Molecular cloning, expression profiling and RNA interference of a vitellogenin gene from Harmonia axyridis (Coleoptera:Coccinellidae)
LlANG Chao, LlU Ting-hui, HAN Shi-peng, HE Yun-zhuan
College of Plant Protection, Hebei Agricultural University, Baoding 071001, P.R.China
Abstract In this study, the Harmonia axyridis vitellogenin gene 2 (HmVg2) sequence was identified from transcriptomic databases of female adult H. axyridis and cloned into pMD18-T vector. The HmVg2 gene was 5 460 bp in length, and formed with an open reading frame (ORF) of 5 361 nucleotides (GenBank accession no. KY794939). The putative molecular weight of the primary HmVg2 protein was 203.459 kDa and the predicted isoelectric point (pI) was 8.59. HmVg2 contained a signal peptide, vitellogenin N-terminal (vitellogenin-N) domain, domain of unknown function 1943 (DUF1934) domain and von Willebrand factor type D (VWD) domain. The developmental expression profiling showed that HmVg2 was extremely highly expressed in female insects, but was expressed at lower levels in male insects. In female insects, HmVg2 was mainly expressed in the wing and fat body. The double-stranded RNA-HmVg2/-GFP was injected into H. axyridis, and qRT-PCR results showed that the HmVg2 gene was specifically silenced. The eggs laid during the first five days and the hatching rate of eggs was lower than controls after dsHmVg2 injection. This investigation demonstrated that the HmVg2 gene plays an important role in H. axyridis reproduction and enriches the function of the insect vitellogenin gene.
Keywords: vitellogenin, Harmonia axyridis, expression profiling, RNAi
1. lntroduction
Vitellogenin (Vg) is a precursor of vitellin (Vn) in almost all oviparous animals, and it is prevalent in the blood of nonmammalian mature ovulating females (Utarabhand and
Bunlipatanon 1996). As an important reproductive protein,Vg plays a critical role in the reproduction and development of oviparous animals. Vg is a large molecular weight glycolipid complex protein, which is the key component of yolk (Dong et al. 2008). The Vg protein usually contains several relatively well-conserved domains or amino acid motifs, such as polyserine tracts, GL/ICG motif, cysteine residues, DGXR motif, DXXR motif, and others. The typical characteristic of insect Vg is polyserine tracts, which usually lie at the N-terminus, with a small amount at the C-terminus(Barr 1991; Rouille et al. 1995; Tufail et al. 2007; Tufail and Takeda 2008). However, some insects’ Vgs contain only one short polyserine tract, such as those of Apis mellifera(Piulachs et al. 2003), Bombus hypocrita (Li et al. 2010), and some insects’ Vgs have no polyserine tracts, such as the Vg of Pimpla nipponica (Nose et al. 1997), Encarsia formosa(Donnell et al. 2004) and Solenopsis invicta (Tufail and Takeda 2008). Insect Vgs normally originate from a 6-7 kb mRNA sequence. To date, cDNAs encoding Vg genes have been cloned and characterized from many insect orders,including Lepidoptera, Diptera, Hymenoptera, Hemiptera and Coleoptera. Until now, insect Vg has not been clearly classified, but it is known that vitellogenin-N domain,DUF1934 domain, and von Willebrand factor type D (VWD)are specific domains for Vg (Marchler-Bauer et al. 2011).
