酸化赤泥吸附环丙沙星的特征、机理及过程优化
史京转,魏 红*,周孝德,史颖娟,郑佳欣
酸化赤泥吸附环丙沙星的特征、机理及过程优化
史京转1,魏 红1*,周孝德1,史颖娟2,郑佳欣1
(1.西安理工大学,省部共建西北旱区生态水利国家重点实验室,陕西 西安 710048;2.陕西水环境工程勘测设计研究院,陕西 西安 710021)
为提高赤泥的资源化利用及抗生素有机废水的深度处理,以酸化赤泥为吸附剂、环丙沙星为目标污染物,研究了酸化赤泥吸附环丙沙星的条件、特征和机理.采用响应面法中Box-Behnken设计方法,以吸附温度、溶液pH值、环丙沙星初始浓度、酸化赤泥投加量为自变量,吸附量为响应值建立4因素3水平优化模型,确定了最佳吸附条件,并对吸附过程的动力学模型、等温线模型、热力学特性及吸附机理进行了研究.结果表明,溶液pH值、环丙沙星初始浓度、酸化赤泥投加量为影响吸附量的显著因素.酸化赤泥吸附环丙沙星的最佳条件为:温度45℃、pH=3.04、环丙沙星初始浓度29.20mg/L,酸化赤泥投加量3.40g/L,预测最大吸附量为7.30mg/g.酸化赤泥吸附环丙沙星的过程遵循伪二级反应动力学模型及Langmuir-Freundilich吸附等温线模型,经过拟合最大吸附量分别为7.90和7.35mg/g.根据Van Tehoff公式计算吸附热力学状态函数Δ0为-82.13~-94.37kJ/mol、Δ0为0.61J/(mol·K)、Δ0为100.25KJ/mol,吸附为自发进行的吸热反应. FTIR表明环丙沙星分子中—COO与酸化赤泥的Al—O键发生络合反应,C=O与Fe—O键发生微弱的静电或内球面键合作用.研究表明,酸化赤泥是一种极具潜力的廉价吸附剂,可用于处理抗生素污染废水.
酸化赤泥;环丙沙星;响应面优化;吸附动力学;吸附热力学
赤泥是工业生产氧化铝过程中产生的固体废渣,主要包含Na、K、Al、Fe、Ni、Si、Cu、Mn、Ti 及 Zn等元素,不同地区铝土矿及氧化铝生产工艺不同而导致赤泥的成分有所差异[1].据统计,2014年世界范围内赤泥的贮存量为35亿t,并以每年1.2亿t的速度增长[2]. 我国每年赤泥的产生量7000万t,累积库存约为6亿t[3].目前,赤泥最普遍的处置方式是筑坝填埋[4],由此导致的环境污染及次生灾害问题时有发生[5].因此,对赤泥进行资源化和无害化利用越来越受到人们的关注[6].近年来,国内外对赤泥的有效利用主要集中在建材生产[7]、稀有金属回收[8]、赤泥基催化剂及吸附材料的制备[9-10]等方面.为了降低赤泥的高碱性危害,研究普遍对赤泥进行酸活化处理.不同酸活化可显著提高赤泥对重金属离子[11]、有机染料[12]及磷酸盐[13]等污染物的吸附性能.但赤泥对新兴污染物如抗生素的吸附还鲜见报道.
近年来,抗生素类有机污染物在环境介质中频繁检出,引起了学者们的广泛关注[14].不仅在河流[15]、污水中[16]有抗生素检出,饮用水中也检测到痕量抗生素.抗生素在环境介质中的“假持久性”[17]和“耐药性”[18]已经严重威胁到人类的健康.环丙沙星作为环境中检出率最高的人畜共用类抗生素之一[19],传统的水处理工艺对其去除效果不佳[20],采取更有效的处理方法已经迫在眉睫. 吸附法具有操作简单、成本低、不添加任何氧化剂等特点而备受青睐[21].
本实验根据响应面法[22]中Box-Behnken设计方法对酸化赤泥吸附环丙沙星的影响因素进行评价、优化, 研究吸附过程的动力学、吸附等温线和吸附热力学特性,并结合傅里叶变换衰减全反射红外光谱(ATR-FTIR)对吸附机理进行分析,以期为赤泥的综合利用和环丙沙星污染水体的修复提供一定科学依据.
1 材料与方法
1.1 仪器与试剂
实验所用试剂均为分析纯;原始赤泥取自三门峡市义翔铝业公司.赤泥浸出液pH值为11.04,经XRD(X-ray diffraction,X射线衍射)分析赤泥的主要组分为水化石榴石、钙霞石、赤铁矿、刚玉等,经BET(Brunauer-Emmett-Teller)多层吸附计算赤泥的比表面积10.96m2/g,平均孔径40.93nm;环丙沙星购于日本东京化成工业株式会社,纯度大于98 %,分子式: C17H18FN3O3, 相对分子量331.35.
1.2 实验方法
1.2.1 酸化赤泥的制备 将5g原始赤泥溶于1.0L超纯水中,调节pH值(6.0±0.2),以100r/min搅拌9h,静置分离,将泥饼冲洗至中性,并收集于100℃烘干,过150目筛(孔径106μm),记为酸化赤泥.
1.2.2 吸附量的测定 将酸化赤泥(3.0,4.0,5.0g/L)和200mL环丙沙星溶液(10,20,30mg/L)混合置于250mL棕色锥形瓶中.用0.1mol/L的HCl和NaOH调节溶液pH值(3.0, 5.0, 7.0, 9.0, 11.0,误差±0.1),密封后放入250r/min的气浴恒温(25,35,45℃)摇床(HZ-8811K,常州德欧)中振荡180min.一定时间间隔取一定混合液, 4000r/min离心10min,过0.22μm滤膜,用高效液相色谱仪(HPLC,Aglient 1200,美国)测定环丙沙星浓度.同时设置空白对照实验(不加吸附剂)和3组平行实验. 吸附量的计算如式(1)所示.

式中:q为时刻的吸附量,mg/g;0为环丙沙星的初始浓度,mg/L;c为吸附时刻环丙沙星的浓度, mg/L;为反应液体积,mL;为酸化赤泥的质量,g.
1.2.3 响应面优化吸附条件 通过前期大量单因素实验分析,以吸附温度、溶液pH值、环丙沙星初始浓度和酸化赤泥投加量为自变量,分别记为A、B、C、D;以酸化赤泥对环丙沙星的吸附量作为响应值,记为Y.使用Design Expert 10.0.7软件中Box- Behnken响应面优化设计方法设计实验方案,各自变量因素及其水平见表1.
