Fabrication of Polypyrrole@CuO Nanotubes and Conversion to Carbon@CuO Nanotubes and Their Electrochemical Catalysis Performance
, ,
(1. Shandong Mingxiang Environmental Technology Limited, Jinan Shandong, 250100, China; 2. Anhui Key Laboratory of Controllable Chemistry Reaction & Material Chemical Engineering, Hefei University of Technology, Hefei Anhui, 230009, China)
Abstract: Polypyrrole@copper oxide nanotubes(PPy@CuO-NTs) as a new high-performance composite material was prepared by in-situ chemical oxidation polymerization of pyrrole on the surface of CuO nanotubes by using L-Lysine as modified surfactants. PPy@CuO-NTs was converted into Carbon@CuO nanotubes(C@CuO-NTs) under high-temperature of 600℃ vacuum treatment by carbonization of the PPy shell. Their electro-catalytic performance was investigated. The result of cyclic voltammograms and electro-catalysis of glucose demonstrated that the material showed good electrochemical catalysis performance compared with bare Cu electrode. Transmission electron microscope(TEM), scanning electron microscopy(SEM), fourier transform infrared spectroscopy(FTIR) and X-ray diffraction(XRD) confirmed the formation of the PPy@CuO-NTs and C@CuO-NTs.
Keywords: PPy@CuO-NTs; composites; C@CuO-NTs; electrochemical catalysis; glucose
1 Introduction
The conducting polymers including polyaniline(PANI)[1], polypyrrole(PPy)[2]and polythiophene(PTh)[3]are most widely studied. Among these conducting polymers, PPy is one of the most extensively studied materials due to its good environmental stability, high conductivity, high potential and biocompatibility[4]. Multifunctionalized PPy nanostructures have been synthesized by blending PPy with electrical, optical and magnetic inorganic nanoparticles to form nanocomposites. Armes and co-workers first prepared colloidal conducting polymers by coating metal oxide nanoparticles with PPy or polyaniline[5-7]. In recent years, considerable attention has been focused on one-dimensional nanostructured materials owing to their unique physical properties and potential applications in sensors, magnetics, electric transportation, optics, and even as building blocks for nanoscale devices[8-11]. In particular, much effort has been devoted to the controllable synthesis of inorganic nanotubes since the discovery of carbon nanotubes in 1991[12]. Unlike nanorods and nanowires, only a few groups have synthesized CuO nanotubes due to fabrication difficulties within hollow nanorods and nanowires. The simplest method of making CuO is from the direct thermal oxidation of copper metal[13], and many research groups have prepared CuO nanowires and nanorods by oxidizing copper foils[14-16]. The crystal of bare CuO nanotubes are of frangibility and low conductivity. The PPy@CuO-NTs are not only can prevent crystal collapsing in redox process but also can offer many electrolyte-filled channels for faster transport. These advantages can improve the electro-catalytic performance of the PPy@CuO-NTs. On the basis of above, the C@CuO-NTs were obtained by carbonization of the PPy@CuO-NTs in vacuum and the eletro-catalytic performance was investigated.
In this work, a novel way of polymerization on the synthesis of a neoteric tubular inorganic-polymer composite is reported. PPy@CuO-NTs were successfully prepared by directly in-situ chemical polymerization of pyrrole monomer on the surface of CuO-NTs via using L-Lysine as modified surfactants. C@CuO-NTs were obtained after the PPy@CuO-NTs beeing carbonized, The formation mechanism of C@CuO-NTs composites was discussed.
2 Experiments
2.1 Preparation of CuO-NTs
Copper nanowires synthesized following the method described by Chang Yu et al[17]were used as starting solid template for fabrication of polycrystalline oxide nanotubes. Uniform CuO nanotubes were obtained via directly oxidizing copper nanowires in vacuum with the condition of 400℃ and 9h. All of the chemical reagents used were analytical grade without further purification.
2.2 Synthesis of PPy@CuO-NTs and C@CuO-NTs
0.2mmol CuO nanotubes were dispersed in deionized water(100mL) within an ultrasound sonication bath for 30min at room temperature. After that, the mixture was removed to a vessel with vigorous stirring and still under ultrasound condition. 0.3mmol L-Lysine was first dissolved into the stirred solution and 0.2mmol HCl was slowly added into the above solution, then 35μL pyrrole monomer was slowly injected into the stirred solution and stirred continuously. At last,amount of 0.2mmol ammonium persulphate(APS) was added to the mixture. The polymerization was carried out for 6h with constant mechanical stirring and ultrasound. The synthesized samples were filtered and rinsed several times with distilled water. Finally, the product was dried under vacuum at 50℃ for 6h and a black powder was obtained. The PPy@CuO-NTs composites were converted to C@CuO-NTs after 3h of carbonization at 600℃ in vacuum.
2.3 Electrode preparation and electrochemical measurements
The working electrodes were prepared by mixing active materials with acetylene black and polyvinylidene fluoride(PVDF) binder(mass concentration is 0.85∶0.10∶0.05) in N-methyl-2-pyrrolidone(PVP) solvent to form a homogeneous slurry, and then the slurry was uniformly coated on an cooper foil. The coated electrodes were dried at 60℃ for 6h in vacuum. The electrolyte was the 100mL aqueous solution containing 0.02g glucose and 0.6g sodium hydroxide. The electrochemical tests were performed using CHI660B electrochemical work station. All electrochemical experiments were carried out using a conventional three-electrode system consisting of an Ag/AgCl(saturated KCl) reference electrode, a platinum foil counter electrode, and a C@CuO-NTs working electrode. A 0.15mol/dm3NaOH solution was used as the supporting electrolyte.
3 Results and discussion
3.1 The Synthesis Mechanism of PPy@CuO-NTs and C@CuO-NTs
Fig.1(page 666) is the schematic formation mechanism of PPy@CuO nanotubes and C@CuO nanotubes. (NH4)2S2O8(APS) is a very effective oxidizer to pyrrole monomers(Py), but it is easily to be hydrolyzed in water. L-Lysine is an alkaline protein. It doesn’t react with CuO-NTs and keeps the solution of the weak alkali together with 1mol/L HCl aqueous solution. At the same time, it is also a kind of surface modification agent, so the surface of CuO-NTs can be covered closely by the oil-base alkyl group layer. The added pyrrole monomers will be absorbed on alkyl group’s chain. Pyrrole monomers would be in-situ polymerized on the surface of CuO-NTs under the trigger effect of the (NH4)2S2O8. Then the PPy was effectively coated on the CuO-NTs, forming PPy@CuO shell/core nanotubes. Finally, The PPy@CuO-NTs composite were converted to C@CuO-NTs after 3h of carbonization at 600℃ in vacuum.