Vg protein plays an important role in insect reproduction.RNA interference is an effective way of studying gene function (Fire 1999). In 2015, a study of the Vg gene in Chrysopa septempunctata revealed that the expression level of the CsVg gene was inhibited by RNA interference. CsVg gene plays a key role in the insect reproduction process,affecting the number and quality of eggs (Liu et al. 2015).In 2014, injection of CceVg double-stranded RNA (dsRNA)into early-emergent females of Corcyra cephalonica reduced CceVg mRNA expression and the CceVg protein was insufficient to ensure normal development of all oocytes,leading to severely abnormal ovaries. The expressionpattern experiment showed that CceVg was first transcribed at a very low level in the early larval stage but disappeared in later larva stage, and it was detected subsequently in the early pupal stage and throughout the adult stage of female insects (Veerana et al. 2014). The expression patterns of the Vg gene from various insect species at different developmental stages show different expression levels.For example, the Vg gene from Spodoptera litura was first detected in the late pupal stage, whereas in Bombyx mori and Antheraea pernyi, Vg genes were first detected at the late larval stage (Yano et al. 1994; Shu et al. 2009). The expression pattern of the Vg gene in adult Apis cerana cerana worker bees exhibits intermittent transcription,where the Vg gene can be detected in the head, thorax and abdomen but its expression abundance is significantly different among these segments (Li 2016). In Harmonia axyridis, Du and Zeng (2016) used a time-series analysis and found three genes related to Vg functions, one Vg receptor (308 bp) and two Vgs (5 471 and 5 505 bp), which play an important role in yolk formation but do not involve the function of genes. Zhang et al. (2017) also found a Vg of H. axyridis (5 403 bp) and the purified soluble Vg fragment(VWD) was included as part of an artificial diet. The result shows that the egg production of H. axyridis was significantly increased. RNA interference technology is a good tool for understanding the function of Vg gene, and was used here to learn more about the effect of Vg gene in H. axyridis.Research on gene expression patterns will contribute to the subsequent study of gene function.
H. axyridis (Coleoptera) is an important biological control predator (natural enemy) for many insect pests (Koch 2003). The expression pattern, molecular characterization,and functional analysis of H. axyridis vitellogenin gene 2(HmVg2) would be helpful to better understand the regulation mechanisms responsible for HmVg synthesis. In this study,we identified and characterized a new Vg encoding gene(HmVg2) from an H. axyridis transcriptome database. In order to understand the phylogenetic relationships of HmVg2 proteins, we constructed a phylogenetic tree with other insect Vg amino acid sequences. We also assessed the developmental and tissue-specific abundance of HmVg2 gene and RNA interference was used to analyze the function of the HmVg2 gene. As H. axyridis is an important natural enemy, understanding the molecular properties of its reproduction will allow us to develop artificial methods to promote its Vgs and mass rearing. This article hypothesizes that Vg is specifically present in female adults of H. axyridis and plays a critical role in the reproductive process. This hypothesis was verified by qRT-PCR and RNA interference.
2. Materials and methods
2.1. Ethics statement
The beetles used in this study were collected from a natural population in a field at Hebei Agricultural University, Baoding City (38°85´N, 115°50´E), Hebei Province, China. The aphids were captured from a specimen garden in Hengshui City(37°73´N, 115°68´E), Hebei Province. The field studies did not involve any endangered or protected species, and no specific permissions were required for this research or the above-mentioned sample collections from these locations.
2.2. lnsects
H. axyridis (Pallas) (Coleoptera: Coccinellidae) were captured from a specimen garden at Hebei Agricultural University. H. axyridis larvae and adults were kept inside a growth incubator at (25±1)°C and (65±5)% relative humidity with 16 h light and 8 h dark photoperiods, and provisioned with bird cherry-oat aphids (Rhopalosiphum padi Linnaeus).
Bird cherry-oat aphids (R. padi L.) were captured from a specimen garden in Hengshui City and were kept inside a growth incubator at (22±1)°C and (65±5)% relative humidity with 16 h light and 8 h dark photoperiods. They were fed on barley seedlings.
2.3. Gene cloning and sequencing
In the transcriptome database, there are two Vg genes named Vg1 and Vg2, respectively. The open reading frame(ORF) of Vg1 and Vg2 were different, and both of them were detected by qRT-PCR. Vg2 was more highly expressed than Vg1 at all stages especially in female adults (Appendix A), so Vg2 was considered as the target gene. Total RNA was extracted from 7-day-old adult female insects with the Beijing Tiangen Total RNA Extraction Kit DP431. First-strand cDNA was synthesized by reverse transcribing 500 ng of total RNA with the PrimeScript HRT Reagent Kit (TaKaRa,Japan). A pair of gene-specific primers (HmVg2; Table 1)was designed to amplify the ORF and PCR was used. The PCR products were purified, recovered and cloned into pMD18-T vector (TaKaRa, Japan) and then transformed into DH5α competent cells. Positive clones were screened, and then sequenced by Beijing Genomics Institute.