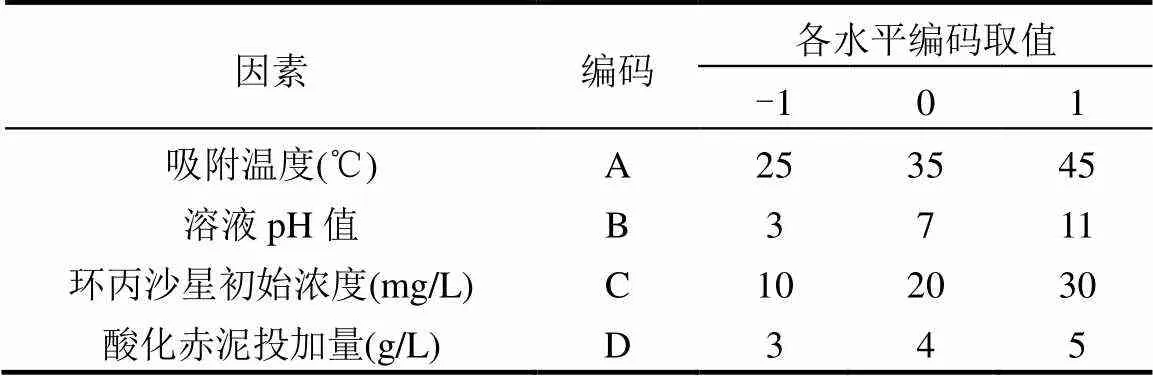
表1 实验自变量因素及其水平表
1.2.4 吸附动力学 将0.68g酸化赤泥加入200mL初始浓度为30mg/L的环丙沙星溶液,调节pH值为3.04, 在45℃、250r/min条件下振荡,一定时间取混合液,方法同1.2.2,测定环丙沙星浓度,设置3组平行.
1.2.5 吸附等温线 将不同初始浓度的环丙沙星溶液(10,20,30,50,100,500mg/L),在不同温度(25,35, 45℃)下振荡180min至吸附平衡,测定环丙沙星浓度,其他条件同1.2.4.
1.2.6 分析方法 HPLC色谱条件为: Eclipse XDB-C18色谱柱(150mm×4.6mm,5µm);流动相:乙腈与0.2%(体积分数)甲酸水溶液(体积比20:80);流速0.2mL/min;检测波长λ=277nm;进样量10μL;柱温30℃.在此条件下,环丙沙星的保留时间R=9.768min.采用ATR法在傅里叶红外光谱仪(VERTEX70, BRUKER,德国)上于400~4000cm-1范围内对吸附前后酸化赤泥样品进行扫描.
1.2.7 数据处理 响应面优化实验数据采用Design Expert 10.0.7软件进行处理,并得出最优吸附条件;吸附动力学、吸附等温线、吸附热力学数据均采用Origin Pro 8软件进行处理和拟合.
2 结果与讨论
2.1 吸附条件的响应面实验结果分析
2.1.1 吸附预测模型建立 采用Box-Behnken实验设计方法对酸化赤泥吸附环丙沙星的条件进行研究并优化,实验结果见表2.
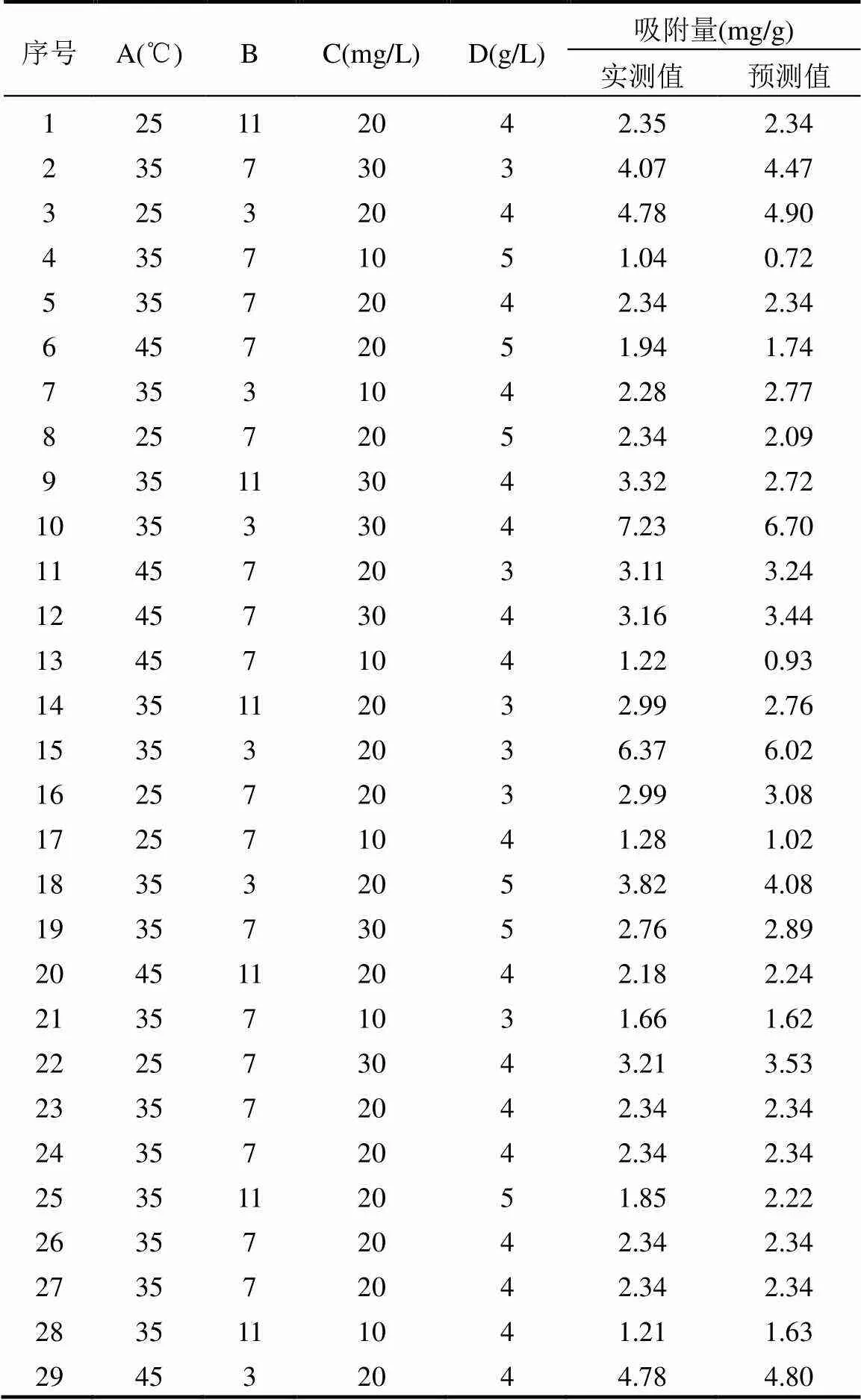
表2 Box-Behnken实验设计及其实验结果
对表2中的结果用Design Expert 10.0.7进行方差分析,结果见表3. 吸附量与4个变量(温度、溶液pH值、环丙沙星初始浓度和酸化赤泥投加量)的二次多项式见式(2):
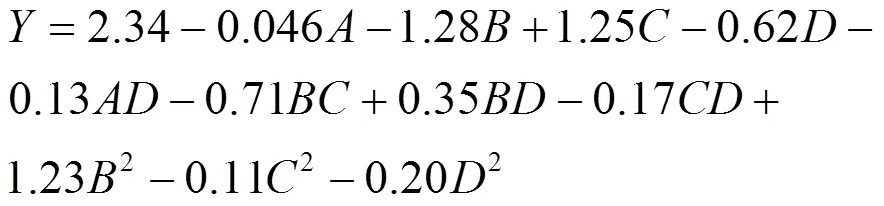
由表3可知,模型中B、C、D、BC、B2参数的值<0.05,说明溶液pH值、环丙沙星初始浓度、酸化赤泥投加量、溶液pH值与环丙沙星初始浓度交互作用、溶液pH值的平方效应对吸附量的影响具有显著性;其他参数的值>0.05,说明其他因素对吸附量的影响不显著.模型的值为40.08,<0.0001,说明模型非常显著.
