茶树根际土壤物质的自毒潜力及其对土壤微生物多样性的影响
王海斌 陈晓婷 赵虎 王裕华 张清旭 汪鹏 叶江华 丁力

摘 要 為了分析茶树根际土壤物质对茶树根际土壤微生物多样性的影响,本研究以植茶年限0、3、9、25 a的铁观音茶树根际土壤为材料,采用不同极性树脂吸附茶树根际土壤物质并洗脱,探讨不同植茶年限茶树根际土壤物质的自毒潜力及其对土壤微生物多样性的影响。结果表明,不同极性树脂吸附洗脱液以ADS-7树脂洗脱液对受体根长的抑制作用最强。ADS-7树脂吸附洗脱液处理重新种植的茶树后,随着土壤植茶年限的增加,茶树根际土壤细菌数量呈现下降趋势。相关性分析结果表明,与土壤年限呈正相关的细菌T-RFs片段15个,涉及8个纲,31种细菌,按照其功能可分为4类,其中病原菌19种,占比61.29%;负相关细菌T-RFs片段18个,涉及11个纲,31种细菌,按照其功能可分为6类,其中与抑制病原菌、碳素循环、氮素循环、硫素循环、土壤质地改善相关的细菌总占比达到83.87%。综上表明,ADS-7树脂洗脱液处理重新种植的茶树后,随着土壤植茶年限的增加,茶树根际土壤病原菌数量大幅上升,益生菌与土壤养分循环相关的细菌数量显著下降,土壤微生物生态系统平衡失调。
关键词 茶树根际土壤;自毒作用;物质;微生物多样性中图分类号 S571.1; S154.3 文献标识码 A
The Autotoxicity ofTea Tree Rhizosphere Soil Chemicals and the Effect of Soil MicrobialDiversity
WANG Haibin1,2, CHEN Xiaoting1,2, ZHAO Hu1,3, WANG Yuhua1, ZHANG Qingxu2, WANG Peng2,YE Jianghua2,4, DING Li1
1. College of Life Sciences, Longyan University, Longyan, Fujian 364012, China; 2. Fujian Provincial Key Laboratory of Agroecological Processing and Safety Monitoring, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, China; 3. College of Horticulture, Gansu Agricultural University, Lanzhou, Gansu 730070, China; 4. College of Tea and Food Science, Wuyi University, Wuyishan, Fujian 354300, China
Abstract In order to analyze the effect of tea tree rhizosphere soil chemicals on the soil microbial diversity, the rhizosphere soils from Tieguanyin tea plantations of 0, 3, 9 and 25 years old were extracted and eluted through different polar resins to discuss the autotoxicity of resins eluted and the effect of soil microbial diversity. The results showed that the inhibitory effect of ADS-7 resin eluent on the root length of receptor was the strongest. After the replanted tea trees was treated by ADS-7 resin eluent, the number of bacteria in the tea trees rhizosphere soil decreased with the increase of planting soil age. Correlation analysis result showed that 15 T-RFs from bacterial community were significantly and positively correlated with soil age, which included 31 species of microbes belonging to 8 classes, respectively. Thirty-one microbes could be divided into 4 types according to the function, among them, 19 species of pathogenic bacteria accounted for 61.29%. In addition, 18 T-RFs were significantly and negatively correlated with soil age, which included 31 species of bacteria belonging to 11 classes, respectively. The thirty-one bacteria could be divided into 6 types according to the function, and total percentage of the bacteria to inhibit 83.87%. In brief, after the replanted tea trees was treated by ADS-7 resin eluent, the number of pathogenic bacteria in tea tree pathogenic bacteria, carbon cycle, nitrogen cycle, sulphur cycle and the bacteria to improve the soil quality accounted for rhizosphere soil was great enhanced and the number of probiotics and soil nutrient cycling bacteria decreased significantly as planting soil age increased, which led to the imbalance of soil microbial ecosystem.