2.4. Sequence analysis
The full-length cDNA sequence of HmVg2 was translated into the deduced amino acid sequence with DNAMAN Software, and the protein molecular weight and isoelectric point (pI) were analyzed. SignalP 4.1 (http://www.cbs.dtu.dk/services/SignalP/) was used for signal peptide prediction. The protein domain search and prediction were carried out with NCBI BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and NetNGlyc (http://www.cbs.dtu.dk/services/NetNGlyc/) programs, respectively. Phylogenetic analysis was performed to investigate the evolutionary relationships among the putative Vg and other published insect Vgs. There are seven insect orders with Vg protein sequences in the GenBank for phylogenetic analyses with HmVg2. The ORF region (5 361 bp) was used for the phylogenetic analysis.Phylogenetic trees were constructed using MEGA 6.0 Software with the neighbor-joining (NJ) method. The bootstrap test with 1 000 replications was used to estimate the stability of the inferred phylogeny. The amino acid sequence was used for phylogenetic analysis.
2.5. Expression profiling analysis of HmVg2
In order to investigate the developmental expression of the HmVg2 gene, the egg, 1st instar, 2nd instar, 3rd instar, 4th instar, pupa, adult male and female H. axyridis samples were collected. The head, thorax, wing, leg, ovary and fat body were dissected from adult females to examine HmVg2 gene expression in specific tissues. The different tissues were taken from female adults on the 9th day after emergence. Each tissue sample represented 100 mg of tissue, and samples of different instars and different stages also represented 100 mg of tissues dissected from individual insects. If the individual insect weight was greater than 100 mg then the whole body was taken as the sample. The experiments were performed in three biological replicates.Total RNA was extracted and cDNA was synthesized with a cDNA Synthesis Kit (TaKaRa, Japan). qRT-PCR was used to confirm the expression profile with HmVg2 gene specific primers (qHmVg2; Table 1) and a pair of H. axyridis elongation factor (HmEF1) gene specific primers (qEF;Table 1). qRT-PCR was performed in a 20-μL reaction volume containing 1 μL cDNAs, 10 μmol L-1of each primer,7 μL H2O and 10 μL SYBR Ex Taq (TaKaRa, Japan) on a Bio-Rad CFX machine (Bio-Rad, USA). PCR conditions were 95°C for 30 s for an initial denaturation step, followed by 40 cycles of denaturing at 95°C for 5 s, annealing at 56°C for 30 s and extending at 72°C for 30 s. The melting curves of amplicons were measured by taking continuous fluorescence readings with increasing temperature from 65 to 95°C, and a dissociation curve analysis was conducted to verify the absence of any nonspecific amplicons. The relative expression of HmVg2 was assessed with the 2-ΔΔCT method with HmEF1 gene as a reference gene.
2.6. In vivo knock-down of HmVg2 by RNAi injection
For dsRNA synthesis, 484 and 423 bp fragments corresponding to HmVg2 and GFP were generated by PCR with dsHmVg2 and dsGFP primers (Table 1). The T7 RiboMAXTMExpress RNAi System (Promega, USA)was used to synthesize dsRNA in accordance with the manufacturer’s instructions. Newly emerged females and males were reared separately and fed with aphids. Six days after emergence, the females were injected with dsHmVg2,and dsGFP was used as a negative control for non-specific effects of dsRNA. A total of 500 ng dsRNA was injected laterally into the 2nd abdominal segment of a female. The experiment was divided into two parts, one was for qRTPCR analysis of the gene silencing effect, and the other was for egg production analysis. Each section consisted of 10 pairs of test insects, and was carried out in triplicate(30 pairs in total for each part). In the silencing effect part,samples were taken at 1, 3 and 5 days after injection fromeach group. Three females were sampled at each time point and the sampling was carried out in triplicate (nine in total).Total RNA was isolated from a pool of three insects. The whole body transcript level of HmVg2 was checked after dsRNA injection. The RNAi efficiency in specific tissues was not detected. The method of taking the samples is the same as in the Section 2.5. The egg production and egg hatching rates were also counted.