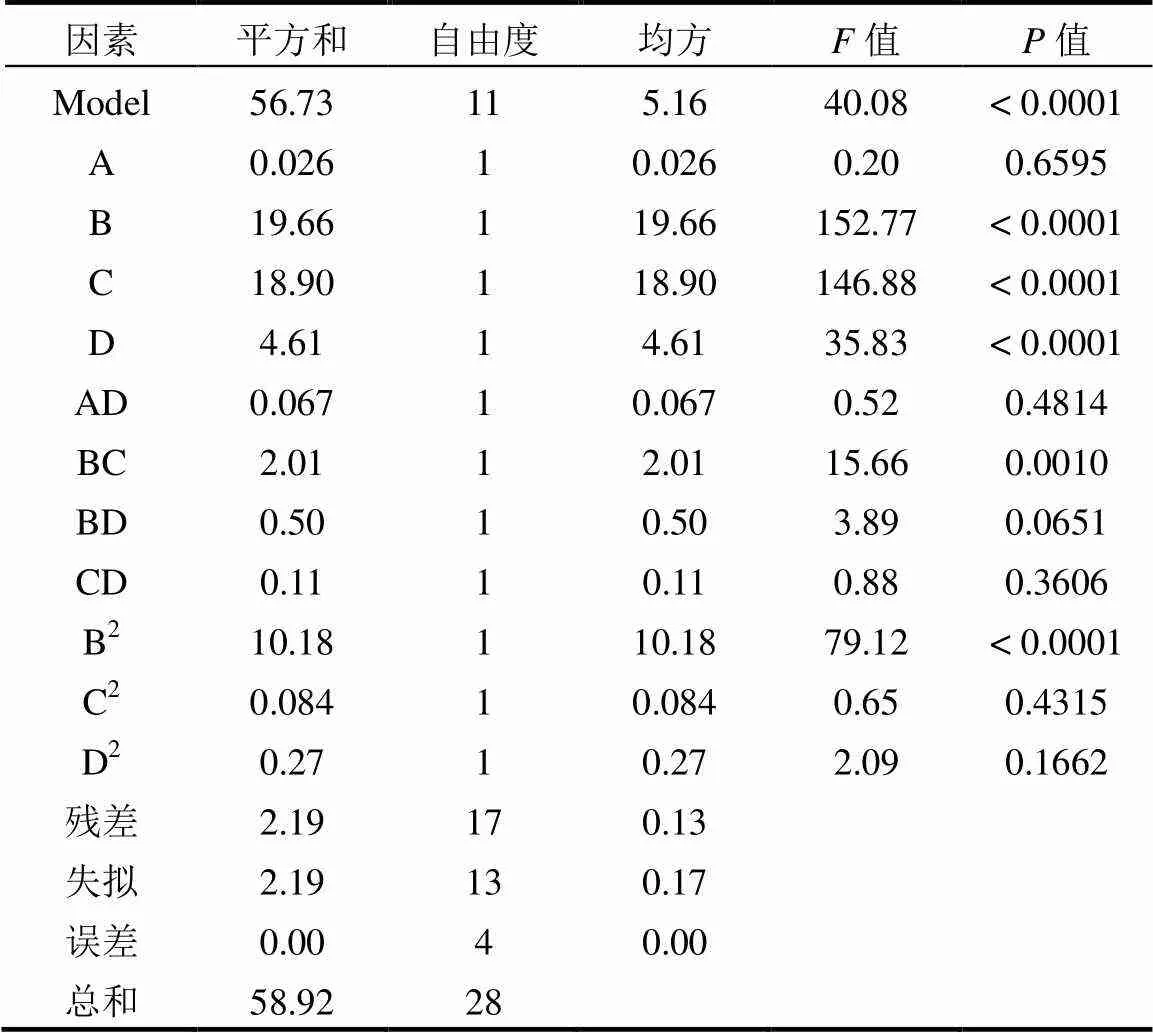
表3 方差分析
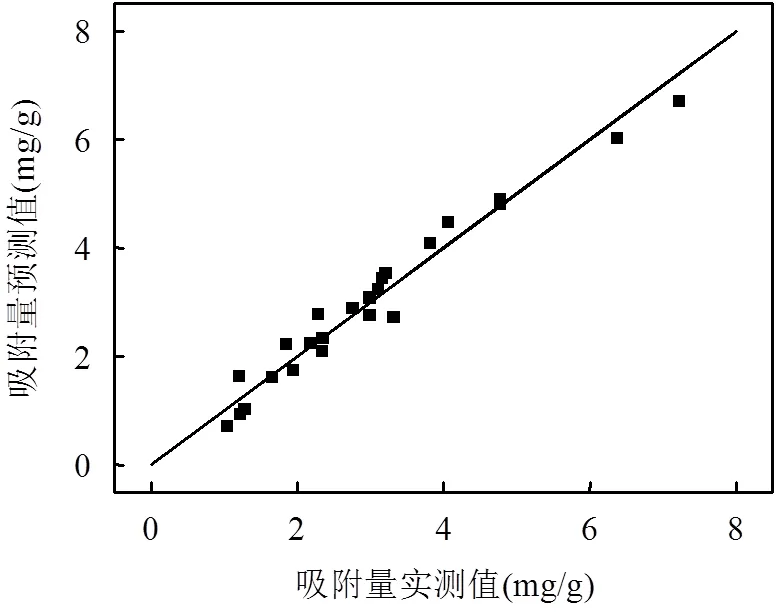
图1 吸附量实测值与预测值比较
图1表明,建立的二次多项式模型计算出的预测值与实测值服从正态分布,决定系数2=0.9629,表明96.3 %的变异能够被解释.校正系数2Adj= 0.9389(接近1),说明模型的拟合程度很高;模型的信噪比为25.92(大于4视为合理),说明模型的可信度高,数据合理[23].因此,该模型建立的方程式[式(2)]能准确合理的反映吸附条件与吸附量之间对应关系.
2.1.2 响应面分析及模型验证 由表3可知,溶液pH值与环丙沙星初始浓度交互作用显著,图2为其对吸附量的三维曲面和对应的等高线图.