Keywords rhizosphere soil of tea tree; autotoxicity; chemical; microbial diversity
DOI10.3969/j.issn.1000-2561.2019.09.025
茶树[Camellia sinensis(L.) O. Kuntze]属于山茶科、山茶属灌木或小乔木茶种,为多年生常绿木本植物。铁观音茶园的开垦、种植到采摘需要2~3年,正常经济效益旺期在7年左右。21世纪初,安溪铁观音进入飞速发展时期。大型茶叶企业纷纷在安溪县及周边县市大量开垦新茶园,种植铁观音茶树,并按公司化方式统一管理,安溪县周围的山地已形成以茶树为主要植物种群的单一群落结构。为此,茶园“土壤病”形成,茶园逐渐退化,茶树病虫害加剧,茶叶单产水平及品质也逐年下降。茶园退化一方面是茶树本身自然衰老,另一方面茶树连年种植后,土壤环境发生变化,不利于茶树生长的因素积累,土壤自毒作用加剧。
王海斌等[1]调查分析了安溪县9个乡镇茶园土壤的酸化情况发现,调查的茶园中37.67%的土壤已经酸化,10.03%的土壤不适宜种植茶树,茶树树龄与其根际土壤pH呈极显著负相关,茶叶的产量、品质与茶树根际土壤pH呈极显著正相关,该研究认为,茶树根际土壤酸度随着茶树树龄的增加而加剧,茶叶产量降低、品质呈现下降趋势。Ye等[2-3]研究也发现,随着茶树树龄的增加,茶树根际土壤酸度加剧,自毒作用潜力增强,茶叶产量和品质降低,这种现象的形成与土壤中酸类物质的积累增加有关。本课题组前期对不同树龄茶树根际土壤物质进行HPLC分析发现,随着茶树树龄的增加,茶树根际土壤中6种酸类物质含量不断上升。此外,进一步采用不同极性树脂吸附土壤物质并进行生物测试发现,以ADS-7树脂吸附后的洗脱液自毒作用能力最强,GC-MS分析ADS-7树脂洗脱液的物质成分,发现13种物质随着茶树树龄的增加呈现上升趋势,其中9种是酸类物质[4-5]。可见,退化茶园“土壤病”的形成与茶树根际土壤物质种类与数量密切相关。
土壤是一个复杂生态系统,存在着丰富的微生物种类,植物释放和积累的物质通过土壤载体进行传播,进而影响土壤中的微生物区系与种类,土壤生态系统朝着专一化的趋势发展[6-8]。综上可见,随着茶树树龄的增加,土壤自毒潜力加剧,茶树根际土壤微生物发生显著变化[9-10];而关于这种变化是否与土壤物质有关,土壤物质对微生物多样性变化是否会产生影响的研究还鲜有报道。据此,本研究以不同树龄铁观音茶树根际土壤为材料,采用不同极性树脂进行吸附并洗脱,土壤洗脱液一方面用于自毒潜力评价,一方面用于处理新种植的茶树并测定茶树根际土壤微生物种类、群落结构及其功能的变化,以期为茶园土壤退化的修复提供一定的理论依据。
1 材料与方法
1.1材料
以福建省泉州市安溪县龙涓乡铁观音茶园为研究地点(东经117°93′、北纬24°97′),收集已种植0、3、9、25年的铁观音茶树根际土壤,用于土壤物质提取及不同极性树脂的吸附与洗脱后的生物测试。土壤取样方法:随机选择3、9、25 a树龄的茶树各100株,去除土壤表层杂质,连根挖出茶树,收集茶树根际土壤;以未种植过茶树的土壤为对照(0 a),首先去除地表植被和杂质,收集15~25 cm范围的土壤,多点随机收集;各样品的取样量均为约15 kg。取样点茶园土壤的基本理化指标:有机质、全氮、全磷、全钾、速效氮、速效磷、速效钾含量,分别为8.34 g/kg、2.17 g/kg、1.05 g/kg、1.46 g/kg,25.3 mg/kg、56.7 mg/kg、264.6 mg/kg。
1.2方法