Table 1 Primers used in PCR amplification and double-stranded RNA synthesis
2.7. Statistical analysis
All data were reported in terms of a mean±SD, unless otherwise stated. The IBM SPSS Statistics version 22 was used for data analysis. Differences between the groups were analyzed using the Duncan’s multiple range test and analysis of variance (ANOVA). The different letters above bars denote significant differences. Statistical differences of the RNA interference experiments were tested by Student’s t-test. P<0.05 was considered statistically significant. To examine how silencing of the HmVg2 gene influenced oviposition and hatching, deposited eggs were counted daily.
3. Results
3.1. Cloning and sequencing of HmVg2
The HmVg2 gene sequence was 5 460 bp in length, with an ORF of 5 361 bp. The specific cDNA sequence of HmVg2 gene was obtained by PCR and is shown in Appendix B.The deduced protein sequence showed that HmVg2 was synthesized as a pre-protein of 1 786 amino acid residues with a predicted molecular weight of 203.459 kDa, and a predicted pI of 8.59. HmVg2 was predicted to contain a 19 amino acid signal peptide by Software SignalP4.1. BLASTp analysis indicated that the HmVg2 has a vitellogenin-N domain, a DUF1934 domain and a von Willebrand factor type D (VWD) domain. The deduced HmVg2 protein contained three putative cleavage recognition sites, RHPR(364-367), RLPR (907-910), and RLVR (1 081-1 084). The sequences of the DGTR motif (1 600-1 603) and the GL motif(1 619-1 620) were also present in HmVg2. The number of cystein residues was nine, and they were located at the C-terminal. In addition, there were five putative glycosylation sites, NFT (215-217), NET (562-564), NRS (902-904), NIT(1 338-1 340) and NAS (1 406-1 408) (Fig. 1).
In order to investigate the evolutionary relationships of HmVg2, a phylogenetic tree was constructed using the deduced amino sequences of HmVg2 and other insect species. The neighbor-joining method was used, and HmVg2 was clearly distant from the Vg2 of other orders,falling into the Coleoptera branch (Fig. 2). Analysis by DNAMAN found HmVgs had the highest similarities of 35.32% with Tribolium castaneum, 35.18% with Anthonomus grandis, 35.14% with Colaphellus bowringi, and 34.71% with Monochamus alternatus. The phylogenetic tree aggregated the same target insects on the same evolutionary branches,reflecting the similarity between the classification of the Vgs and the traditional phylogenetic classification of the insect species.
3.2. Expression profiling of HmVg2 gene
In this study, the expression of HmVg2 was detected by qRT-PCR. As shown in Fig. 3-A, the expression of HmVg2 was extremely high in female adults and lower in male adults. HmVg2 was expressed at low levels at other stages.However, HmVg2 nearly disappeared during 1st, 2nd, 3rd and 4th instar and pupa stages. The head, thorax, wing,leg, ovary and fat body tissues were dissected from adult female insects to study tissue-specific expression. The results showed that HmVg2 was expressed in all of the above-mentioned tissues, but mainly in the wing and fat body tissues (Fig. 3-B).
3.3. Effects of HmVg2-directed RNA interference
In order to investigate the function of HmVg2 gene, dsHmVg2 was injected into the H. axyridis female adults. A dsRNA directed at green fluorescent protein (GFP) was used as negative control. Compared with the control, the target gene expression was significantly reduced after dsRNA injection.Compared with dsGFP injection, the lowest relative level of HmVg2 gene expression was detected on the first day after females were injected with dsHmVg2. After the third day following injection, the relative expression level increased but it remained lower than after the dsGFP injection. The situation on the fifth day was similar to the one on the third day (Fig. 4-A; 1st day, F=23343.64, P=1.10E-08; 3rd day,F=292.63, P=6.85E-05; 5th day, F=152.05, P=2.49E-04).The number of eggs laid by H. axyridis during the first five days after dsRNA injection was counted, and the total number of eggs of the target gene injection group was significantly lower than that of the control group (Fig. 4-B;F=137.98, P=3.01E-04). In addition, the egg hatching rate of the dsHmVg2 injection group was significantly lower than that of the dsGFP injection group (Fig. 4-C; F=208.09,P=1.34E-04). These results revealed that HmVg2 gene silence may affect both the total number of eggs laid and egg quality, thereby affecting the final hatching rate.
