天然微藻水热炭理化特性及热解动力学研究
刘慧慧,曲 磊,陈应泉,张文楠,杨海平,王贤华,陈汉平
天然微藻水热炭理化特性及热解动力学研究
刘慧慧1,曲 磊1,陈应泉1,张文楠2,杨海平1,王贤华1※,陈汉平1
(1. 华中科技大学煤燃烧国家重点实验室,武汉 430074;2. Department of Chemical Engineering, Mid Sweden University, Sundsvall SE-85170, Sweden)
为探索天然微藻资源化的利用途径,该文以天然栅藻为原料,采用傅立叶转换红外线光谱分析, X射线衍射分析,X射线荧光光谱分析, 环境扫描电子显微镜与热重分析仪对水热炭进行测试分析。研究结果表明,随着水热温度的升高,水热炭产率从47.29%(180℃)降低至43.01%(240%);水热炭的O/C摩尔比从1.45减小至0.28,碳化程度加强,水热炭具有应用于固体燃料的潜力。鉴于水热炭含有大量灰分,其热值为8.43~9.67 MJ/kg,因此脱灰预处理是必要的过程。经过水热碳化处理,天然栅藻的比表面积从4.36 m2/g增加到35.26 m2/g。热解动力学结果表明随着水热温度的提高,水热炭的热稳定性增强。研究结果对天然微藻的资源化利用提供了一定的理论参考。
碳化;热解;动力学;天然栅藻;水热炭;理化特性
0 引 言
微藻作为第三代生物质燃料有很大的能源价值和环境效益[1-2]。相比于传统生物质,微藻生长在水环境中,不占用农业耕地面积[3];光合固碳效率高[4-5],单位时间单位面积生物质产量高[6];易获得低成本。因此微藻应用于热化学转化领域受到人们广泛关注。其中,水热工艺是微藻热化学转化利用的重要方式。水热工艺可以生产高能量密度以及高附加值化学品[7]。与其他热化学转化工艺相比,水热工艺不需要对原料进行干燥,降低了过程能耗,适用于含水率较高的生物质能源;对物质质量传递没有限制,不受物料含水率制约;反应过程简单,反应条件温和;水热产物易分离[8]。因此水热工艺被公认为是高含水率生物质能源化利用较为理想的方法。水热碳化过程中以大分子解聚为小分子以及小分子片段重新聚合为大分子为主要过程,包含了水解、脱水、脱羧、缩聚和芳香化等反应[9],可生产具有疏水性,易干燥和粉碎以及官能团丰富的焦炭[10-12]。
在微藻水热领域,国内外学者对水热工艺应用于微藻进行了大量的研究。Cheng等[13]评估了高蛋白质含量的红藻的物质产率,能量回收率以及化学组成。Heilmann等[14]对衣藻进行碳化研究,其水热炭的热值达31.58 MJ/kg,且碳元素的收率为60%。Xu等[15]在低温阶段(180~210 ℃)分析铜藻水热碳化特性,水热炭的最大热值达25.1 MJ/kg。Park等[16]通过对小球藻进行水热炭化处理,发现其水热炭的热值高达29.8 MJ/kg,能量回收率为90%,水热炭化处理有效的将微藻转化为高效节能的可再生燃料资源。Lee等[17]将脂质提取后的微藻()进行水热炭化处理,发现水热炭在高温区具有稳定的燃烧特性。Marin-Batista等[18]研究发现微藻经过水热炭化处理后,其水热炭的碳含量和热值均大于原料。在水热工艺中,温度是影响水热过程的重要因素[19]。在高温高压环境下,水的离子特性改变,大量H+和OH-解离促进了有机物的异构化,解聚和再聚合作用。同时,为了确保微藻转化率达到最大值,充足的反应时间也是必须的。
目前,微藻的水热工艺研究主要是利用实验室理想环境培养的微藻作为原料。然而,天然藻类水热特性研究还较少。由于生长环境复杂,天然藻类的组成与培养藻类有很大差异,本研究选用天然栅藻作为原料,其脂类和蛋白质含量很少,分别为1.4%和15.1%,灰分质量分数高达44.66%,而培养栅藻的脂类,蛋白质和碳水化合物的质量分数分别为20.2%、48.1%和9.86%,灰分质量分数仅为3.46%[20]。不同的组分组成导致其水热特性也不尽相同。本文研究了天然栅藻()的水热特性,分析了其水热炭物化特性以及热解动力学变化过程,利用傅立叶转换红外线光谱分析(Fourier transform infrared spectrometer),X射线衍射分析(X-ray diffraction),X射线荧光光谱分析(X-ray fluorescence),环境扫描电子显微镜(environmental scanning electron microscope,ESEM)和热重分析(thermogravimetric analysis)对水热炭进行测试分析,为天然栅藻水热碳化利用提供一定的理论基础,促进了天然栅藻的资源化利用过程。
1 栅藻水热碳化特性试验
1.1 试验原料
天然栅藻由瑞典农业科技大学提供,生长在瑞典的Umeå(63°87′N,20°80′E),将其自然晾干后运送至实验室。栅藻经粉碎,筛分至粒径小于0.125 mm。将原料放置于干燥箱内,55℃干燥至质量恒定。
1.2 试验方法
微藻水热装置为100 mL的自搅拌高温高压反应釜(北京世纪森朗实验仪器有限公司,SLM-100)。将干燥的天然栅藻和去离子水以1:15(g/mL)添加入反应釜中,其中原料的质量为3 g,经超声震荡10 min后,组装密封反应釜装置,用氩气排空后升压至2 MPa,将反应釜升温至反应温度(180、200、220、240、260 ℃),在反应温度下保温4 h。反应完成后,将反应釜迅速置于冰水混合物中进行冷却。待冷却至室温后,打开泄气阀将釜体内的气体排出,拆卸反应釜装置,用真空抽滤装置将反应产物固液分离,将固体产物105 ℃烘干12 h,每组工况至少重复2次。NM表示天然栅藻,反应产物用HC-进行标记,其中表示反应温度。水热炭产量、固存率、高位发热量(high heating value,HHV),能量回收率通过以下公式[21-22]进行计算