1.2.1 不同极性树脂洗脱液对受体莴苣(Lactuca saliva)根长的抑制率分析 土壤样品置于阴凉处自然风干,研磨过40目筛,称取0、3、9、25 a茶树根际土壤各5 kg,加入20 L蒸馏水,360 W超声提取1 h(每隔10 min均匀搅拌1次),120 r/min振荡1 h,重复5次,过滤,提取液于45 ℃下旋转蒸发浓缩至5 L;此时,每毫升浸提液约含有1 g土壤物质[11]。
先将不同极性的树脂ADS-7、ADS-21、ADS-F8、ADS-17、ADS-8(天津南开合成科技有限公司)采用纯乙醇浸泡活化24 h,蒸馏水浸泡清洗至没有乙醇。取收集的不同样品提取浓缩液各5 L,分成5组,每组1 L,采用5种不同极性树脂分别进行静态吸附,方法为:每升提取浓缩液加入200 g树脂,置于摇床上120 r/min振荡吸附24 h,弃上清液,树脂中加入600 mL甲醇,置于搖床上120 r/min洗脱12 h,收集甲醇洗脱液过滤并浓缩至200 mL,用于不同极性树脂洗脱液对受体莴苣根长的抑制率测定,具体参照Wang等[12]的方法进行测定,根长抑制率的计算公式为,相对抑制率=(1-处理值/对照值)×100%。
1.2.2 外源添加不同极性树脂洗脱液处理新种植的茶树 取不同极性树脂吸附后的洗脱液各50 mL,于45 ℃下旋转蒸发浓缩至10 mL,加无菌蒸馏水定容至2 L,-20 ℃保存备用。
取未种植过茶树的土壤风干研磨并过40目筛;将土壤装入盆中,每盆10 kg,选择1年生、长势相对一致的铁观音茶苗并移栽到盆中,每盆6株,移栽后,恢复生长30 d。适当搅动、松动种植土壤,将配置好的树脂洗脱液2 L缓慢倒入盆中,尽量使其在土壤中分布均匀,继续常规种植茶树60 d,收集茶树根际土壤,用于土壤微生物的T-RFLP分析,每种处理种植3盆,即3个重复。种植土壤的基本理化指标为:有机质、全氮、全磷、全钾、速效氮、速效磷、速效钾含量,分别为9.02 g/kg、1.03 g/kg、0.56 g/kg、1.85 g/kg、89.46 mg/kg、15.28 mg/kg、179.62 mg/kg。
1.2.3 土壤微生物的T-RFLP分析 采用CTAB-蛋白酶K-液氮冻融法直接抽提不同样品的土壤微生物总DNA,用于微生物的16S rDNA的扩增和酶切[13]。16S rDNA扩增的PCR反应程序为:94 ℃ 5 min;94 ℃ 45 s,52 ℃ 45 s,72 ℃ 1.5 min,30个循环后,72 ℃ 10 min。其中,扩增引物采用带有FAM荧光标记的细菌通用引物,分别为8F-FAM(5′-AGAGTTTGATCCTGGCTCA G-3′)和926R(5′-CCGTCAATTCCTTTRAGTT T-3′),PCR反應体系的总体积为25 μL,反应体系中各成分含量为:2.5 μL 10×PCR Buffer、正反向引物(10 μmol/L)各0.8 μL、2.0 μL dNTP(25 μmol/L)、0.2 μL BSA、17.05 μL ddH2O、0.15 μL rTaq、1.5 μL DNA模板。PCR结束后,电泳检测,UNIQ-10柱式DNA胶回收试剂盒回收PCR产物中1000 bp左右的片段,用于酶切。
酶切采用HaeIII和MspI 2种内切酶进行消化,其中HaeIII酶切体系为:HaeIII 1 μL、H×Buffer 2 μL、ddH2O 7 μL、PCR产物10 μL;MspI酶切体系为:MspI 1 μL、T-Buffer 2 μL、BSA 2 μL、ddH2O 5 μL、PCR产物10 μL。将配置好的2种酶切体系分别置于37 ℃水浴酶切5 h,酶切后的产物采用ABI自动测序分析仪(Model 3130 Applied Biosystems)测定。
1.3数据处理