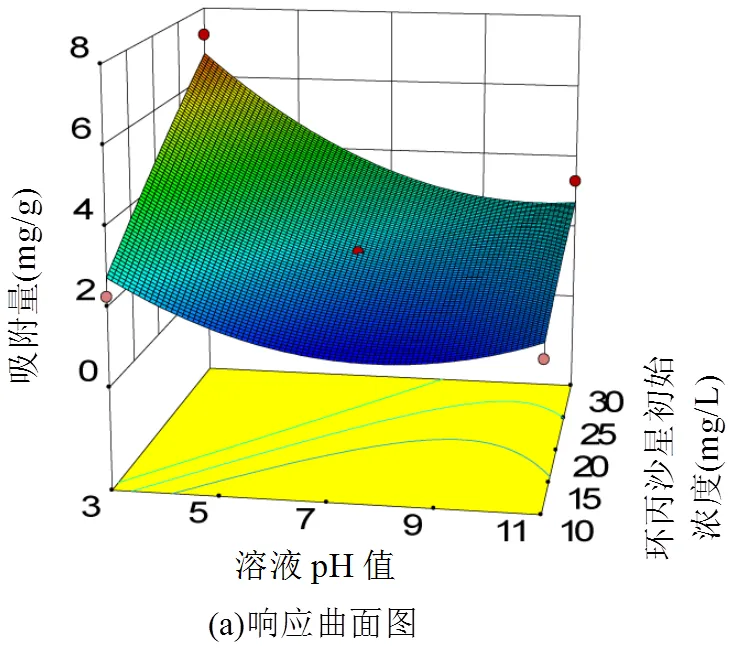
由图2可知,酸化赤泥对环丙沙星的吸附量随环丙沙星初始浓度的升高而升高;随溶液pH值的升高而降低.这是因为在不同pH值条件下,环丙沙星的解离形态不同(图3所示).当溶液pH值较低时,环丙沙星的解离形态主要为H4CIP3+,由于H4CIP3+的化学活性较强于H3CIP2+离子,有利于与酸化赤泥中的活性物质发生吸附作用[24];当溶液偏碱性时,酸化赤泥中的活性成分主要以沉淀的形式存在,从而降低了酸化赤泥对环丙沙星的吸附性能.
2.1.3 最佳吸附条件确定 根据模型预测出酸化赤泥对环丙沙星的最佳吸附条件为:吸附温度45℃,溶液pH值3.04,环丙沙星初始浓度29.20mg/L,酸化赤泥投加量为3.40g/L,最大吸附量为7.30mg/g.
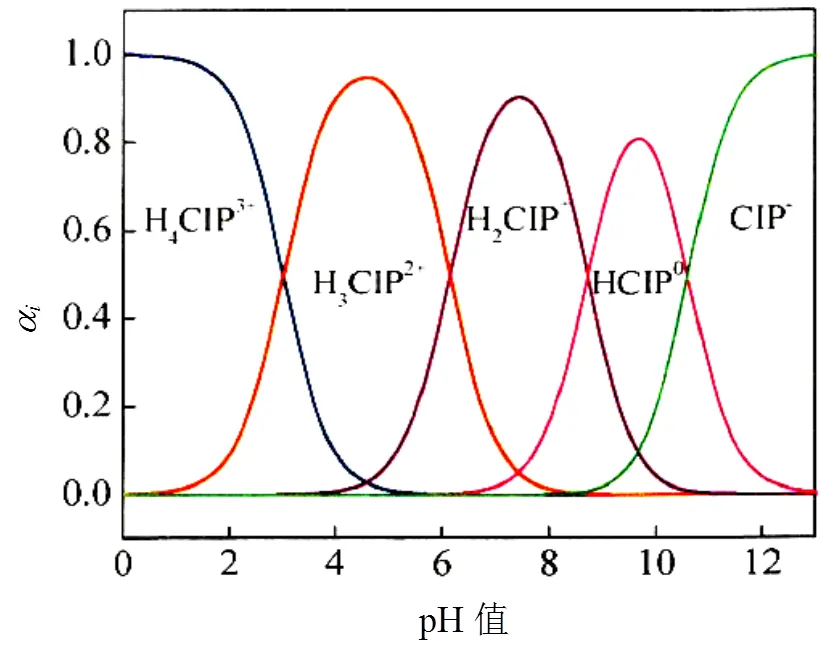
图3 不同pH值环丙沙星的解离形态
2.2 吸附动力学
吸附动力学主要研究吸附剂吸附溶质的速率快慢.通过动力学模型对实验数据进行分析拟合,从而探讨其吸附机理.常用的吸附动力学模型有:伪一级动力学模型、伪二级动力学模型、颗粒内扩散模型、Elovich动力学模型[式(3)~(6)].
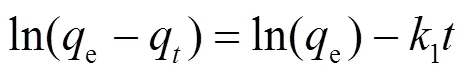
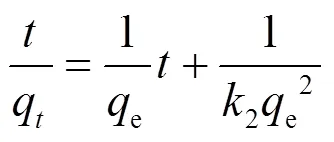
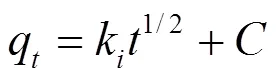

式中:e是达到吸附平衡时的吸附量, mg/g;q是时刻的吸附量,mg/g;1是伪一级吸附动力学反应速率常数,min-1;2是伪二级反应动力学速率常数, g/(mg·min);是涉及到厚度、边界层的常数;k为粒子内扩散常数, mg/(g·min1.5);是Elovich常数,表示初始吸附速率,mg/(g·min2);是Elovich常数,表示解吸脱附系数,mg/(g·min).
图4(a)为酸化赤泥对环丙沙星的吸附结果;(b)~(e)分别为不同动力学模型拟合情况,具体拟合结果见表4.
由图4(a)可知,吸附初期,酸化赤泥吸附环丙沙星非常迅速,5min的吸附量达到6.54mg/g; 100min后,反应体系达到动态吸附平衡,最大吸附量为7.84mg/g.出现这一现象的原因可能是,反应刚发生时,体系内酸化赤泥有足够多的吸附点位,环丙沙星的浓度也很高,反应速度非常快,随着吸附点位和环丙沙星浓度的降低,吸附反应相对缓慢,最后达到动态平衡.