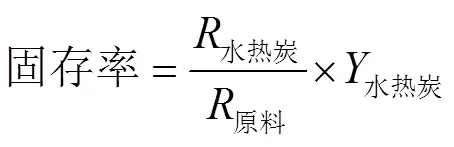
HHV=0.338 3C+1.443H+0.094 2S−0.1803O (3)

1.3 分析方法
采用SDTGA型工业分析仪(西班牙Las Navas公司)和EL-2元素分析仪(德国Vario公司)对样品进行工业分析和元素分析。通过X’Pert PRO型X射线衍射仪(荷兰帕纳科公司PANalytical生产)对样品晶相成分进行分析,扫描步长为0.0170°,阳靶极为Cu,操作条件为:40 mA,40 kV,2角度范围为10°~80°。物相结构分析则采用X'Pert High Score Plus 软件。利用EAGLE III X射线荧光探针(美国伊达克斯有限公司EDAX Inc.生产)对样品灰分金属盐含量进行分析,其微聚焦X光管最大功率40 kV,1.0 mA。采用VERTEX 70傅立叶变换显微红外(德国Bruker 公司)分析样品官能团演变过程,其光谱范围为50~12,500 cm-1。利用ASAP2020型比表面积及孔径分析仪(美国Micromeritics公司生产),通过Brunauer-Emmett-Teller(BET)方程进行线性回归计算比表面积,Barrett-Joyner-Halenda模型计算总孔容。利用Quanta 200环境扫描电子显微镜(ESEM,荷兰FEI公司)。
1.4 动力学方程
为了研究水热炭的失重特性,对失重剧烈的阶段构建热解的表观动力学模型以及求解主要的反应动力学参数。对栅藻进行反应动力学分析。将样品从室温以10 ℃/min升温速率,在氮气氛围条件下从室温升至800 ℃。根据文献[23]中动力学分析方法,假设微藻热重分析为一级反应模型[24],则反应转化率的变化率可表述为
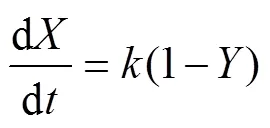
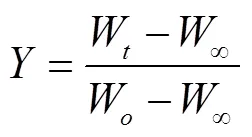
式中为反应速率常数;为反应转化率;M为反应时刻样品质量,mg;M为反应结束后最终固体质量,mg;M为初始样品质量,mg。
根据Arrhenius方程
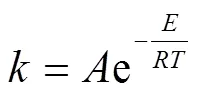
可确定热解过程的表观反应为
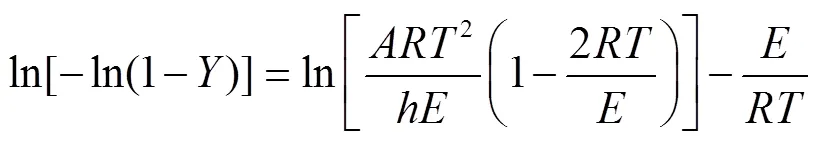
式中为指前因子,为表观活化能,kJ/mol;为气体常数,8.314 J/(mol·K)。由于升温速率(=d/d)确定,ln[−ln(1−)]与1/呈线性关系,可计算出表观活化能。
2 结果与分析
2.1 天然栅藻及其水热炭基本特性分析
天然栅藻及不同水热温度条件下的水热炭物化特性如表1所示。随着水热温度的增加,水热炭的产率逐渐从47.29%(180 ℃)降低至43.01%(240 ℃),这是因为高温为微藻发生水解、脱水和脱羧基反应提供更多的能量,高聚物降解作用增强;当温度升高至260 ℃时,水热炭产率有增加趋势,这可能是由于水热的中间产物发生缩聚反应,导致水热炭产率增加。
当温度由180 ℃升高至240 ℃,挥发分质量分数由55.34%降至28.53%,而260 ℃时,挥发分质量分数增加为31.10%;与此同时,灰分质量分数由44.66%增至71.47%。当温度为260 ℃时,灰分质量分数为68.90%。这说明天然微藻中的灰分多为难溶于水的组分,利用X射线荧光光谱分析(X-ray fluorescence)对灰分化学元素组分含量进行分析发现(图1),经过水热处理后Na、K、Cl元素含量急剧降低,而其他元素含量仅有微量变化。天然栅藻及其水热炭的XRD图谱如图2所示。从图2中可以看出,天然栅藻中含有方解石(Mg0.064Ca0.936CO3)、SiO2、NaCl、Al2O3、CaSO4、Mg3S2O8(OH)2。经过水热处理后,NaCl溶解于水溶液中,其衍射峰消失;随着水热温度的增加,各种不溶盐的衍射峰增强,这是由于水热碳化过程中,由于有机组分分解,方解石(Mg0.064Ca0.936CO3)等无机矿物组分被富积下来,衍射峰增强,这与XRF对于灰分组分的分析结果一致。因此,与其他原料不同[25-27],根据GB/T 28731-2012固体生物质燃料工业分析方法测量,天然栅藻和水热炭均不含固定碳。通常释放的挥发分会在固体表面沉降聚合形成固定碳[28],但由于天然微藻中含有大量的灰分,且多为难溶性组分,导致固定碳在工业分析中未能检测到。
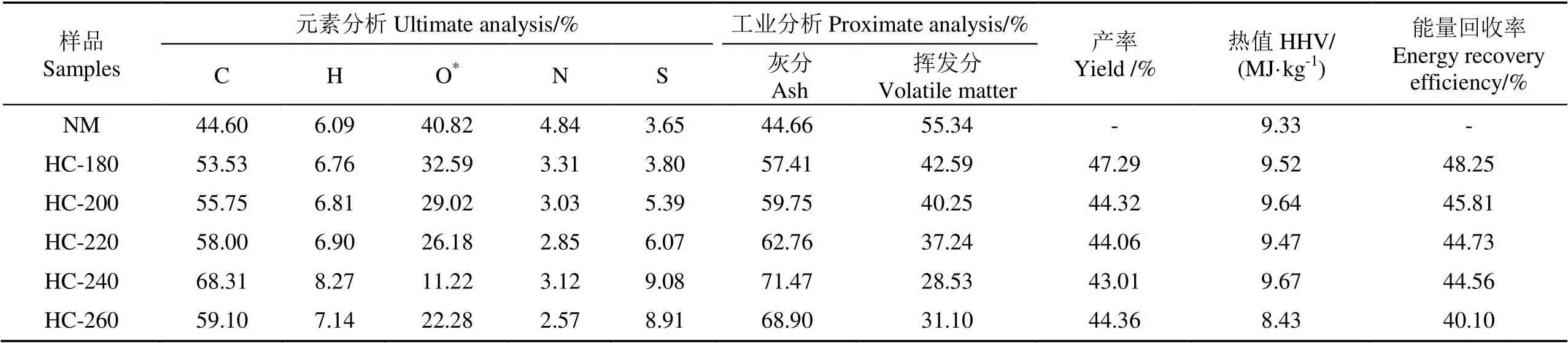
表1 不同温度下天然栅藻及其水热炭元素分析、工业分析、产率、热值和能量回收率
注:*:差减法;元素分析:干燥无灰基;工业分析:干燥基。
Note: *: Calculated by minusing. Ultimate analysis: dry ash-free basis; Proximate analysis: dry basis.