土壤微生物测序结果分析采用GeneMarker V1.2软件,分析参数参照SoftGenetics Application Note July, 2006。酶切获得的T-RFs片段分析采用Ribosomal Database Project II数据库比对法,获取T-RFs双酶切片段所对应的微生物物种。T-RFs片段丰度计算公式为:T-RFs片段丰度=ni/N×100,式中ni代表可分辨的T-RF的峰面积,N代表所有T-RF峰面积的总和[14]。其余常规的数据分析、方差分析、显著性分析及变化分析等采用Excel软件和DPS数据处理系统进行处理。
2 结果与分析
2.1不同极性树脂洗脱液对受体莴苣根长的抑制率分析
不同极性树脂洗脱液对受体莴苣根长影响的分析结果表明(表1),不同极性树脂洗脱液对受体根长均存在一定的抑制作用,以25 a茶树根际土壤的树脂吸附洗脱液抑制作用最强,表现为ADS-8、ADS-17、ADS-F8、ADS-21、ADS-7树脂洗脱液对莴苣根长的抑制率分别为15.76%、19.68%、20.57%、29.07%、43.76%。其中以25 a茶树根际土壤经ADS-7树脂吸附的洗脱液抑制作用最强。
2.2 ADS-7树脂洗脱液处理后茶树根际土壤细菌多样性分析
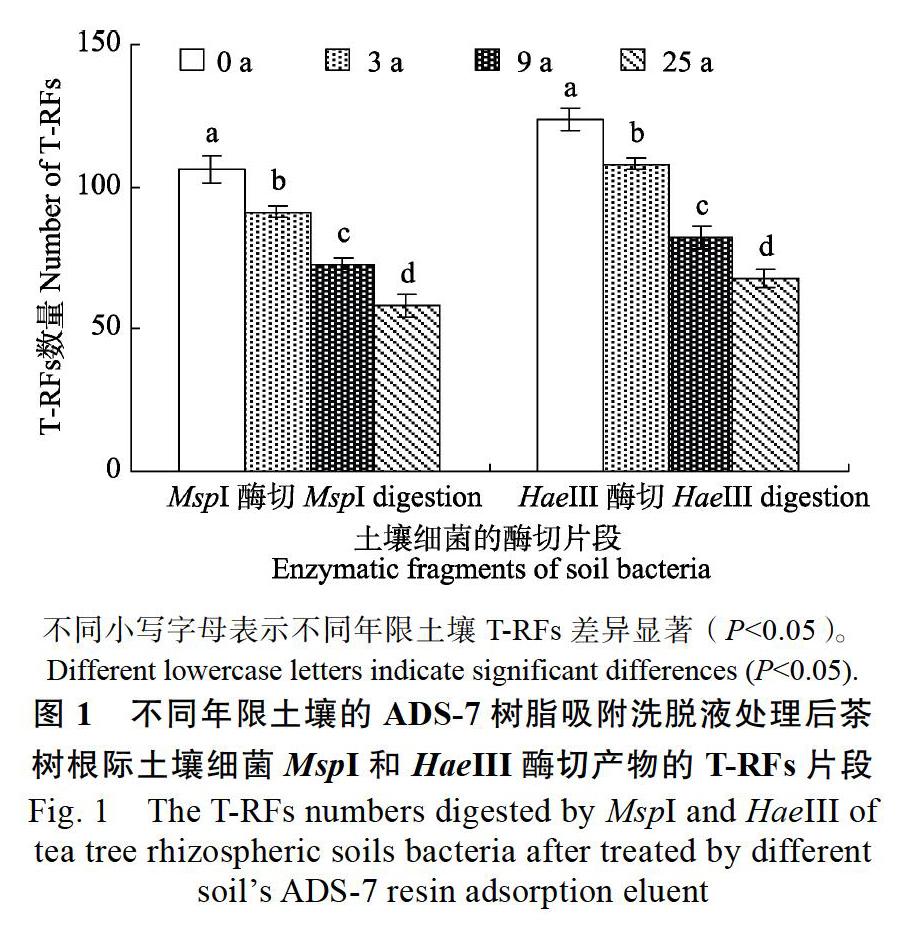
由图1可见,0、3、9、25 a茶树根际土壤经ADS-7树脂洗脱液处理后,根际土壤细菌HaeIII和MspI酶切产物的T-RFs片段,随着土壤年限的增加呈现下降趋势,表现为HaeIII酶切产物的T-RFs片段数量从106个下降至58个,而MspI酶切产物的T-RFs片段数量则从124个下降至68个。可见,不同种植年限土壤的ADS-7树脂吸附洗脱液处理后,茶树根际土壤细菌多样性发生显著的变化。
2.3 ADS-7树脂洗脱液处理后茶树根际土壤正、负相关细菌分析
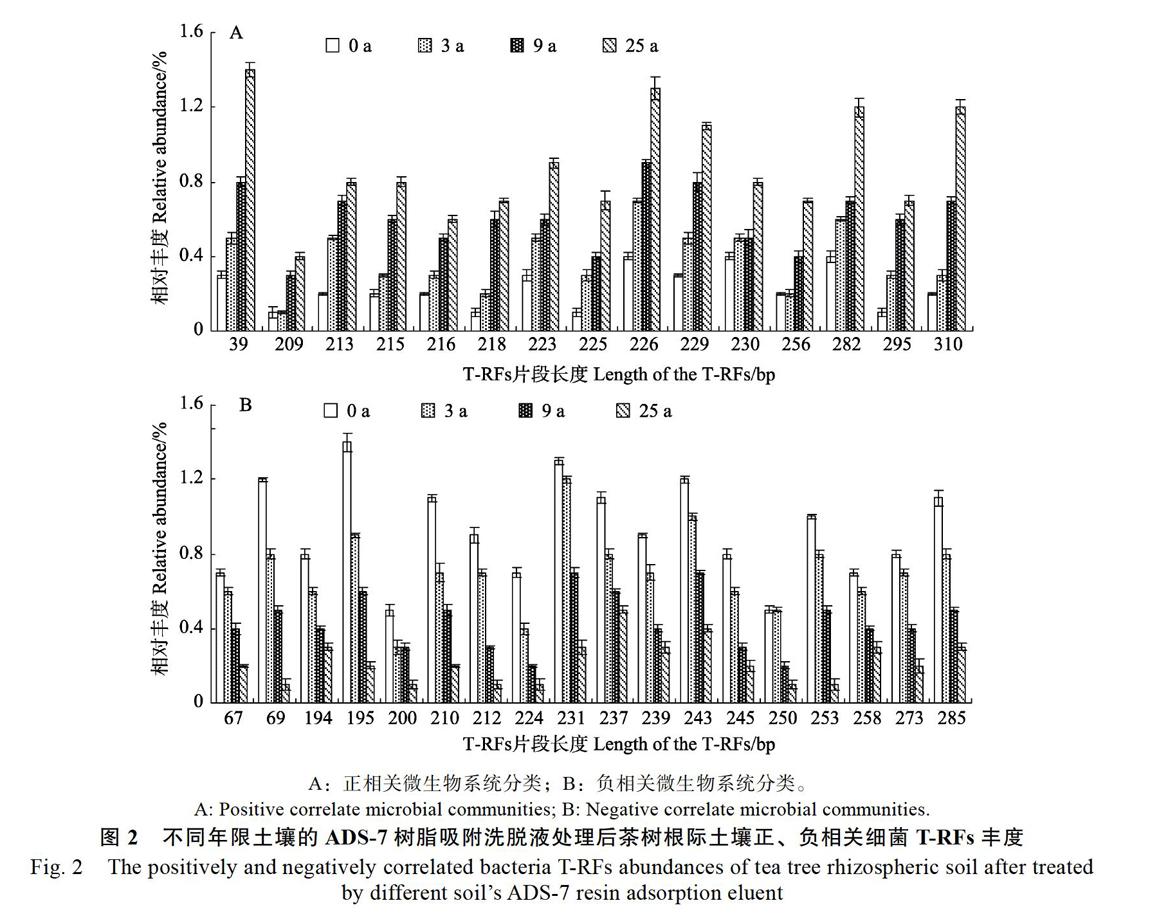
以不同茶树根际土壤细菌HaeIII酶切后的T-RFs片段丰度进行相关性分析发现(图2),不同年限茶树根际土壤的ADS-7树脂吸附洗脱液处理后,与土壤种植年限呈显著或极显著正相关的细菌T-RFs片段共15个,呈显著或极显著负相关的有18个。
进一步将T-RFs片段与Ribosomal Database Project II数据库进行比对,结果表明(图3),正相关细菌T-RFs片段15个,涉及31种细菌,由8个纲组成,占比分别为β-变形菌纲12.90%、γ-变形菌纲16.13%、δ-变形菌纲6.45%、芽孢杆菌纲38.71%、放线菌亚纲9.68%、鞘脂杆菌纲9.68%、梭菌纲3.23%、螺旋体纲3.23%;负相关细菌T-RFs片段18个,涉及31种细菌,由11个纲组成,占比分别为α-变形菌纲25.81%、β-变形菌纲6.45%、γ-变形菌纲12.90%、放线菌亚纲19.35%、梭菌纲12.90%、芽孢杆菌纲6.45%、螺旋体纲3.23%、柔膜菌纲3.23%、鞘脂杆菌纲3.23%、红色杆菌亚纲3.23%、梭杆菌纲3.23%。可见,不同种植年限土壤的ADS-7树脂吸附洗脱液处理后,茶树根际土壤细菌种类发生显著变化。
2.4 ADS-7树脂洗脱液处理后茶树根际土壤正相关细菌功能分析
由表2可见,0、3、9、25年茶树根际土壤经ADS-7树脂洗脱液处理后,茶树根际土壤正相关细菌有31种,按照其功能可分为4大类:病原菌19种,占比61.29%;抑制病原菌相关细菌3种,占比9.68%;碳素循环相关细菌4种,占比12.90%;改善土壤质地相关细菌5种,占比16.13%。可见,不同种植年限土壤的ADS-7树脂吸附洗脱液处理后,茶树根际土壤不同功能类别的细菌数量发生显著变化,特别是病原菌数量显著增加,表现为正相关细菌中病原菌占比最大。
2.5 ADS-7树脂洗脱液处理后茶树根际土壤负相关细菌功能分析