4. Discussion

Fig. 1 The amino acid sequences of vitellogenin gene 2 deduced from Harmonia axyridis. The stop codon TAG is indicated with an asterisk. The putative signal peptide is underlined. The shaded regions represent three domains, vitellogenin-N (gray),DUF1934 (black), and VWD (light gray). Three putative cleavage sites RXXR are shown with bold underline. The GL and DGTR motifs are shown with light-shaded frame. The nine conserved cysteine residues at the C-terminus are shown with bold. Five putative glycosylation sites are bold and italic.
Vg is a critical precursor protein of egg yolk Vn that provides nutrition for the development of the embryo and serves as an energy reserve in many oviparous species (Tiu et al. 2009).In this study, a Vg gene (HmVg2) from the coleopteran insect H. axyridis was identified. So far, more than 50 types of insect Vg gene sequences from 7 orders have been cloned. The sequence analysis has mainly concentrated on Lepidoptera, Diptera, Hymenoptera and Hemiptera(Li et al. 2012). Results showed that HmVg2 was most homologous with the Tribolium castaneum sequence. The Vg protein reflecting the gene is in fact a vitellogenin from a beetle. Insects have multiple Vg genes and sequences are usually highly similar to each other, retaining conserved functional domains and participating in Vgs (Fire 1999).HmVg2 is substantially the same as other Vgs. Vg has some diversities in the number of cleavage sites, and (R/K)XX(R/K) cleavage recognition sites have been found in almost all insect Vg sequences that have been identified(Sappington and Raikhel 1998). For example, the deduced CceVg protein from C. cephalonica contained five putative cleavage sites (RHIR, RVRR, RLCR, REPR, and RPER)(Veerana et al. 2014), while the lone putative cleavage site of Vg from C. septempunctata was RSTR at position 371-374(Liu et al. 2015). The deduced HmVg2 protein contained three putative cleavage recognition sites, which most likely would be separated from the first site.

Fig. 2 Phylogenetic tree of vitellogenins from Harmonia axyridis and some other insects. The phylogenetic tree was constructed by MEGA6 Software with the neighbor-joining method. The numbers at the nodes are the bootstrap values with bootstrap analysis of 1 000 replications. The GenBank accession numbers are followed with the gene names.
This study has pointed out that most of the insect Vg synthesized in the female fat body was first secreted into the hemolymph and then taken up into the oocyte by endocytosis. However, more in-depth research revealed that Vg synthesis shows tissue, sex and developmental stage specificity. The expression profiles of HmVg2 in different tissues show that the wings have a higher level of expression. Currently, the study of Vgs has mainly targeted the fat body and ovary. While Vg has rarely been reported in wings, some studies can provide references. Amongst the various tissues tested in Aphis (Toxoptera) citricidus, the AcVg gene was expressed in wings. The expression level had no significant differences between the wings and either muscle, integument, gut, or salivary gland. The Vg receptor was also expressed in the wings, and the expression level was not significantly different from that of other tissues(Shang et al. 2018). In Bombyx mandarina, BmaVgR was also detected in wings (Qian et al. 2015). Chen et al.(2012) strongly suggested that most of the crossveinless d(Cv-d) produced in fat body and wing is supplied via the hemolymph. Cv-d can promote posterior cross vein (PCV)development from outside the wing. Cv-d is a Vg-like lipoprotein, similar to the Vgs, which are major constituents of yolk in many animals (Chen et al. 2012). Determining the function of Vg gene expression in wings is still in need of further research.