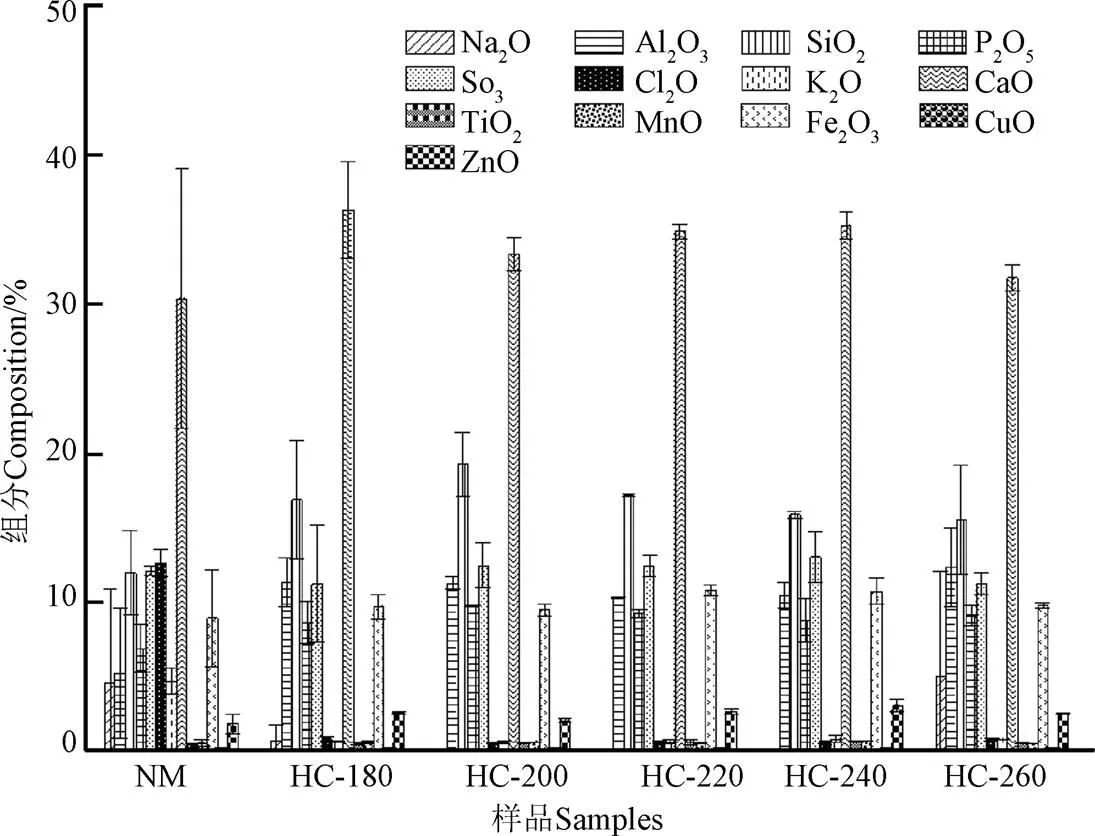
图1 天然栅藻及水热炭XRF含量分布图
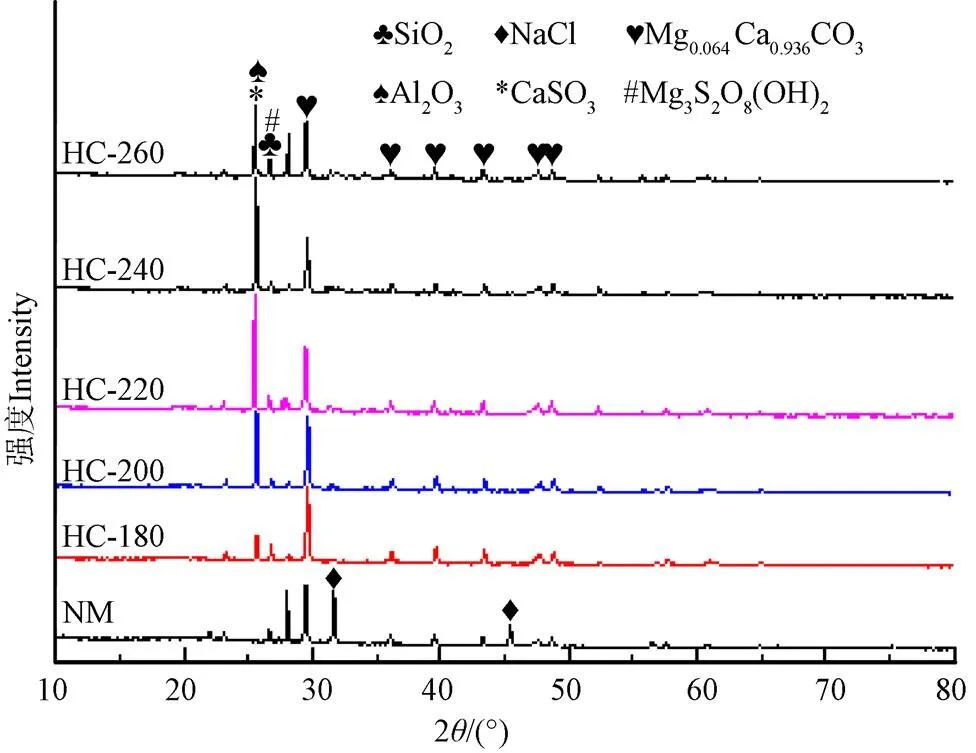
图2 天然栅藻及水热炭XRD图谱
元素分析结果显示,与天然栅藻相比,在180~240 ℃范围内,水热炭的C和H元素随着温度升高而增加,而O元素质量分数减少,在脱水和脱羧基作用下以H2O和CO2形式脱除。当温度从180 ℃升高至240 ℃时,C元素和H元素质量分数分别由44.6%和6.09%,增加至68.31%和8.27%,O元素质量分数由40.82%降至11.22%;而当温度升至260 ℃时,C、H、O元素质量分数有相反的变化趋势。天然栅藻及其水热炭的C、H、O元素固存率如图3所示。在水热过程中,C元素的固存率最大,H元素次之,O元素最小。随着温度的增加,水热炭中的C和H元素固存率分别由43.69%和40.41%,降至33.04%和29.20%;O元素固存率从29.06%(180 ℃)降至6.09%(24 ℃),而在260 ℃时增加至13.61%。这表明当240 ℃时,天然栅藻中69.88%的H元素和93.88%的O元素被脱除,C的固存率为33.97%。
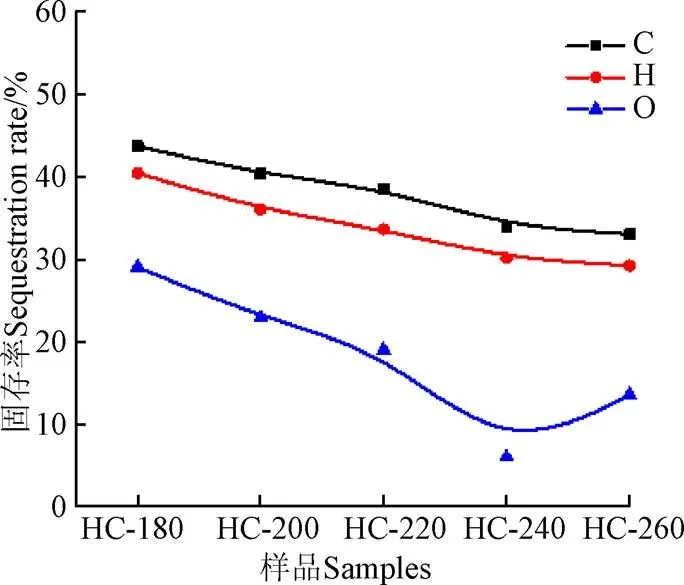
图3 天然栅藻水热炭中C、H、O固存率
天然栅藻的热值为9.33 MJ/kg,随着水热温度增加,水热炭的热值减小。Van Krevelen图用来描述反应过程中的脱水、脱羧基和脱甲基过程[29],如图4所示。H/C摩尔比从1.64减小至0.69,O/C摩尔比从1.45减小至0.28。从图4中可看出,脱水和脱羧基是天然栅藻水热过程中的主要路径,脱甲基路径可忽略;O/C一般用于反映碳化程度[8],O/C的摩尔比从1.45减小至0.28。说明随着水热温度的升高,水热碳化程度加强,表明水热炭有应用于固体燃料的潜力,鉴于水热炭含有大量灰分,脱灰预处理是必要的过程。

注:NM为天然栅藻。下同。
2.2 天然栅藻及其水热炭化学结构特性分析
天然栅藻及其不同水热温度条件下的水热炭的FTIR结果如图5所示。
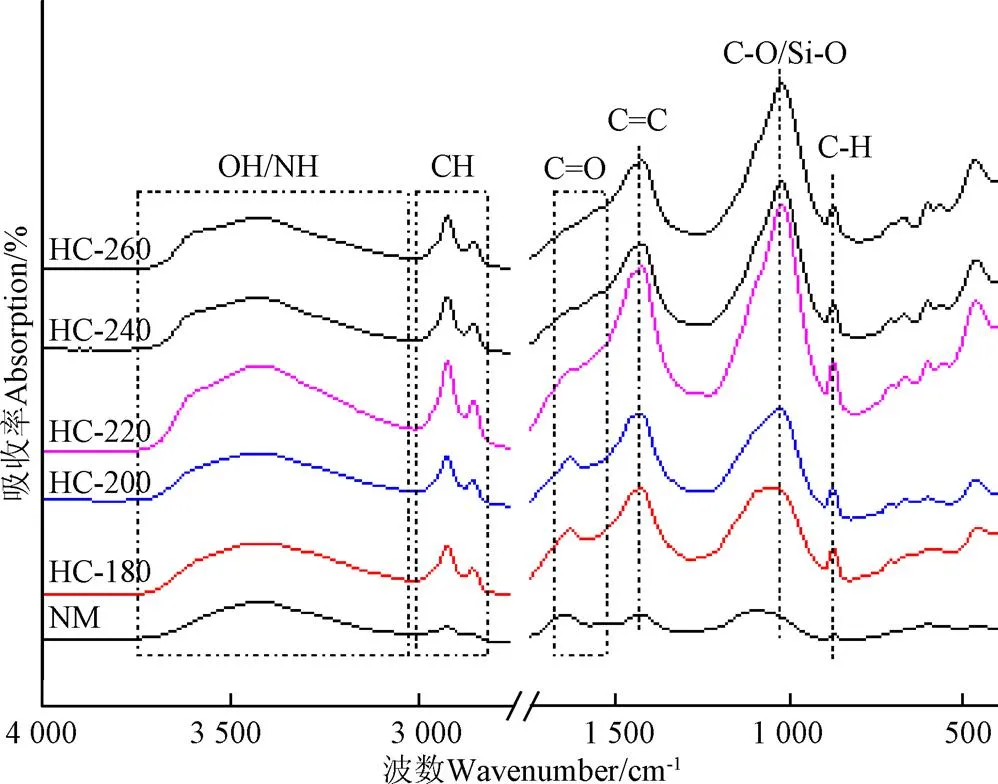
图5 天然栅藻及水热炭FTIR吸收峰图谱
根据文献[30-36],对样品进行官能团种类及演变过程进行分析。在3 000~3 800 cm-1处的强宽峰是羟基、羧基或氨基中的OH和NH伸缩振动的吸收峰,2 800~3 000 cm-1处的吸收峰为脂肪族CH2、CH3的不对称伸缩振动峰,1 646 cm-1处的吸收峰主要是酮基和酰胺基中的C=O伸缩振动峰,1 535 cm-1处的吸收峰羧基中C=O的不对称伸缩振动,1 424 cm-1处的吸收峰是芳香环中C=C的伸缩振动峰,1 094 cm-1处的吸收峰为醚键中C-O-R或醇类中C-O键的伸缩振动,同时,该出峰也常表示Si-O的吸收峰,874 cm-1处的吸收峰是芳香环侧链中C-H的伸缩振动。