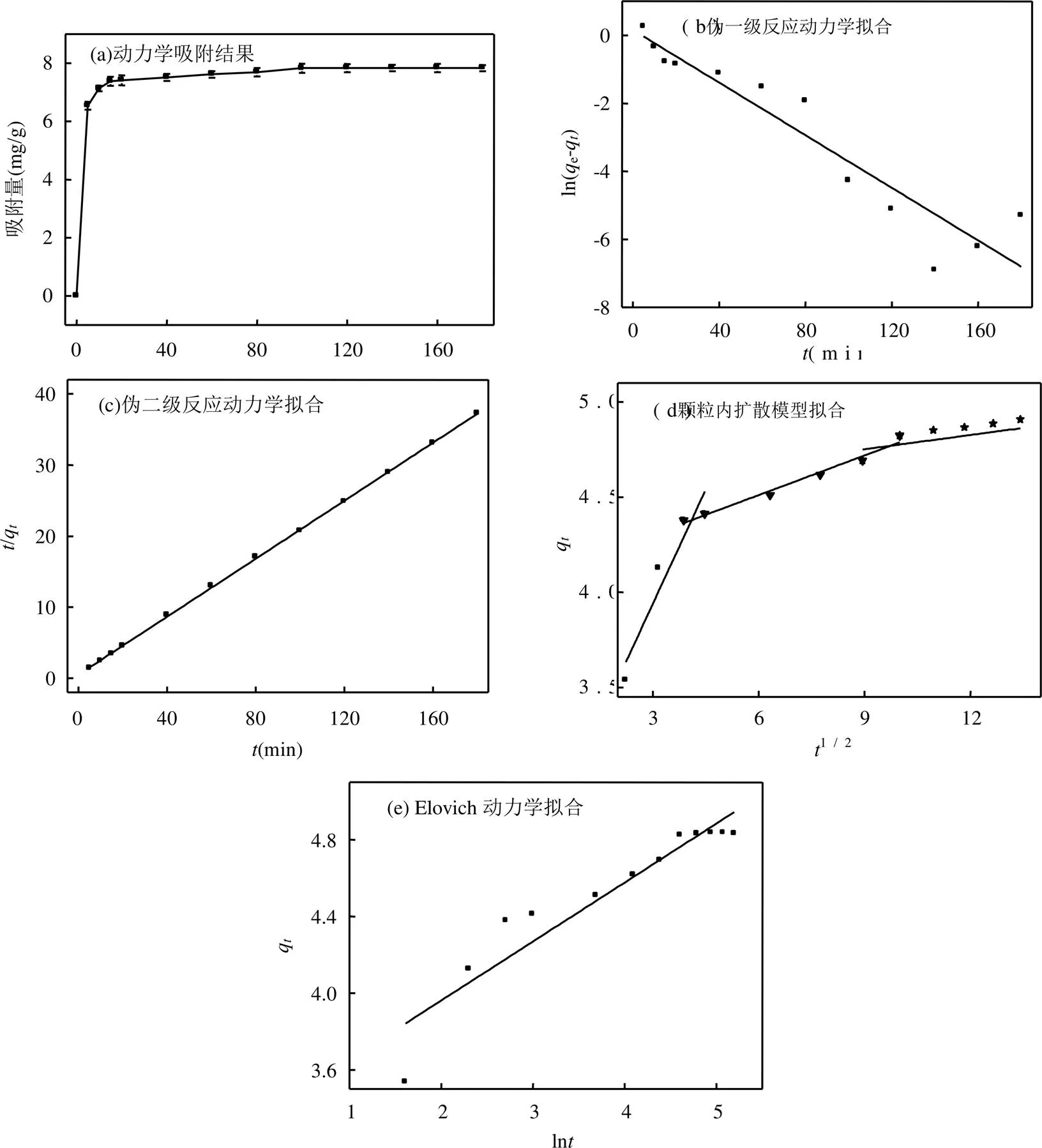
图4 吸附结果(a)及吸附动力学模型(b~e)拟合情况
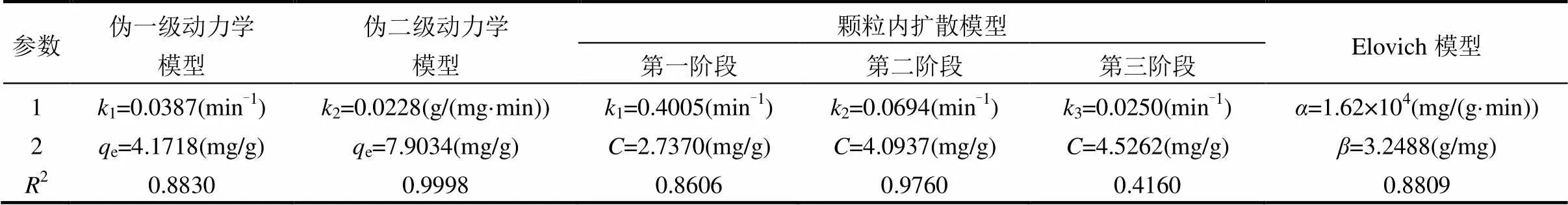
表4 吸附动力学模型拟合参数
由图4(b)、(c)、(e)和表4的拟合结果可知,酸化赤泥对环丙沙星的吸附过程遵循伪二级吸附动力学模型,2=0.9998,且拟合的e值和实验值较吻合,这表明酸化赤泥吸附环丙沙星是一个化学吸附过程[25].这可能是由于赤泥中含有多种过渡金属元素,容易和环丙沙星中含富电子,N、O元素的官能团配位.为进一步了解吸附过程机理,用颗粒内扩散模型进行拟合分析,结果见图4(d).整个吸附过程包括快速吸附、慢速吸附、颗粒内扩散3个阶段,多种吸附机理同时参与反应;拟合曲线不经过原点进一步说明除了颗粒内扩散外,还有其他的速控步骤参与[26].
2.3 吸附等温线
吸附等温线是在一定温度下,溶质在两相界面上达到吸附平衡时的浓度关系曲线.常用的等温线模型有Langmuir、Freundlich和Langmuir-Freundlich [式(7)~(9)].
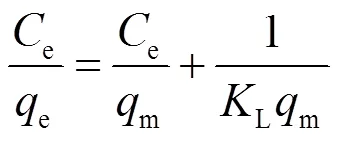
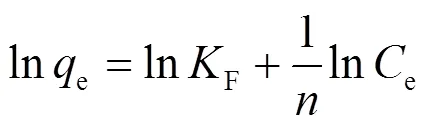
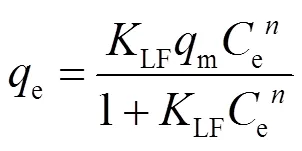
式中:e为溶质的平衡浓度,mg/L;e是平衡吸附量, mg/g;m是理论上的最大单分子层吸附量,mg/g;L是与吸附能相关的Langmuir吸附常数,L/mg;F是Freundlich常数,与溶质的移除效率有关;是表征吸附强度的常数;LF是Langmuir-Freundlich常数.
图5为Langmuir-Freundlich模型的拟合结果,其他拟合见表6.