由表3可见,0、3、9、25 a茶树根际土壤经ADS-7树脂洗脱液处理后,茶树根际土壤负相关细菌31种,按照其功能可分为6大类:病原菌5种,占比16.13%;抑制病原菌相关细菌4种,占比12.90%;碳素循环相关细菌10种,占比32.26%;氮素循环相关细菌8种,占比25.81%;改善土壤质地相关细菌3种,占比9.68%;硫素循环细菌1种,占比3.23%。可见,年限土壤经ADS-7树脂吸附洗脱液处理后,茶树根际土壤不同功能类别的细菌数量发生显著变化,特别是与抑制病原菌、碳素循环、氮素循环、土壤质地改善、硫素循环相关的细菌数量显著下降,总占比达到83.87%。
3 讨论
植物土壤生态系统,主要涉及植物、土壤、微生物三者,三者之间相互协调,影响着植物的生长,土壤的质量,微生物的生存[63]。茶树长期种植后,根系分泌物在土壤中大量积累,使土壤微生物在选择性压力影响下发生了显著的变化,这种变化可能朝着对茶树生长有利或有弊的方向发展。本研究结果表明,随着茶树树龄的增加,茶树根际土壤不同极性树脂吸附洗脱液对受体莴苣根长存在一定的抑制作用,以ADS-7树脂洗脱液的抑制作用最强。其次,不同种植年限土壤的ADS-7树脂吸附洗脱液处理重新种植的茶树后发现,随着土壤种植年限的增加,茶树根际土壤细菌数量呈现下降趋势。众多学者在研究不同作物——太子参、山银花、茶树等连续种植后,土壤微生物数量变化时也发现类似的趋势[64-66]。可见,ADS-7树脂洗脱液处理对茶树根际土壤微生物数量产生影响,这种影响与不同年限茶树原位种植现象类似。
作物长期种植,土壤物质积累后会对微生物数量与种类产生影响。Wang等[12]研究发现,百香果连续种植后,产量和品质呈现下降趋势;郝慧荣等[67]研究发现,牛膝连年种植后,反而有利于促进其生长和品质提高。可见,深入分析作物连续种植后土壤微生物种类及功能变化对于明晰“土壤病”的形成具有重要的意义。本研究结果表明,不同种植年限土壤的ADS-7树脂吸附洗脱液处理重新种植的茶树后,随着土壤年限的增加,与其正相关细菌T-RFs片段15个,涉及8个纲、31种细菌,其中病原菌19种,占比61.29%。可见,ADS-7树脂吸附洗脱液处理后,茶树根际土壤的病原菌数量增多,进而影响茶树的生长。此外,进一步分析发现,随着土壤年限的增加,与其负相关细菌T-RFs片段18个,涉及11个纲、31种细菌,其中与抑制病原菌、碳素循环、氮素循环、土壤质地改善、硫素循环相关的细菌数量显著下降,总占比达到83.87%。可见,ADS-7树脂吸附洗脱液处理后,茶树根际土壤的益生菌与土壤养分循环相关的细菌数量下降,茶树根际土壤质地变劣,养分循环受阻。
土壤微生物是土壤生态系统的重要组成部分,其数量及多样性水平高低对于生态系统稳定具有重要的作用,丰富的土壤微生物有利于降低“土壤病”的发生机率,反之则提高[68-69]。可见,不同年限土壤的ADS-7树脂吸附洗脱液处理重新种植的茶树后,茶树根际土壤细菌多样性及群落结构失去平衡,进而可能导致茶树生长受阻。
综上,本研究探讨了茶树根际土壤物质的自毒潜力及其对土壤微生物多样性的影响,结果表明,ADS-7树脂吸附洗脱液对受体莴苣根长的抑制作用最强,ADS-7树脂洗脱液处理重新种植的茶树后,随着土壤年限的增加,茶树根际土壤病原菌数量大幅上升,益生菌与土壤养分循环相关的细菌数量显著下降,土壤微生物生态系统平衡失调,“土壤病”形成,进而可能导致茶树生长和品质受阻。然而,对于茶树—土壤—微生物,三者之间是如何实现相互调控与影响,还需进一步深入研究。
参考文献
- 王海斌, 陈晓婷, 丁 力, 等. 福建省安溪县茶园土壤酸化对茶树产量及品质的影响[J]. 应用与环境生物学报, 2018, 24(6): 1398-1403.