与天然栅藻相比,水热炭主要吸收峰位置变化不大,但强度变化较明显,说明水热炭化处理后,官能团种类没有变化,但含量发生了变化。相比于天然栅藻,水热炭在3 000~3 800 cm-1处的OH、NH吸收峰,2 800~3 000 cm-1处的CH吸收峰,1 424 cm-1处的C=C吸收峰,1 094 cm-1处的C-O吸收峰,874 cm-1处的C-H吸收峰均有明显增加,说明相比于天然栅藻,水热炭的官能团更丰富,芳构化程度更强,水热炭的炭化程度增加;酮基和酰胺基中的C=O(1 646 cm-1)和羧基中C=O(1 535 cm-1)吸收峰有降低趋势,说明水热过程中有明显的脱羧基和脱羰基作用,O元素质量分数降低,这与元素分析结果一致。
表2和图6分别为不同温度条件下制备水热炭的结构特征和吸附-脱附等温线图。从表2中可看出,相比于天然栅藻,水热炭比表面积有明显的增大,水热炭的比表面积范围为28.7~35.26 m2/g,说明水热工艺有利于改善其孔结构特性。对比不同水热温度条件下制备的水热炭发现,不同水热温度对孔结构的影响较小。图6中,水热炭的等温吸附-脱附曲线为V型等温线中的H3型回滞环。当相对压力(0)大于0.2时出现回滞环,这是由于水热炭中的毛细凝聚。回滞环的形状反映了孔的存在结构。原料栅藻的吸脱附能力很小,水热炭的吸脱附能力明显增强。
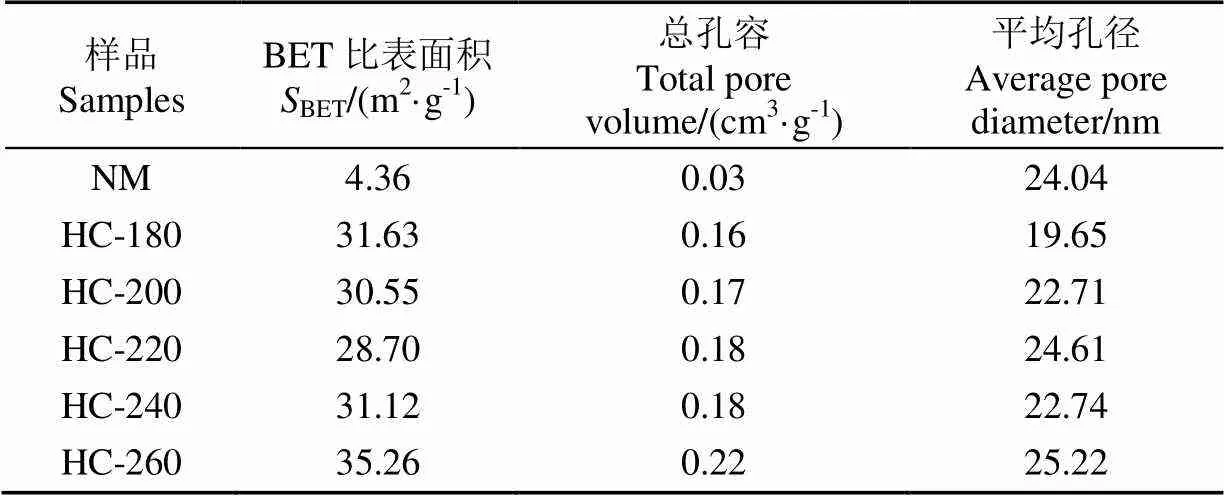
表2 天然栅藻及水热炭BET比表面积、总孔容及平均孔径
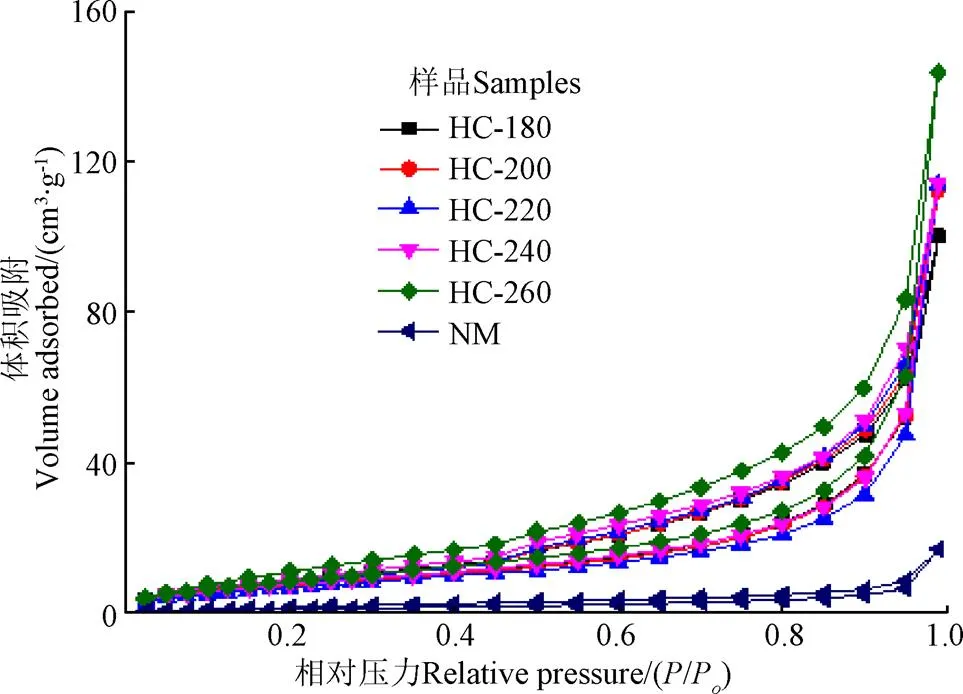
图6 天然栅藻及水热炭氮气吸附-脱附等温曲线
图7为天然栅藻及其水热炭的SEM图。天然栅藻呈密实的块状无孔道结构(图7a),从图7b和图7c中可看出,经过水热碳化处理后,样品表面形貌有较大变化,水热炭的破碎度和孔隙度增大,这是由于水热过程中挥发分析出以及天然微藻基质的化学分解,这与前文相应的孔隙结构分析结果一致。

图7 天然微藻及其水热炭电镜图片
2.3 栅藻及水热炭热解动力学特性
图8为天然栅藻及其在不同温度条件下水热炭的热重及失重速率曲线。
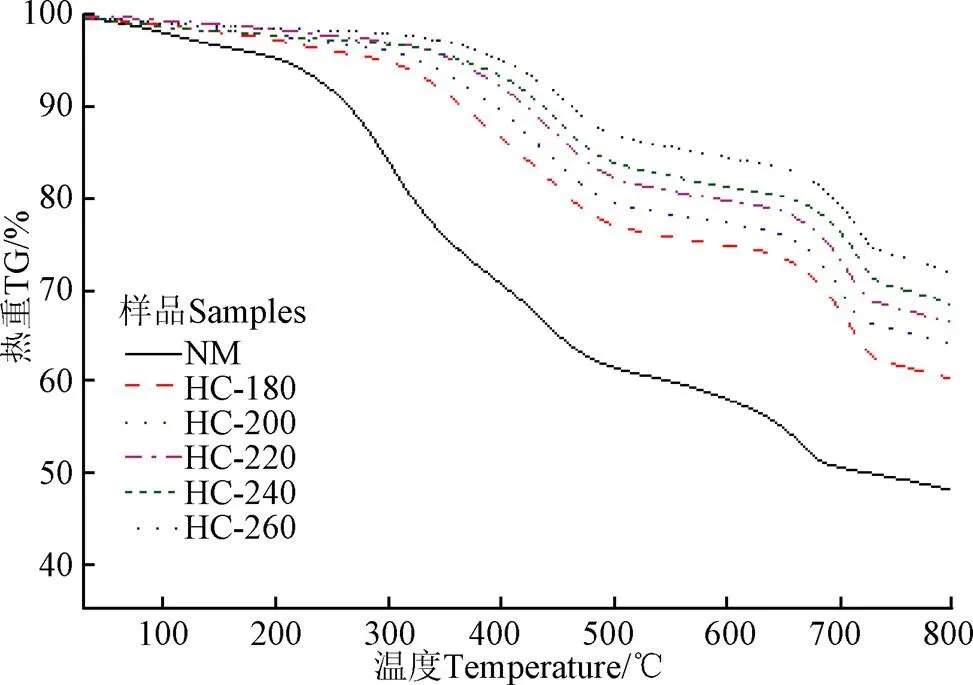
a. TG
b. DTG
图8 天然栅藻和不同水热温度下水热炭的TG和DTG曲线
Fig.8 TG-DTG curves of raw and hydrochars at different temperatures