根据式(7)中L得到一个表征吸附分离难易的无因次分离因子L[式(10)]:
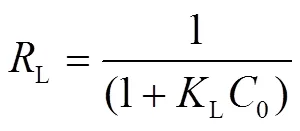
L是一个无量纲常数,L>1说明过程不利于吸附;L=1是线性的;0 式(8)中是表征吸附强度的常数,一般在1~10之间说明有利于吸附;值更高或者1/更小,说明吸附剂和溶质之间有很强的相互作用;=1时是线性吸附,说明所有的位点都有相同的吸附量[27].根据计算可知(表5)值为1~10,说明该吸附有利于进行. 图5 Langmuir-Freundlich吸附等温线 根据Langmuir-Freundlich模型的计算结果,最大吸附量7.35mg/g与实测值较符合,2为0.9999,因此该模型能更好地拟合该吸附过程. 根据文献报道,高岭土对环丙沙星的最大吸附量为0.143mg/g[28];湖泊底泥吸附环丙沙星的最大吸附量为6.13mg/g[29];微波辅助合成的磁性生物炭对环丙沙星的吸附量为8.30mg/g[30].本实验中酸化赤泥对环丙沙星的最大吸附量为7.84mg/g,稍低于磁性生物炭材料.这可能与酸化赤泥比表面积不高有关,后续将围绕优化赤泥结构,提高比表面积和暴露活性点位开展进一步研究. 表5 不同吸附等温线模型的拟合参数 吸附热力学通过Van Tehoff方程依据不同温度下Langmuir吸附等温线常数计算[式(11~12)]. 式中:L是Langmuir吸附平衡系数. lnL对1/作图,结果见图6,根据直线斜率和截距求算的结果见表6. 由表6可知,不同温度下,Δ0均为负值,这说明酸化赤泥吸附环丙沙星的过程是自发进行的.随着温度升高|Δ0|增大,说明温度升高吸附剂的吸附性能增大[31].物理吸附Δ0一般认为在0~20kJ/mol,化学吸附在-80~400kJ/mol之间[32],因此环丙沙星在酸化赤泥上的吸附为化学吸附.Δ0>0,则说明吸附过程为吸热反应[33]. 图6 温度对吸附平衡常数的影响 表6 吸附热力学状态函数 通过FTIR(图7)分析,1614cm-1处的吸收峰为环丙沙星中C=O的伸缩振动峰;1372和1588cm-1为环丙沙星的—COO的对称和反对称伸缩振动峰[34]; 1498cm-1为O—H的伸缩振动峰. 图7 环丙沙星及酸化赤泥吸附环丙沙星前后的红外光谱 (before)表示吸附环丙沙星之前的酸化赤泥;(after)表示吸附环丙沙星之后的酸化赤泥 酸化赤泥的FTIR有几个重要的吸收峰,其中1628cm-1是Fe—O键(赤铁矿)的伸缩振动峰; 1394cm-1为Al—键(一水软铝石)的伸缩振动峰; 1504cm-1处为O—H键的弯曲振动,说明酸化赤泥中有吸附水的存在[35]. 对比环丙沙星和吸附前后酸化赤泥的FTIR可知,1394cm-1处的Al—O键特征峰位移至1380cm-1处,说明赤泥的Al—O键与环丙沙星的—COO发生表面络合作用[36]. 1504cm-1处的O—H键发生位移,说明赤泥中的吸附水被消耗[37]; 1628cm-1处的Fe—O键发生的位移较小,说明环丙沙星的C=O与赤泥的Fe—O键发生微弱的静电作用或与赤泥的内球面相键合[38].这也印证了前面吸附动力学和热力学认为吸附是一个化学吸附过程. 3.1 基于Box-Behnken设计方法建立的响应面优化模型对酸化赤泥吸附环丙沙星进行预测模拟.得到响应面优化的最佳吸附条件为:吸附温度45℃,溶液pH=3.04,环丙沙星初始浓度29.20mg/L,酸化赤泥投加量3.40g/L,预测最大吸附量7.30mg/g. 3.2 酸化赤泥对环丙沙星的吸附过程遵循伪二级反应动力学模型(2=0.999),拟合的最大吸附量为7.90mg/g,与实测最大吸附量7.84mg/g相符. 3.3 酸化赤泥对环丙沙星的吸附等温线遵循Langmuir-Freundlich模型(2=0.9998),拟合得到最大吸附量为7.35mg/g. 3.4 吸附热力学表明,酸化赤泥吸附环丙沙星的过程是自发进行的吸热反应. 3.5 ATR-FTIR分析表明,酸化赤泥吸附环丙沙星的机理为环丙沙星的—COO与酸化赤泥发生表面络合作用,同时C=O可能与酸化赤泥发生微弱的静电作用或内球面键合作用. [1] Paramguru R K, Rath P C, Misra V N. Trends in red mud utilization-a review [J]. Mineral Processing and Extractive Metallurgy, 2005,26:1-29. [2] Ren J, Chen J, Guo W, et al. Physical, chemical, and surface charge properties of bauxite residue derived from a combined process [J]. Journal of Central South University, 2019,26(2):373-382. [3] Xue S G, Zhu F, Kong X F, et al. A review of the characterization and revegetation of bauxite residues (Red mud) [J]. Environmental Science and Pollution Research, 2016,23(2):1120-1132. [4] Schmalenberger A, O'sullivan O, Gahan J, et al. Bacterial communities established in bauxite residues with different restoration histories [J]. Environmental Science & Technology, 2013,47(13):7110-7119. [5] Ren J, Chen J, Han L, et al.Spatial distribution of heavy metals, salinity and alkalinity in soils around bauxite residue disposal area [J]. Science of the Total Environment, 2018,628-629:1200-1208. [6] Hua Y, Heal K V, Friesl H W. The use of red mud as an immobiliser for metal/metalloid-contaminated soil: A review [J]. Journal Hazard Mater, 2017,325:17-30. [7] Gu H N, Wang N, Liu S R. Radiological restrictions of using red mud as building material additive [J]. Waste Management & Research, 2012,30(9):961-965. [8] Zhu X, Li W, Tang S, et al. Selective recovery of vanadium and scandium by ion exchange with D201 and solvent extraction using P507 from hydrochloric acid leaching solution of red mud [J]. Chemosphere, 2017,175:365-372. [9] 燕希敏,苗 鹏,常国璋,等.Fe/赤泥催化水蒸气气化煤焦的反应性与微结构特性[J]. 化工进展, 2018,37(5):1753-1759. Yan X M, Miao P, Chang G Z, et al. Characteristics of microstructures and reactivities during steam gasification of coal char catalyzed by red mud [J]. Chemical Industry And Progress, 2018,37(5):1753-1759. [10] Wang Y, Yu Y, Li H, et al. Comparison study of phosphorus adsorption on different waste solids: Fly ash, red mud and ferric-alum water treatment residues [J]. Journal of Environmental Science (China), 2016,50:79-86. [11] Genç-Fuhrman H, Tjell J C, McConchie D. Increasing the arsenate adsorption capacity of neutralized red mud (Bauxsol) [J]. Journal of Colloid and Interface Science, 2004,271(2):313-320. [12] 黄 凯,李一飞,焦树强,等.柠檬酸活化赤泥对亚甲基蓝染料废水的吸附净化作用[J]. 