Fig. 3 qRT-PCR analysis of Harmonia axyridis vitellogenin gene 2 (HmVg2) at different developmental stages and tissues.Expression levels of the HmVg2 gene in different stages and tissues were quantified by qRT-PCR, where expression levels of HmEF1 gene were used as an internal standard. A, different stages; E, egg; 1st, 1st instar; 2nd, 2nd instar; 3rd, 3rd instar; 4th,4th instar; P, pupa; M, male; F, female. B, tissue-specific expression patterns of vitellogenin genes HmVg2 in the female adult.Bars represent the SD obtained from duplications of qRT-PCR analysis. Different letters indicate significant differences (P<0.05).
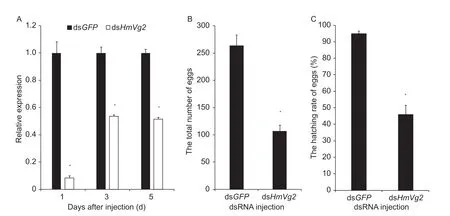
Fig. 4 Influence of double-stranded RNA (dsRNA) of Harmonia axyridis vitellogenin gene 2 (dsHmVg2) treatment of females on reproduction. A, the effects of dsRNA injection on the fecundity of Harmonia axyridis. Six days after emergence female adults were injected with dsRNA. Double-stranded green fluorescent protein (dsGFP) was injected as a negative control. HmEF1 with the same cDNA template served as an internal control. B, the total eggs laid during the first five days in H. axyridis after dsRNA injection. C, the hatching rate of H. axyridis eggs after dsRNA injection. Bars represent the SD obtained from duplications of qRT-PCR analysis. The asterisks indicate significant differences between the group of dsGFP and dsHmVg2 injection (P<0.05).
Based on the results of this study, HmVg2 was highly expressed in fat bodies, instead of the high expression expected in ovaries. Other studies have found similar results. The expression of Apolygus lucorum Vg gene was significantly higher in the fat body than in other tissues(ovary, hemolymph, gut and malpighian tubules) in females(Li et al. 2016). In the reproducing queens and workers of Bombus terrestris, the strongest expression occurred in the fat bodies (Jedlicka et al. 2016). In B. mori, BmVg was only detected in the fat body of the female transgenic silkworms and was not detected in ovaries and midgut (Xu et al. 2014).In Dermacentor variabilis, Northern blot shows that DvVg2 mRNA is detected in the fat body of pre-ovipositing females while not detected in the ovary (Khalil et al. 2011).
The expression of insect vitellogenin varies from species to species. The Vg gene of most insects is synthesized by female fat bodies, but there are exceptions. Follicular cells of Leptinotarsa decemlineata (Peferoen and Loof 1986)and Coccinella septempunctata (Zhai et al. 1984) can also synthesize Vg. According to Chen (2011) on the female adults of H. axyridis, on the third day after eclosion, little vitellin was discovered in fat body, while there was no vitellin in ovary or hemolymph; and then vitellin was detected in ovary and hemolymph, with the peak of vitellin in fat body followed by the peak in ovary (Chen 2011). This pattern suggested that the vitellin was synthesized by fat body, and then secreted into the hemolymph, and at last transferred to the ovary. Our results are consistent with Chen (2011).