从图8的热重曲线中可看出,与天然栅藻相比,水热炭表现出不同的热解特性;不同的水热温度下水热炭的失质量过程差异也较大,失质量率范围为33%~43%,原料的失质量率为57%,这是由于水热处理后,天然栅藻中有机组分发生水解、脱水、脱羧、芳香化、缩聚等反应。从失质量速率曲线中可以看出,可以将整个热解过程分为4个失质量区间。这些失质量区间对应不同的有机物的分解,第1个失质量阶段(30~200 ℃)是样品的脱水阶段;第2个失质量阶段(200~400 ℃)是原料和水热炭中的无定形结构的快速热解阶段。最大失质量速率温度为300 ℃。随着水热温度的升高,天然栅藻碳化程度增强,挥发分含量减少,导致该阶段的失质量峰逐渐减小,当水热温度大于220 ℃时,水热炭在300 ℃处的失质量峰消失,最大失质量速率峰向高温区移动。第3个失质量阶段(400~550 ℃)是由于焦炭的失质量引起的;第4个失质量阶段(550~800 ℃)是由于残余焦炭以及灰分中盐类的失质量引起的。对比天然栅藻,水热炭的失质量峰明显增强。由以上分析可知,高灰分含量的栅藻经处理后,最大失质量速率峰值向高温区移动,栅藻中的有机易分解组分向难分解残炭转化,一定程度上提高了固体产物的热稳定性。
DTG峰高表现的热解速率与化学反应活性成正比,反应温度与反应活性成反比[37]。对不同水热温度条件下水热炭进行热解动力学分析,为详细研究水热炭的热解动力过程,将失重阶段分为4个阶段,即30~200、200~400、400~550和550~800 ℃,各阶段表观活化能分别用1、2、3、4表示,结果如表3所示。对比分析天然栅藻及其水热炭的表观活化能发现,水热炭1小于天然栅藻1,说明水热炭的疏水性优于原料[38],易于运输和储存。随着水热温度的升高,2减小,3值增,说明水热炭热稳定性增强。

表3 不同热重阶段天然栅藻及水热炭的表观活化能
注:1~4表示不同阶段的活化能。
Note:1to4indicate the activation energy at different stages.
3 结 论
1)天然栅藻灰分质量分数为44.66%,脂类和蛋白质质量分数为1.4%和15.1%。天然微藻灰分组分多为难溶于水的组分,天然微藻水热碳化后,水热炭灰分含量增加。240 ℃时,天然微藻中69.88%的H元素和93.88%的O元素被脱除,C的固存率为33.97%。O/C摩尔比从1.45减小至0.28,水热碳化程度加强。水热炭有应用于固然燃料的潜力,鉴于水热炭含有大量的灰分,脱灰预处理是必要的过程。
2)水热碳化处理有效提高了水热炭的孔隙结构,水热炭的吸脱附能力明显增强,相比于天然栅藻(4.36 m2/g),水热炭的比表面积范围为28.7~35.26 m2/g。天然栅藻呈密实的块状无孔道结构,而水热炭的破碎度和孔隙率增大。
3)水热炭热重分析发现,随着水热温度升高,300 ℃处的失质量峰逐渐消失,栅藻中的易分解组分向难分解残炭转化,提高了固体产物的热解稳定性。热解动力学结果显示,水热炭疏水性优于天然栅藻。