- Ye J H, Wang H B, Kong X H, et al. Soil sickness problem in tea plantations in Anxi county, Fujian province, China[J]. Allelopathy Journal, 2016, 39(1): 19-28.
- Ye J H, Wang H B, Yang X Y, et al. Autotoxicity of the soil of consecutively cultured tea plantations on tea (Camellia sinensis) seedlings[J]. Acta Physiologia Plantarum, 2016, 38(8): 195.
- Jia X L, Ye J H, Zhang Q, et al. Soil toxicity and microbial community structure of Wuyi rock tea plantation[J]. Allelopathy Journal, 2017, 41(1): 113-126.
- Jia X L, Wang H B, Ye J H, et al. Identification of Allelochemicals responsible for soil degradation in continuously cropped Tea plantations[J]. Allelopathy Journal, 2018, 45(1): 1-12.
- Travis S W, Harsh P B, Erich G, et al. Root Exudation and rhizosphere biology[J]. Plant Physiology, 2003, 132(1): 44-51.
- Vivanco J M, Bais H P, Stermitz F R, et al. Biogeographical variation in community response to root allelochemistry: novel weapons and exotic invasion[J]. Ecology Letters, 2004, 7(4): 285-292.
- Marschner P, Timonen S. Interactions between plant species and mycorrhizal colonization on the bacterial community composition in the rhizosphere[J]. Applied Soil Ecology, 2005, 28(1): 23-36.
- 王海斌, 陈晓婷, 丁 力, 等. 连作茶树根际土壤自毒潜力、酶活性及微生物群落功能多样性分析[J]. 热带作物学报, 2018, 39(5): 852-857.
- 王海斌, 陈晓婷, 丁 力, 等. 不同树龄茶树根际土壤细菌多样性的T-RFLP分析[J]. 应用与环境生物学报, 2018, 24(4): 775-782.
- Huang L F, Song L X, Xia X J, et al. Plant-soil feedbacks and soil sickness: From mechanisms to application in agriculture[J]. Journal of Chemical Ecology, 2013, 39(S1): 232-242.
- Wang H B, Chen X T, Ding L, et al. Replant problem and soil toxicity of passion fruit (Passiflora edulis Sims) in China[J]. Allelopathy Journal, 2018, 44(1): 1-11.
- Wang H B, Zhang Z X, Li H, et al. Characterization of metaproteomics in crop rhizospheric soil[J]. Journal Proteome Research, 2011, 10(3): 932-940.
- Horswell J, Cordiner S J, Maas E W, et al. Forensic comparison of soils by bacterial community DNA profiling[J]. Journal of Forensic Sciences, 2002, 47(2): 350-353.
- Thanigaivel S, Vijayakumar S, Gopinath S, et al. In vivo and in vitro antimicrobial activity of Azadirachta indica (Lin) against Citrobacter freundii isolated from naturally infected Tilapia (Oreochromis mossambicus)[J]. Aquaculture, 2015, 437: 252-255.
- Reyes F, Singh N, Khurram N A, et al. Strongyloides hyperinfection syndrome causing fatal meningitis and septicemia by Citrobacter koseri[J]. IDCases, 2017, 10: 102-104.
- Nunney L, Schuenzel E L, Scally M, et al. Large-scale intersubspecific recombination in the plant-pathogenic bacterium Xylella fastidiosa is associated with the host shift to mulberry[J]. Applied and Environmental Microbiology, 2014, 80(10): 3025-3033.
- Nazareno E S, Kersey C M, Dumenyo C K. Characterization of the incompatible interaction between Erwinia tracheiphila and non-host tobacco (Nicotiana tabacum)[J]. Physiology and Molecular Plant Pathology, 2016, 96: 85-93.
- Bhullar K, Zarepour M, Yu H B, et al. The serine protease autotransporter pic modulates Citrobacter rodentium pathog ene sis and its innate recognition by the host[J]. Infection and Immunity, 2015, 83(7): 2636-2650.
- Vincenzo S, Chiara C, Azaira B, et al. Epidemiology, pathogenicity and emerging resistances in Staphylococcus pasteuri: From mammals and lampreys, to man[J]. Recent Patents on Anti-Infective Drug Discovery, 2009, 4(2): 123-129.
- Prosekov A, Milentyeva I, Sukhikh S, et al. Identification of probiotic strains isolated from human gastrointestinal tract and investigation of their antagonistic, antioxidant and antiproliferative properties[J]. Biology Medicine, 2015, 7(5): 1-5.
- Fotoglidis A, Pagourelias E, Kyriakou P, et al. Endocarditis caused by unusual streptococcus species (Streptococcus pluranimalium)[J]. Hippokratia, 2015, 19(2): 182-185.
- Li J Q, Tan B P, Mai K S, et al. Comparative study between probiotic bacterium Arthrobacter XE-7 and chloramphenicol on protection of Penaeus chinensis post-larvae from pathogenic vibrios[J]. Aquaculture, 2006, 253(1-4): 140-147.