The Vg gene from Apis mellifera can be detected not only in the fat body and ovary of queen bees, but also in the fat body of worker bees and drones, which do not produce eggs(Guidugli et al. 2005). CceVg gene from C. cephalonica can be detected in mating males (Veerana et al. 2014). In 2014, it was found that BmVg should be considered as a female-specific high expression gene, for which synthesis mainly starts in the fat body of the female larva-pupa and is then sustained all throughout the silkworm pupation (Yang et al. 2014). The expression of Vg gene in Nilaparvata lugens started on the third day after eclosion in short wing forms, and the expression increased with the days after eclosion, whereas the NlVg gene in long wing forms of N. lugens began expression on the fourth day after eclosion(Tufail et al. 2010). In Plutella xylostella, microvillia Vg gene expression can be detected from the egg until before larval pupation stages (Zou et al. 2015). In this study, the expression of HmVg2 in different stages was studied, and results showed that the expression of HmVg2 was extremely high in the female adult and lower in the male adult. The HmVg2 was expressed at low levels in eggs, and HmVg2 was not detected in 1st, 2nd, 3rd and 4th instar or pupa stages. The HmVg2 gene was mainly expressed in wing and fat body of the female adults. As expected, the results revealed that the main location of HmVg2 protein synthesis is the fat body of female adults. Then, like in other insects,HmVg2 was transported via the hemolymph to the oocytes(Sappington and Raikhel 1998). Finally, it was found that HmVg2 participated in the formation of eggs. Therefore,HmVg2 mRNA expression was not required in male adults and other stages. RNA interference is a good method to study gene function. The RNAi experiment in C. cephalonica showed that the silencing of CceVg led to the deformation of ovaries and consequently, the production of CceVg protein was insufficient to ensure normal development of all oocytes (Veerana et al. 2014). The silencing of BmVg will affect the number and color of eggs, demonstrating that BmVg is necessary for the egg formation and embryonic development of silkworms (Yang et al. 2014). Injection of PxVg siRNA caused severe suppression of Vg expression within 24 h, and the number of eggs laid by females within five days was significantly decreased, but no significant effect was observed on the hatching rate of the eggs (Zou et al. 2015). In this study, dsRNA injection of HmVg2 gene clearly decreased the relative expression of the HmVg2 gene, the total number of eggs laid and the egg hatching rate within five days. This may be because Vg protein deposited in oocytes was not enough for normal embryogenesis. If the Vg reserve was sufficient, the egg hatching rate should not be decreased. The hatching rate of eggs was different in PxVg and HmVg2 after RNAi. This phenomenon may be caused by the difference of dsRNA and siRNA injection.Fat body can absorb harmful macromolecules from the hemolymph, therefore, the injected dsRNA may act on other tissues through the fat body. That is, fat body could process the dsRNA in hemolymph into small interfering RNA or other small fragment signaling molecules (Boutla et al.2002). Injecting siRNA does not require this intermediate step. Similar results also occurred in C. septempunctata,where RNAi mediated by injection of dsRNA depleted CsVg transcripts, significantly reduced egg-laying amount, and decreased egg hatching rate (Liu et al. 2015).
These results demonstrated that HmVg2 plays an important role in H. axyridis reproduction, and will provide a foundation for further physiological and artificial feeding studies on H. axyridis. Taken together, the experimental results demonstrated that HmVg2 most likely plays an essential role in reproduction and it may be of critical importance to H. axyridis utilization as a biological control agent.
5. Conclusion
In this study, we cloned a vitellogenin gene from H. axyridis(HmVg2). Its expression patterns and basic functions have been preliminarily studied, and results showed that HmVg2 gene plays an important role in the fecundity and hatching rate of H. axyridis. These results provide the basis for further study of the physiological function of Vg in the reproduction of H. axyridis.
Acknowledgements
This project was supported by the National Natural Science Foundation of China (31272095), the National Key Research and Development Program of China (2017YFD0201004)and the Hebei Modern Agriculture Industry Technology System, China (HBCT2018060204).
Appendicesassociated with this paper can be available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
 Journal of Integrative Agriculture2019年10期
Journal of Integrative Agriculture2019年10期
- Journal of Integrative Agriculture的其它文章
- Application of virus-induced gene silencing for identification of FHB resistant genes
- Dynamic changes of root proteome reveal diverse responsive proteins in maize subjected to cadmium stress
- Strategies to enhance cottonseed oil contents and reshape fatty acid profile employing different breeding and genetic engineering approaches
- Maize/peanut intercropping increases photosynthetic characteristics,13C-photosynthate distribution, and grain yield of summer maize
- Rhizosphere soil bacterial community composition in soybean genotypes and feedback to soil P availability
- Effect of biochar on grain yield and leaf photosynthetic physiology of soybean cultivars with different phosphorus efficiencies