[1] Teymouri A, Stuart B J, Kumar S. Effect of reaction time on phosphate mineralization from microalgae hydrolysate[J]. Acs Sustainable Chemistry & Engineering, 2018, 6(1): 618-625.
[2] Bhattacharya S, Maurya R, Mishra S K, et al. Solar driven mass cultivation and the extraction of lipids from chlorella variabilis: A case study[J]. Algal Research, 2016(14): 137-142.
[3] 徐玉福,俞辉强,朱利华,等. 小球藻粉水热催化液化制备生物油[J]. 农业工程学报,2012,28(19):194-199.
Xu Yufu, Yu Huiqiang, Zhu Lihua, et al. Preparation of bio-fuel from Chlorella pyrenoidosa by hydrothermal catalytic liquefaction[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2012. 28(19): 194-199. (in Chinese with English abstract)
[4] 曲磊,崔翔,杨海平,等. 微藻水热液化制取生物油的研究进展[J]. 化工进展,2018,37(8):2962-2969.
Qu Lei, Cui Xiang, Yang Haiping, et al. Review on the preparation of bio-oil by microalgae hydrothermal liquefaction[J]. Chemical Industry and Engineering Progress, 2018, 37(8): 2962-2969. (in Chinese with English abstract)
[5] 徐春明,焦志亮,王晓丹,等. 微藻作原料生产生物柴油的研究现状和前景[J]. 现代化工,2015,35(8):1-5.
Xu Chunming, Jiao Zhiliang, Wang Xiaodan, et al. Biodiesel production from microalgae: Current status and potential[J]. Modern Chemical Industry, 2015, 35(8): 1-5. (in Chinese with English abstract)
[6] 张冀翔,蒋宝辉,王东,等. 微藻水热液化生物油化学性质与表征方法综述[J]. 化工学报,2016(5):1644-1653.