- Jansson E, Lindberg L, S?ker E, et al. Diagnosis of bacterial kidney disease by detection of Renibacterium salmoninarumby real-time PCR[J]. Journal of Fish Diseases, 2008, 31(10): 755-763.
- 李璐瑶, 刘梦佳, 滕明明, 等. 霍氏肠杆菌生物学特性的研究[J]. 畜牧兽医杂志, 2017, 36(4): 1-2, 6.
- Mack D, Becker P, Chatterjee I, et al. Mechanisms of biofilm formation in Staphylococcus epidermidis and Staphylococcus aureus: functional molecules, regulatory circuits, and adaptive responses[J]. International Journal of Medical Microbiology, 2004, 294(2-3): 203-212.
- 徐 佳. 奶牛乳腺炎葡萄球菌种类、毒力基因及抗性基因与耐药性的调查分析[D]. 扬州: 扬州大学, 2016.
- Kumar V, Kumar M, Sharma S, et al. Probiotics in agroecosystem[M]. Singapore: Springer, 2017: 451-467.
- Bark S W, Kim K B W R, Kim M J, et al. Optimization and characterization of conditions for cellulose-degrading crude enzymes produced by Cellulophaga lytica PKA 1005[J]. Korean Journal of Microbiology and Biotechnology 42(1): 18-24.
- Xia F, Zou B, Shen C, et al. Complete genome sequence of Methylophilus sp. TWE2 isolated from methane oxidation enrichment culture of tap-water[J]. Journal of Biotechnology, 2015, 211: 121-122.
- Pablos T E, Sigala J C, Borgne S L, et al. Aerobic expression of Vitreoscillahemoglobin efficiently reduces overflow metabolism in Escherichia coli[J]. Journal of Biotechnology, 2014, 9(6): 791-799.
- Noparat P, Maneerat S, Saimmai A. Application of biosurfactant from Sphingobacterium spiritivorum AS43 in the biodegradation of used lubricating oil[J]. Applied Biochemistry and Biotechnology, 2014, 172(8): 3949-3963.
- 刘志丹, 周 良, 杜竹玮, 等. 异化金属还原菌的研究进展[J]. 微生物学通报, 2005, 32(5): 156-159.
- Salam L, Obayori O, Campbell C, et al. Pyrene biodegradation potentials of an actinomycete, microbacterium esteraromaticum isolated from tropical hydrocarbon-contaminated soil[J]. Journal of Microbiology, Biotechnology and Food Sciences, 2017, 6(4): 995-1000.
- Kurniyati K, Kelly J F, Vinogradov E, et al. A novel glycan modifies the flagellar filament proteins of the oral bacterium Treponema denticola[J]. Molecular Microbiology, 2017, 103(1): 67-85.
- Raffel S J, Battisti J M, Fischer R J, et al. Inactivation of genes for antigenic variation in the relapsing fever spirochete Borrelia hermsii reduces infectivity in mice and transmission by ticks[J]. PLoS Pathogens, 2014, 10(4): e1004056.
- Yang P, Hung G C, Lei H Y, et al. Genome sequencing and annotation of Afipia septicemium strain OHSU_II[J]. Geno m ics Data, 2014, 2: 123-126.
- Han Y W. Fusobacterium nucleatum: a commensal-turned pathogen[J]. Current Opinion in Microbiology, 2015, 23: 141-147.
- Xiu Y J, Wu T, Meng X H, et al. Identification and isolation of a spiroplasma pathogen from diseased oriental river prawn, Macrobrachium nipponense, in China: A new freshwater crustacean host[J]. Aquaculture, 2015, 437: 270-274.
- Messmer V D, Bioemberg G V, Ritter C, et al. Diagnostic molecular mycobacteriology in regions with low tuberculosis endemicity: Combining real-time PCR assays for detection of multiple mycobacterial pathogens with line probe assays for identification of resistance mutations[J]. EBioMedicine, 2016, 9: 228-237.
- Lusby P E, Coombes A L, Wilkinson J M. Bactericidal activity of different honeys against pathogenic bacteria[J]. Archives of Medical Research, 2005, 36(5): 464-467.
- 鲁红学, 周 燚. 类芽孢杆菌在植物病害防治和环境治理中的应用研究进展[J]. 安徽农业科学, 2008, 36(30): 13244-13247.
- Floate K D, Poku G K K, Coghlin P C. Overview and relevance of Wolbachia bacteria in biocontrol research[J]. Biocontrol Science and Technology, 2006, 16(8): 767-788.
- Khasnobis S, Vincent E E, Chatterjee D. Emerfing therapeutic targets in tuberculosis: post-genomic era[J]. Expert Opinion on Therapeutic Targets, 2002, 6(1): 21-40.
- Tourova T P, Spiridonova E M, Berg I A, et al. Phylogeny and evolution of the family Ectothiorhodospiraceae based on comparison of 16S rRNA, cbbL and nifH gene sequences[J]. International Journal of Systematic and Evolutionary Microbiology, 2007, 57: 2387-2398.
- Hervé V, Junier T, Bindschedler S, et al. Diversity and ecology of oxalotrophic bacteria[J]. World Journal of Microbiology & Biotechnology, 2016, 32: 28-35.