中国有色金属学报, 2011,21(12):3182-3188.Huang K, Li Y F, Jiao S Q, et al. Adsorptive removal of methylene blue dye wastewater from aqueous solution using citric acid activated red mud [J]. The Chinese Journal of Nonferrous Metals, 2011,21(12):3182-3188. [13] Ye J, Cong X, Zhang P, et al. Interaction between phosphate and acid-activated neutralized red mud during adsorption process [J]. Applied Surface Science, 2015,356:128-134. [14] Tang J, Shi T, Wu X, et al. The occurrence and distribution of antibiotics in Lake Chaohu, China: Seasonal variation, potential source and risk assessment [J]. Chemosphere, 2015,122:154-161. [15] Zhang H, Du M, Jiang H, et al. Occurrence, seasonal variation and removal efficiency of antibioticsand their metabolites in wastewater treatment plants, Jiulongjiang River Basin, South China [J]. Environmental Science Processes & Impacts, 2015,17(1):225-234. [16] Han Y R, Wang Q J, Mo C H, et al. Determination of four fluoroquinolone antibiotics in tap water in Guangzhou and Macao [J]. Environmental Pollution, 2010,158(7):2350-2358. [17] Watkinson A J, Murby E J, Kolpin D W, et al. The occurrence of antibiotics in an urban watershed:from wastewater to drinking water [J]. Science of The Total Environment, 2009,407(8):2711-2723. [18] Bengtsson P J, Larsson D G J. Concentrations of antibiotics predicted to select for resistant bacteria: Proposed limits for environmental regulation [J]. Environment International, 2016,86:140-149. [19] Liu X, Steele J C, Meng X Z. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: A review [J]. Environmental Pollution, 2017,223:161-169. [20] Gu X Y, Tan Y Y, Tong F, et al. Surface complexation modeling of coadsorption of antibiotic ciprofloxacin and Cu(II) and onto goethite surfaces [J]. Chemical Engineering Journal, 2015,269:113-120. [21] Ni F, He J, Wang Y, et al. Preparation and characterization of a cost-effective red mud/ polyaluminum chloride composite coagulant for enhanced phosphate removal from aqueous solutions [J]. Journal of Water Process Engineering, 2015,6:158-165. [22] 刁 硕,王红旗,吴枭雄,等.基于响应面法优化一株低温耐盐芘降解菌共代谢条件的研究 [J]. 中国环境科学, 2017,37(1):345-351. Diao S, Wang H Q, Wu X X, et al. Optimization for pyrene bacteria cometabolism degradation under the low temperature and high salt environment through response surface [J]. China Environmental Science, 2017,37(1):345-351. [23] 王雅辉,邹雪刚,舒冉君,等.胡敏素对Pb2+吸附的响应面优化及机理[J]. 中国环境科学, 2017,37(5):1814-1822.Wang Y H, Zou X G, Shu R J, et al. Adsorption of Pb(Ⅱ) from aqueous solutions by humin: optimization and mechanism [J]. China Environmental Science, 2017,37(5):1814-1822. [24] Saha S, Sarkar P. Arsenic remediation from drinking water by synthesized nano-alumina dispersed in chitosan-grafted polyacry- lamide [J]. Journal of Hazardous Materials, 2012,227-228:68-78. [25] Hu X, Wang J, Liu Y, et al. Adsorption of chromium (VI) by ethylenediamine-modified cross-linked magnetic chitosan resin: Isotherms, kinetics and thermodynamics [J]. Journal of Hazardous Materials, 2011,185(1):306-314. [26] Ofomaja A E. Kinetic study and sorption mechanism of methylene blue and methyl violet onto mansonia (Mansonia altissima) wood sawdust [J]. Chemical Engineering Journal, 2008,143(1-3):85-95. [27] Febrianto J, Kosasih A N, Sunarso J. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: A summary of recent studies [J]. Journal of Hazardous Materials, 2009,162:616-645. [28] 高 鹏,莫测辉,李彦文,等.高岭土对喹诺酮类抗生素吸附特性的初步研究[J]. 环境科学, 2011,32(6):1740-1744.Gao P, Mo C H, Li Y W, et al. Preliminary study on the adsorption of quinolones to kaolin [J]. Environmental Science, 2011,32(6):1740-1744. [29] 王富民,马秀兰,边炜涛,等.湖库底泥对环丙沙星吸附特性的研究[J]. 水土保持学报, 2016,30(2):312-316,322.Wang F M, Ma X L, Bian W T, et al. The adsorption characteristic of reservior sediment to ciprofloxacin [J]. Journal of Soil and Water Conservation, 2016,30(2):312-316,322. [30] 张学良,徐 建,占新华,等.微波辅助合成γ-Fe2O3/花生壳磁性生物炭对水体中环丙沙星吸附的研究[J/OL]. 