Fig.1 The synthesis mechanism schematic diagram of PPy@CuO-NTs and C@CuO-NTs
3.2 Morphology of CuO-NTs, PPy@CuO-NTs and C@CuO-NTs

Since PPy is one of the carbon sources[18], PPy can be converted to the corresponding carbon by thermal carbonization of the PPy. Xuan S.H gave the thermal gravimetric analysis(TGA) of PPy[19]under the condition of 600℃. The TEM image of C@CuO-NT is shown in Fig.2(c). What is important, the PPy@CuO-NTs must be under a vacuum sealed tube in the process of thermal treatment. The principle process of experiment is shown in scheme1.

Fig.2 TEM images of (a) CuO-NTs, (b) PPy@CuO-NTs and (c) C@CuO-NTs
3.3 XRD Analysis
X-ray powder diffraction measurements were used to identify the crystalline phase of the pure PPy and PPy@CuO-NTs composite, as displayed in Fig.3. The XRD pattern of pure PPy shows a broad peak located in the range of 15~30° and centered at 2θ=21°, which can be ascribed to the characteristic peak of amorphous PPy. In the XRD profile of CuO nanotube, all the peaks observedcan be indexed to monoclinic CuO (JDPDS NO: 80-1916). The XRD pattern shows the characteristic broad features of PPY and also some sharp peaks associated with the monoclinic CuO. The diffraction peaks of CuO are stronger than those of the PPY, which can be attributed tothe residual inorganic materials.
3.4 FT-IR Spectra Analysis
FT-IR spectra of the prepared PPy and PPy@CuO-NTs composite are shown in Fig.4. The main characteristic peaks of PPy are assigned as follows: the band at 1565cm-1is attributed to the antisymmetric and symmetric pyrrole ring vibration. The bands located at 1307 and 1213cm-1are ascribed to the =C-H in-plane deformation and the C-N stretching vibration, while other bands at 1047 and 925cm-1reflect N-H in-plane deformation vibration and the C-H out-of-plane vibration, respectively, which implies the doping state of PPy[20]. The peaks centering at 789cm-1are assigned to the hydroxyl group peaks. The results are in agreement with the reported literature[21]. The PPy@CuO-NTs composite has identical characteristic vibrations for PPy, but some peaks of PPy slightly shift to high wave number by the incorporation of CuO nanotubes.