- Kurata A, Matsumoto M, Kobayashi T, et al. Hyaluronate lyase of a deep-sea bacillus niacini[J]. Marine Biotechnology, 2015, 17(3): 277-284.
- Lin S, Huangpu J J, Chen T, et al. Analysis of soil microbial community structure and enzyme activities associated with negative effects of pseudostellaria heterophylla consecutive monoculture on yield[J]. Pakistan Journal of Botany, 2015, 47(2): 761-769.
- Rawat S, Johri B N. Role of Thermophilic Microflora in Composting[M]//Satyanarayana T, Littlechild J, Kawarabayasi Y. Thermophilic Microbes in Environmental and Industrial Biotechnology. Dordrecht: Springer, 2013: 137-169.
- Nguyen N L, Yu W J, Yang H Y, et al. A novel methanotroph in the genus Methylomonas that contains a distinct clade of soluble methane monooxygenase[J]. Journal of Microbiology, 2017, 55(10): 775-782.
- Hiraishi A, Okamura K. Proposal of Rhodoplanes tepidamans sp. nov. to accommodate the thermotolerant phototrophic bacterium previously referred to as Rhodoplanes (Rhodopseudomonas) cryptolactis[J]. International Journal of Systematic and Evolutionary Microbiology, 2017, 67: 1540-1545.
- Mellbye B L, Giguere A T, Bottomley P J, et al. Quorum quenching of Nitrobacter winogradskyi suggests that quorum sensing regulates fluxes of nitrogen oxide(s) during nitrification[J]. mBio, 2016, 7(5): e01753-e01756.
- Subramanian P, Kim K, Krishnamoorthy R, et al. Endophytic bacteria improve nodule function and plant nitrogen in soybean on co-inoculation with Bradyrhizobium japonicum MN110[J]. Plant Growth Regulation, 2015, 76(3): 327-332.
- Wang J, Bao J T, Su J Q, et al. Impact of inorganic nitrogen additions on microbes in biological soil crusts[J]. Soil Biology & Biochemistry, 2015, 88: 303-313.
- Depraect O G, Barajas C G, Roblero J J, et al. Characterization of a marine microbial community used for enhanced sulfate reduction and copper precipitation in a two-step process[J]. Applied Biochemistry and Biotechnology, 2017, 182(2): 452-467.
- Zamani M, diCenzo G C, Milunovic B, et al. A putative 3-hydroxyisobutyryl-CoA hydrolase is required for efficient symbiotic nitrogen fixation in Sinorhizobium meliloti and Sinorhizobium fredii NGR234[J]. Environmental Microbio logy, 2017, 19(1): 218-236.
- Ali S S, Abomohra A E, Sun J Z. Effective bio-pretreatment of sawdust waste with a novel microbial consortium for enhanced biomethanation[J]. Bioresource Technology, 2017, 238: 425-432.
- Lennon J T. Diversity and metabolism of marine bacteria cultivated on dissolved DNA[J]. Applied and Environmental Microbiology, 2007, 73(9): 2799-2805.
- Arora P K, Srivastava A, Singh V P. Bacterial degradation of nitrophenols and their derivatives[J]. Journal of Hazardous Materials, 2014, 266: 42-59.
- Hemmann J L, Saurel O, Ochsner A M, et al. The one-carbon carrier methylofuran from Methylobacterium extorquens AM1 contains a large number of α- and γ-linked glutamic acid residues[J]. Journal of Biological Chemistry, 2016, 291(17): 9042-9051.
- Orsi W D, Barker J?rgensen B, Biddle J F. Transcriptional analysis of sulfate reducing and chemolithoautotrophic sulfur oxidizing bacteria in the deep subseafloor[J]. Environmental Microbiology Reports, 2016, 8(4): 452-460.
- Kim J N, Henriksen E D C, Cann I K, et al. Nitrogen utilization and metabolism in Ruminococcus albus 8[J]. Applied and Environmental Microbiology, 2014, 80(10): 3095-3102.
- Marschner P. Plant-microbe interactions in the rhizosphere and nutrient cycling [C]//Marschner P, Rengel Z. Nutrient cycling in terrestrial ecosystems. Beilin: Springer, 2007: 159-182.
- 王海斌, 陳晓婷, 丁 力, 等. 土壤酸度对茶树根际土壤微生物群落多样性影响[J]. 热带作物学报, 2018, 39(3): 448-454.
- 吴林坤, 吴红淼, 朱 铨, 等. 不同改良措施对太子参根际土壤酚酸含量及特异菌群的影响[J]. 应用生态学报, 2016, 27(11): 3623-3630.
- 张珍明, 乐 乐, 林昌虎, 等. 不同种植年限山银花根区土壤生物特性[J]. 水土保持通报, 2015, 35(5): 71-76.
- 郝慧荣, 李振方, 熊 君, 等. 连作怀牛膝根际土壤微生物区系及酶活性的变化研究[J]. 中国生态农业学报, 2008, 16(2): 307-311.
- Girvan M S, Campbell C D, Killham K, et al. Bacterial diversity promotes community stability and functional resilience after perturbation[J]. Environmental Microbiology, 2005, 7(3): 301-313.
- Latz E, Eisenhauer N, Rall B C, et al. Plant diversity improves protection against soil-borne pathogens by fostering antagonistic bacterial communities[J]. Journal of Ecology, 2012, 100(3): 597-604.