Zhang Yixiang, Jiang Baohui, Wang Dong, et al. Chemical properties and characterization methods for hydrothermalliquefaction bio-crude from microalgae: A review[J]. CIESC Journal, 2016(5): 1644-1653. (in Chinese with English abstract)
[7] Peterson A A, Vogel F, Lachance R P, et al. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies[J]. Energy & Environmental Science, 2008, 1(1): 32-65.
[8] 王定美,王跃强,袁浩然,等. 水热炭化制备污泥生物炭的碳固定[J]. 化工学报,2013(7):2625-2632.
Wang Dingmei, Wang Yueqiang, Yuan Haoran, et al. Carbon fixation of sludge biochar by hydrothermal carbonization[J]. CIESC Journal, 2013(7): 2625-2632. (in Chinese with English abstract)
[9] Funke A, Ziegler F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering[J]. Biofuels Bioproducts & Biorefining-Biofpr, 2010, 4(2): 160-177.
[10] 李音,单胜道,杨瑞芹,等. 低温水热法制备竹生物炭及其对有机物的吸附性能[J]. 农业工程学报,2016,32(24):240-247.
Li Yin, Shan Shengdao, Yang Ruiqin, et al. Preparation of bamboo biochars by low-temperature hydrothermal method and its adsorption of organics[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2016, 32(24): 240-247. (in Chinese with English abstract)
[11] Cao X, Ro K S, Chappell M, et al. Chemical structures of swine-manure chars produced under different carbonization conditions investigated by advanced solid-state C-13 nuclear magnetic resonance (NMR) spectroscopy[J]. Energy & Fuels, 2011, 25(1): 388-397.
[12] Falco C, BaccileN, Titirici M M. Morphological and structural differences between glucose, cellulose and lignocellulosic biomass derived hydrothermal carbons[J]. Green Chemistry, 2011, 13(11): 3273-3281.
[13] Cheng F, Cui Z, Chen L, et al. Hydrothermal liquefaction of high- and low-lipid algae: Bio-crude oil chemistry[J]. Applied Energy, 2017, 206: 278-292.
[14] Heilmann S M, Davis H T, Jader L R, et al. Hydrothermal carbonization of microalgae [J]. Biomass & Bioenergy, 2010, 34(6): 875-882.
[15] Xu Q, Qian Q, Quek A, et al. Hydrothermal carbonization of macroalgae and the effects of experimental parameters on the properties of hydrochars[J]. ACS Sustainable Chemistry & Engineering, 2013, 1(9): 1092-1101.
[16] Park K Y, Lee K, Kim D. Characterized hydrochar of algal biomass for producing solid fuel through hydrothermal carbonization[J]. Bioresource Technology, 2018, 258: 119-124.
[17] Lee J, Lee K, Sohn D, et al. Hydrothermal carbonization of lipid extracted algae for hydrochar production and feasibility of using hydrochar as a solid fuel[J]. Energy, 2018, 153: 913-920.
[18] Marin-Batista J D, Villamil J A, Rodriguez J J, et al. Valorization of microalgal biomass by hydrothermal carbonization and anaerobic digestion[J]. Bioresource Technology, 2019, 274: 395-402.
[19] 张进红,林启美,赵小蓉,等. 水热炭化温度和时间对鸡粪生物质炭性质的影响[J]. 农业工程学报,2015,31(24):239-244.
Zhang Jinhong, Lin Qimei, Zhao Xiaorong, et al. Effect of hydrothermal carbonization temperature and time on characteristics of bio-chars from chicken manure[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2015, 31(24): 239-244. (in Chinese with English abstract)
[20] Kohansal K, Tavasoli A, Bozorg A. Using a hybrid-like supported catalyst to improve green fuel production through hydrothermal liquefaction ofmicroalgae[J]. Bioresource Technology, 2019, 277: 136-147.
[21] Channiwala S A, Parikh P P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels[J]. Fuel, 2002, 81(8): 1051-1063.
[22] 王庆峰. 中高硫煤浮选脱硫脱灰试验研究[D]. 青岛:青岛理工大学,2013.
Wang Qingfeng. Research on Desulphurization and Deashing for Medium-High Sulphur Coal with Flotation[D]. Qingdao: Qingdao Technological University, 2013. (in Chinese with English abstract)
[23] Jaber J O, Probert S D. Non-isothermal thermogravimetry and decomposition kinetics of two Jordanian oil shales under different processing conditions[J]. Fuel Processing Technology, 2000, 63(1): 57-70.
[24] White J E, Catallo W J, Legendre B L. Biomass pyrolysis kinetics: A comparative critical review with relevant agricultural residue case studies[J]. Journal of Analytical and Applied Pyrolysis, 2011, 91(1): 1-33.
[25] 张进红,林启美,赵小蓉,等. 不同炭化温度和时间下牛粪生物炭理化特性分析与评价[J]. 农业机械学报,2018,49(11):298-305.
Zhang Jinhong, Lin Qimei, Zhao Xiaorong, et al. Physico-chemical characteristics and evaluation of cow manure hydrocharat different carbonization temperatures and durations[J]. Journal of Agricultural Machinery, 2018, 49(11): 298-305. (in Chinese with English abstract)
[26] 马腾,郝彦辉,姚宗路,等. 秸秆水热生物炭燃烧特性评价[J]. 农业机械学报,2018,49(12):340-346.
Ma Teng, Hao Yanhui, Yao Zonglu, et al. Evaluation on combustion characteristics of straw hydrothermal bio-char[J]. Journal of Agricultural Machinery, 2018, 49(12): 340-346. (in Chinese with English abstract)
[27] 张曾,单胜道,吴胜春,等. 炭化条件对猪粪水热炭主要营养成分的影响[J]. 浙江农林大学学报,2018,35(3):398-404.
Zhang Zeng, Shan Shengdao, Wu Shengchun, et al. Carbonization with main nutrients in pig manure hydrochar[J]. Journal of Zhejiang A&F University, 2018, 35(3): 398-404. (in Chinese with English abstract)
[28] He C, Zhao J, Yang Y, et al. Multiscale characteristics dynamics of hydrochar from hydrothermal conversion of sewage sludge under sub- and near-critical water[J]. Bioresource Technology, 2016, 211: 486-493.
[29] Van Krevelen D W. Graphical-statistical method for the study of structure and reaction processes of coal[J]. Fuel, 1950, 29: 228-269.
[30] Zhang B, Feng H, He Z, et al. Bio-oil production from hydrothermal liquefaction of ultrasonic pre-treated[J]. Energy Conversion and Management, 2018, 159: 204-212.