Fig.3 XRD patterns of pure PPy and PPy@CuO-NTs

Fig.4 FT-IR spectra of pure PPy, PPy@CuO- NTs and C@CuO-NTs
The spectrum obtained for C@CuO-NTs sample showsthat the characteristic peak at 3660cm-1corresponds to O-H stretching vibration of the surface hydroxyl groups[22].
3.5 Electrocatalytic Performance Analysis
First of all, the electrocatalytic activity of the C@CuO-NTs modified electrode towards the oxidation of glucose in an alkaline solution was investigated. Fig.5 displays the cyclic voltammograms (CVs) of 1.0mmol/dm3glucose in 0.15mmol/dm3NaOH at the C@CuO-NTs modified and bare Cu electrode respectively.
The Cu electrode shows an oxidation peak at approximately 0.48Vvs. Ag/AgCl while the C@CuO-NTs modified Cu electrode displays an oxidation peak centered at approximately 0.37Vvs. Ag/AgCl. the C@CuO-NTs elecirode, as shown in Fig.4(a), substantial negative shift of the anodic peak potential .The obvious decrease in the anodic overpotential to 0.37V shows a strong catalytic function of C@CuO-NTs in the direct oxidation of glucose. The shifts in this overpotential may be due to a kinetic effect by an increase in the electroactive surface area and the rate of electron transfer from glucose to the C@CuO-NTs modified Cu electrode. Furthermore, the CVs of glucose solution at different scan rates were recorded as depicted in Fig.6. The peak current for the anodic oxidation of glucose is proportional to the square root of the scan rate, indicating that the electrocatalytic reaction is diffusion controlled, which is ideal for quantitative analysis in practical applications.

Fig.5 Cyclic voltammograms (CVs) of 1.0mmol/dm3 glucose in 0.15mmol/dm3 NaOH at the (A) C@CuO-NTs modified and (B) bare Cu electrodes
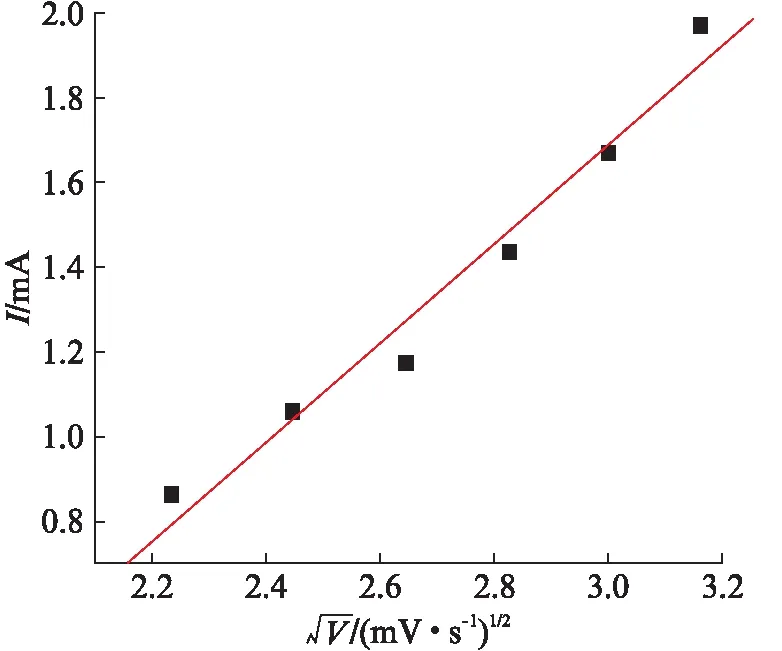
Fig.6 Peak current as a function of sweep rate at the C@CuO- NTs modified electrode subject to 1.0mmol/dm3 glucose in 0.15mmol/dm3 NaOH
4 Conclusions
In summary, uniform CuO nanotubes were obtained via direct oxidizing copper nanowires in vacuum. CuO-NTs were successfully coated with ultrathin, homogeneous, and well-adhered layers of a conducting polymer PPy with the thickness of 20nm by in-situ chemical oxidation polymerization of pyrrole. It is the first time to report that PPy@CuO-NTs and C@CuO-NTs are with tubular morphology. The method presented in this paper may be extended to synthesize other one-dimensional nanoscale composites. C@CuO-NTs have been successfully prepared by thermal carbonization of PPy and showed good electro-catalysis properties, and these new materials will open up opportunities for the development of new carriers for catalysts.

