 are the most representative inflammatory factors and are also indicators of early inflammation[36]. Persistent inflammatory response could damage lung tissue cells and cause excessive cell repair, thus leading to pulmonary fibrosis or other lung diseases[37,38]. We found that administration of PM increased the expression of inflammatory factors, which was consistent with the results observed in pathological sections and previous reports[20].Additionally, researches have shown that massive accumulations of NO in lung tissue aggravate lung injury and promotes the proliferation of lung fibroblasts[39,40]. The pathological results of Masson staining in this study showed that PM also caused the occurrence of lung tissue fibrosis. From our animal experimental results, AO was found to slow down NO accumulation, decrease inflammatory factors expressions and relive pulmonary fibrosis significantly, indicating that AO was a promising therapeutic reagent for PM-induced lung injury.
Currently, most researchers focus on the influence and possible pathogenic mechanism of PM, and only summarize physical ways to prevent lung disease. Others conducted studies on searching effective extracts treating LPS-induced acute lung injury[41,42]. For instance,Liou C J et al. found that the water extract of Helminthostachys zeylanica could attenuate LPS-induced acute lung injury in mice by modulating NF-κB and MAPK pathways[41]. And Aruanã P et al.discovered Punica granatum L. leaf extract reduced inflammation in LPS-induced lung injury mice[42]. Unfortunately, these studies have not shown the beneficial effects of extracts on PM induced longterm chronic lung injury. In this study, we carried out researches on PM induced chronic lung injury in mice, in which PM was used as an analogue of air pollutants, and found that AO could effectively improve lung injury. The results can provide guidance for the treatment of PM induced lung injury. In conclusion, our study confirmed that AO had the potential to alleviate PM-induced lung injury through reversing cell membrane damage, reducing oxidative stress and inflammation. However, the present study did not provide detailed signal pathways, and further studies are needed to reveal specific mechanisms.
Conflict of interest statement
We declare that we have no conflict of interest.
Authors’ contributions
Y. X. Z. and L. Z. put forward the idea and designed experiments.Y. X. Z., W. J. R. and W. L. X. carried out experiments and collected data. Y. X. Z. analyzed and explained the data, and wrote the first draft of the article. Y. X. Z. and L. Z. made critical revision of the article, and L.Z. finally approved the version to be released.
 Asian Pacific Journal of Tropical Medicine2019年12期
Asian Pacific Journal of Tropical Medicine2019年12期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Multi-targeting cytotoxic drug leads from mushrooms
- Spectrum of thalassemia mutations in fetuses of Han and Li ethinicities in Hainan province, China
- Seroprevalence of brucellosis among exposed agro-pastoral communities in southern Saudi Arabia
- Effect of El Niño Southern Oscillations on the incidence of enteric fever in Ahmedabad, India from 1985 to 2017
- Prevalence and risk factors of avian influenza H9N2 among backyard birds in Iran in 2015
- Cutaneous leishmaniasis in an indigenous infant with Down's syndrome:A case report