[31] Sabio E, Alvarez-Murillo A, Roman S, et al. Conversion of tomato-peel waste into solid fuel by hydrothermal carbonization: Influence of the processing variables[J]. Waste Management, 2016, 47: 122-132.
[32] Yuan J H, Xu R K, Zhang H. The forms of alkalis in the biochar produced from crop residues at different temperatures[J]. Bioresource Technology, 2011, 102(3): 3488-3497.
[33] Vyazovkin S, Burnham A K, Criado J M, et al. Kinetics committee recommendations for performing kinetic computations on thermal analysis data[J]. Thermochimica Acta, 2011, 520(1/2): 1-19.
[34] Maliutina K, Tahmasebi A, Yu J. Pressurized entrained-flow pyrolysis of microalgae: Enhanced production of hydrogen and nitrogen-containing compounds[J]. Bioresour Technol, 2018, 256: 160-169.
[35] Zhao H, Yan H, Dong S, et al. Thermogravimetry study of the pyrolytic characteristics and kinetics of macro-algae Macrocystis pyrifera residue[J]. Journal of Thermal Analysis and Calorimetry, 2013, 111(3): 1685-1690.
[36] Liu H M, Li M F, Yang S, et al. Understanding the mechanism of cypress liquefaction in hot-compressed water through characterization of solid residues[J]. Energies, 2013, 6(3): 1590-1603.
[37] 武宏香,李海滨,赵增立. 煤与生物质热重分析及动力学研究[J]. 燃料化学学报,2009(5):538-545.
Wu Hongxiang, Li Haibin, Zhao Zengli. Thermogravimetricanalysisandpyrolytickineticstudyoncoal/biomassblends[J]. Journal of Fuel Chemistry and Technology, 2009(5): 538-545. (in Chinese with English abstract)
[38] 范方宇,邢献军,施苏薇,等.水热生物炭燃烧特性与动力学分析[J]. 农业工程学报,2016,32(15):219-224.
Fan Fangyu, Xing Xianjun, Shi Suwei, et al. Combustion characteristic and kinetics analysis of hydrochars[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2016, 32(15): 219-224. (in Chinese with English abstract)
Physicochemical characteristics and pyrolysis kinetics of hydrothermal carbon from natural
Liu Huihui1, Qu Lei1, Chen Yingquan1, Zhang Wennan2,Yang Haiping1, Wang Xianhua1※, Chen Hanping1
(1.,,430074,; 2.,,SE-85170,)
In order to explore the utilization of natural microalgae, the naturalwas selected to carry out hydrothermal carbonization experiments, and the characterization of its hydrochars was determined using Fourier transform infrared spectroscopy,-ray diffraction analysis,-ray fluorescence spectroscopy, environmental scanning electron microscopy and thermogravimetric analyzer. The results showed that the ash content of naturalwas 44.66%, and the lipid and protein content of naturalwere 1.4% and 15.1%, respectively. The natural microalgae ash components were mostly water-insoluble components. The main components included (Mg0.064Ca0.936CO3), SiO2, NaCl, Al2O3, CaSO4, Mg3S2O8(OH)2. After hydrothermal carbonization treatment, NaCl was dissolved in water, and the water-insoluble components were enriched in hydrochars. Compared with the natural, the ash content of hydrochars increased, in the range from 57.41% to 71.47%. It was worth noting that the naturaland its derived hydrochars had no fixed carbon. With the increase of hydrothermal temperature, the hydrothermal carbon yield decreased from 47.29% (180℃) to 43.01% (240℃). This phenomenon was on account of the organic components in the naturalunderwent hydrolysis, dehydration, decarboxylation, aromatization, condensation and polymerization. The carbon remaining ratio was the largest, the oxygen was the smallest, and the remaining ratios of carbon, hydrogen and oxygen decreased as the hydrothermal temperature increased. For HC-240, the removal rates of H and O were 69.88% and 93.88%, respectively, and the C remaining ration rate was 33.97%. The O/C molar ratio of hydrochars decreased from 1.45 to 0.28. Dehydration and decarboxylation were the main pathways in hydrothermal carbonization of the natural, and the demethylation pathway was negligible. Oxygen was removed in the form of H2O and CO2. The degree of carbonization was enhanced and hydrochars had the potential to be applied to solid fuels. Since hydrochars contained a large amount of ash, its calorific value was in the range of 8.43-9.67 MJ/kg. Hence, the pretreatment of deashing was a necessary process. The hydrothermal carbonization treatment effectively improved the pore structure of hydrochars, and the absorption-desorption capacity of hydrochars was obviously enhanced. Compared with natural(4.36 m2/g), the specific surface area of hydrochars was in the range of 28.7-35.26 m2/g. The naturalhad a dense block-like without pores or pathways. However, the morphologies of hydrochars changed significantly. The fragmentation and porosity of hydrochars increased, which attributed to the release of volatile matter during hydrothermal carbonization process and chemical bond decomposition of feedstock. The thermogravimetric analysis experiments were carried out to reveal the pyrolysis characteristics of hydrochars. It was found that the weight loss peak at 300 ℃ gradually disappeared with the increased of hydrothermal temperature. This was owing to the degree of naturalincreased and the volatile matter content decreased. When the hydrothermal temperature was higher than 220 ℃, the maximum weight loss rate peak moved to the high temperature zone. The pyrolysis kinetics results showed that the thermal stability of hydrochars increased with the increase of hydrothermal temperature. The hydrochars were more hydrophobic than that of the natural. The research results provide a theoretical reference for the resource utilization of natural microalgae.
carbonization; pyrolysis; kinetics; natural; hydrochar; physicochemical characteristics
2019-01-05
2019-06-27
国家自然科学基金:生物质热化学转化基础(51622604)
刘慧慧,博士生,主要从事生物质水热综合利用研究工作。Email:liuhh@hust.edu.cn
王贤华,副教授,博士生导师,主要从事生物质热化学利用研究。Email:wangxianhua@hust.edu.cn.
10.11975/j.issn.1002-6819.2019.14.030
TK16
A
1002-6819(2019)-14-0235-08
刘慧慧,曲 磊,陈应泉,张文楠,杨海平,王贤华,陈汉平. 天然微藻水热炭理化特性及热解动力学研究[J]. 农业工程学报,2019,35(14):235-242. doi:10.11975/j.issn.1002-6819.2019.14.030 http://www.tcsae.org
Liu Huihui, Qu Lei, Chen Yingquan, Zhang Wennan, Yang Haiping, Wang Xianhua, Chen Hanping. Physicochemical characteristics and pyrolysis kinetics of hydrothermal carbon from natural[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2019, 35(14): 235-242. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2019.14.030 http://www.tcsae.org