环境科学学报: 1-11 [2019-07-30]. https://doi.org/10.13671/j.hjkxxb.2019.0176. Zhang X L, Xu J, Zhan X H, et al. Adsorption of ciprofloxacin on magnetic γ-Fe2O3/peanut shell biochar prepared by microwave- assisted synthesis in aqueous [J/OL]. Acta Scientiae Circumstantiae, 1-11 [2019-07-30]. https://doi.org/10.13671/j.hjkxxb.2019.0176. [31] Reza R A, Ahmaruzzaman M, Sil A K, et al. Comparative adsorption behavior of ibuprofen and clofibric acid onto microwave assisted activated bamboo waste [J]. Industrial and Engineering Chemistry Research, 2014,53:9331-9339. [32] Liu C C, Ming K W, Li Y S. Removal of nickel from aqueous solution using wine processing waste sludge [J]. Industrial and Engineering Chemistry Research, 2005,44:1438-1445. [33] Kumar R, Rashid J, Barakat M A. Synthesis and characterization of a starch-AIOOH-FeS2nanocomposite for the adsorption of congo red dye from aqueous solution [J]. RSC Advances, 2014,4:38334-38340. [34] Rakshit S, Sarkar D, Elzinga E J, et al. Mechanisms of ciprofloxacin removal by nano-sized magnetite [J]. Journal of Hazardous Materials, 2013,(246-247):221-226. [35] Deihimi N, Irannajad M, Rezai B. Characterization studies of red mud modification processes as adsorbent for enhancing ferricyanide removal [J]. Journal of Environmental Management, 2018,206:266-275. [36] Venkatesan G, Narayanan S L. Synthesis of Fe2O3-coated and HCl-treated bauxite ore waste for the adsorption of arsenic (Ⅲ) from aqueous solution: isotherm and kinetic models [J]. Chemical Engineering Communications, 2018,205(1):34-46. [37] Castaldia P, Silvetti M, Enzob S, et al. Study of sorption processes and FT-IR analysis of arsenate sorbed onto red muds (a bauxite ore processing waste) [J]. Journal of Hazardous Materials, 2010,175:172–178. [38] Paras T, Dharni V. Spectroscopic investigation of ciprofloxacin speciation at the goethite-water interface [J]. Environmental Science & Technology, 2007,41(9):3153-3158. Adsorption of ciprofloxacin by acidified red mud: characteristic, mechanism and process optimization. SHI Jing-zhuan1, WEI Hong1*, ZHOU Xiao-de1, SHI Ying-juan2, ZHENG Jia-xin1 (1.State Key Laboratory of Eco-Hydraulics in Northwest Arid Region, Xi’an University of Technology, Xi’an 710048, China;2.Shaanxi Reconnaissance Design & Research Institute of Water Environmental Engineering, Xi’an 710021, China)., 2019,39(11):4689~4696 In this paper, the adsorption conditions, characteristic and mechanism of ciprofloxacin on the acidified red mud were studied.A four-factor and three-level optimization model based on Box-Behnken design method was established to determine the optimum adsorption condition, andadsorption temperature, solution pH, ciprofloxacin initial concentration and acidified red mud dosage were as arguments and adsorption capacity as the response value. The kinetic model, isotherm model, thermodynamic property and mechanism of the adsorption process were discussed as well. The results showed that solutionpH, ciprofloxacin initial concentration, acidified red mud dosage had significant effect on the adsorption process.The predicted maximum adsorption reached 7.30mg/g under the optimized conditions of 45℃, pH 3.04, ciprofloxacin initial concentration of 29.20mg/L, and acidified red mud dosage 3.40g/L. The adsorption was well fitted the pseudo-second-order reaction kinetics and Langmuir-Freundilich isotherm model, with the maximum adsorption capacity were 7.90 and 7.35mg/g, respectively. Δ0, Δ0and Δ0were calculated by Van Tehoff equation as -82.13~94.37kJ/mol, 0.61J/(mol·K) and100.25kJ/mol, respectively. Ciprofloxacinadsorption on acidified red mud was a spontaneous endothermic process.Infrared spectrum showed that the complexation between carboxylate group of ciprofloxacin and Al-O bond of acidified red mud, and the weak electrostatic or inner-sphere bonding between keto group in ciprofloxacin and Fe-O in acidified red mud were attributed to the adsorption. This study showed that acidified red mud is a potentially low-cost absorbent for the treatment of antibiotic-contaminated wastewater. acidified red mud;ciprofloxacin;response surface optimization;adsorption kinetics;adsorption thermodynamics X703 A 1000-6923(2019)11-4689-08 史京转(1985-),女,陕西渭南人,西安理工大学博士研究生,主要从事生态环境修复及有机污染有效控制研究.发表论文5篇. 2019-04-19 国家自然科学基金资助项目(51979223);陕西省自然科学基金资助项目(2017JM5082);陕西省水利科技项目(2013slkj-07) * 责任作者, 教授, weihong0921@163.com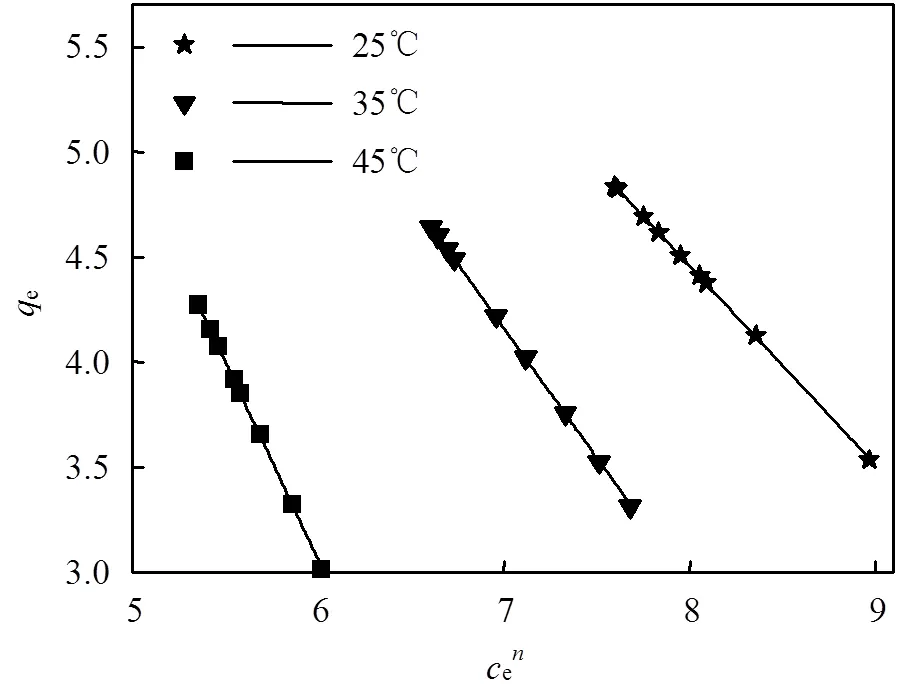
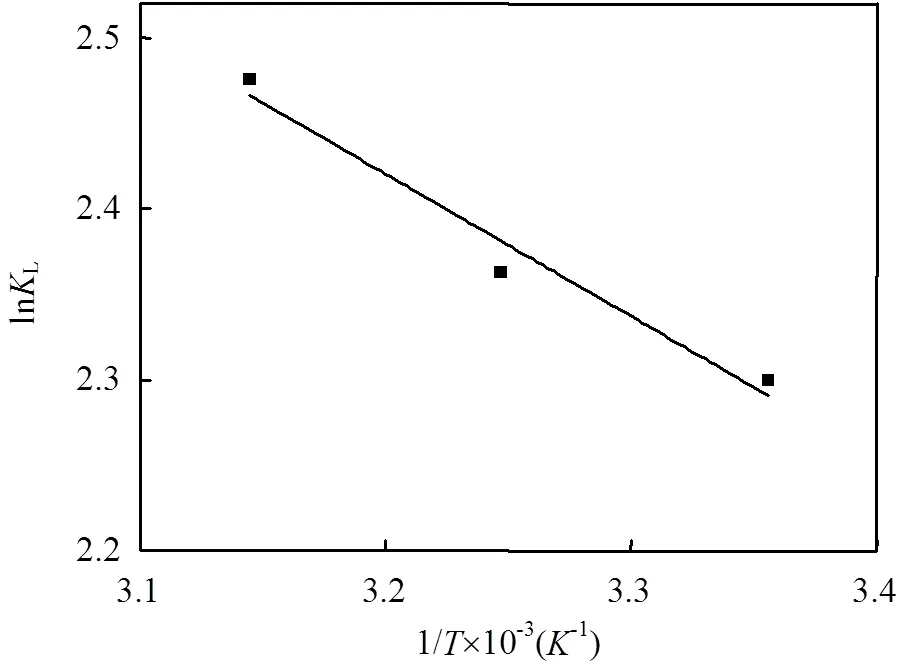
2.4 吸附热力学

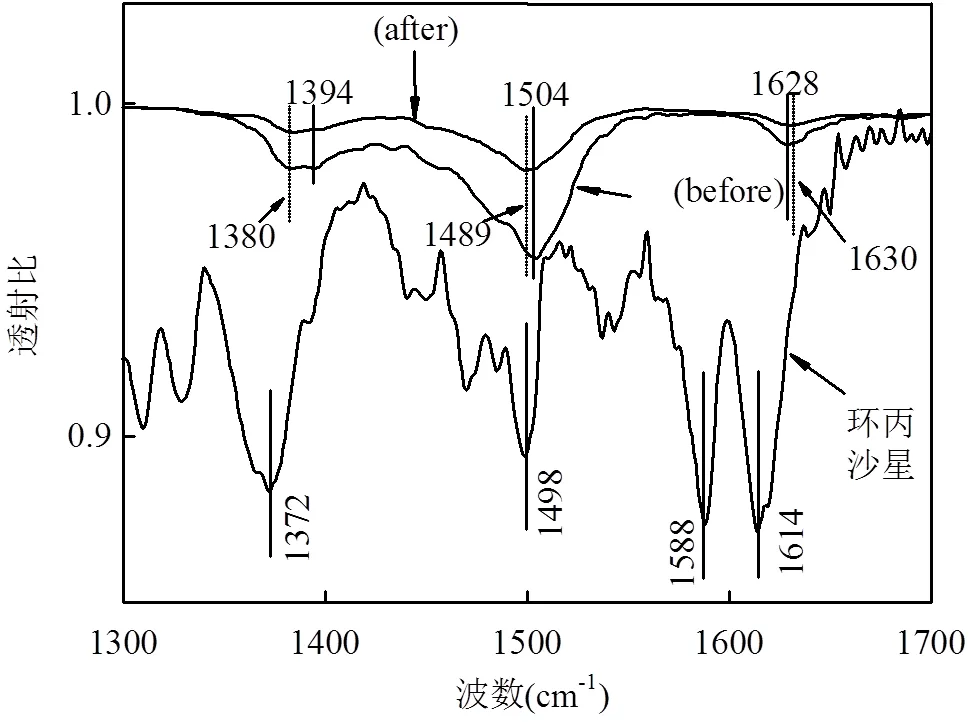
2.5 ATR-FTIR分析
3 结论

