Application of virus-induced gene silencing for identification of FHB resistant genes
FAN Yan-hui, HOU Bing-qian, SU Pei-sen, WU Hong-yan, WANG Gui-ping, KONG Ling-rang, MA Xin, WANG Hong-wei
1 State Key Laboratory of Crop Biology/Shandong Key Laboratory of Crop Biology/College of Agronomy, Shandong Agricultural University, Tai’an 271018, P.R.China
2 Shandong Agricultural University Fertilizer Science Technology Co., Ltd., Feicheng 271600, P.R.China
Abstract Virus-induced gene silencing (VIGS) showed several advantages to identify gene functions such as short experimental cycle, more broad hosts, etc. In this study, the feasibility and efficiency of employing Barley stripe mosaic virus (BSMV)-based VIGS system to evaluate Fusarium head blight (FHB) resistance were explored in wheat. With variable conditions tested, it showed that the maximal silencing efficiency 78% on spike was obtained when the recombinant BSMV was inoculated on flag leaf at flagging stage. However, the plant may reduce its own immunity to FHB when inoculated with BSMV. To induce this impact, different Fusarium graminearum strains were tested and SF06-1 strain was selected for FHB resistance evaluation. Using this system, TaAOC, TaAOS, and TaOPR3 involved in jasmonic acid (JA) signaling pathway were identified to positively regulate FHB resistance, which was underpinned by the results when silencing TaAOS in wheat by stable transgenic plants.
Keywords: Triticeae, Fusarium head blight (FHB), BSMV-VIGS, RNAi
1. lntroduction
In recent decades, conventional breeding approaches by manipulating genetic variation have been made great progress in improving the agronomic properties of crops, the next waves of crop improvement will require much greater knowledge of gene function (Cahidet et al. 2010). As the major crops, such as rice, maize, and wheat, having been sequenced, a huge number of genes have been isolated and their functions have been characterized. However,the process of identifying genes with specific functions is very slow and complicated. For accelerating the speed of function examining of genes in model species, such as Arabidopsis and rice, there are two traditional methods, one of which is T-DNA knockout libraries (Kaiser and Siddiqi 2002), while the other one is T-DNA activation libraries(Weigel et al. 2002). Nevertheless, since the genome size of allohexaploid wheat, consisting of three closely related sub genomes of A, B, and D, is quite large, and the low transformation efficiency in wheat, none of these methods is applicable for analyzing gene functions in wheat. Besides,expression of homeologous genes will mask loss-of-function phenotypes in mutations of hexaploid wheat, which could complicate gene function determination (Cahid et al. 2010).
Recently, virus-induced gene silencing (VIGS), an appealing reverse-genetic strategy, has been developed as a powerful functional genomics tool to assess gene function for species not amenable to stable genetic transformation.The mechanism of VIGS is based on innate immune system in plants against viruses that target RNAs will be degraded while antiviral responses is activated (Ruiz et al. 1989). It exploits the fact that infection of plants by viruses activates post-transcriptional gene silencing (PTGS) defence response (Waterhouse et al. 2001), which is triggered by accumulation of double-stranded RNAs (dsRNA)appearing in the infection cycle. In VIGS, a short fragment of a transcribed sequence of a plant gene is inserted into a cloned virus genome and the recombinant virus is then inoculated onto test plants (Lee et al. 2012). Gene silencing,initiated by recombinant virus, will be triggered and spread systemically from the infection site to the newly grown tissues of the plants (Voinnet et al. 2000; Schwachet et al.2005). Both of the inserted gene sequence of plant, as well as the corresponding endogenous gene, and virus genomes can become the target for gene silencing through the PTGS.By taking advantage of VIGS approach, almost any gene of interest could be down-regulated or silenced if a suitable vector is present for the plant species under investigation.
Barley stripe mosaic virus (BSMV), a type member of Horde virus genus with tripartite genome comprising RNAs α, β and γ, is a kind of highly acclaimed vector using in VIGS for cereals, especially for barley and wheat (Yuan et al. 2011;Lee et al. 2012; Ma et al. 2012). Generally, the BSMV can be easily transmitted by mechanical means in the field,and it usually causes mild to moderate mosaic symptoms on most cultivars. Besides, the seed is also known as an efficient way to transmit virus (Jackson et al. 2009). What’s more, Nicotiana benthamiana, a dicot species, can also be infected by BSMV. BSMV-VIGS system possesses many advantages for gene function studies in plant. For instance, all functionally active homeologous gene copies can be effectively silenced at the same time, especially in wheat, since its hexaploidy greatly complicates mutational analysis. Additionally, wheat transformation costs lots of time and money currently, while VIGS is a rapid process that does not require generation of transgenic plants.Even more importantly, it can silence more than one gene simultaneously with a single VIGS construct (Cakir and Scofield 2008), which means the BSMV-VIGS system is efficient and highly cost effective compared with stable transformation.
In recent decades, many plant fungous diseases, such as Fusarium head blight (FHB) and powdery mildew, as well as wheat rust, are becoming increasingly severe. They can result in destructive effect on cereal production such as yield loss and quality deterioration, and even mycotoxin contamination, which can be detrimental to both humans and livestock (Fung and Clark 2004). Nevertheless, since resistance to these diseases in wheat is polygenic and of quantitative trait, it’s quite difficult to explore genes accurately related to corresponding disease. Recently,BSMV-VIGS system is widely applied to identify genes that contribute to FHB resistance as well as other disease resistance (Hein et al. 2005; Scofield et al. 2005), as its high efficiency for 5 to 6 weeks for a test cycle.
Jasmonic acid (JA) regulates various plant defense responses through signaling pathways. The role of JA in plant defense has been extensively reviewed. JA is an important regulator of plant defenses against necrotrophic fungal pathogens (Glazebrook et al. 2005). The JA pathway mutants of Arabidopsis thaliana, opr3, coi1, and jar1 show hyper-resistance to Fusarium graminearum, indicating that this pathway contributes to susceptibility to this fungus(Ragiba et al. 2010). A. thaliana mutants that are impaired in JA production (e.g, fad3-2 fad7-2 fad8 triple mutant) exhibit enhanced susceptibility to a variety of virulent fungal and bacterial pathogens (Staswick et al. 1998). Allene oxide synthase (AOS), allene oxide cyclase (AOC), and 12-oxophytodienoic acid reductase (OPR) participate in the JA biosynthesis. The bread wheat genes TaOPR1 and TaAOC1 have been shown to enhance salinity tolerance (Zhao et al.2014). Solanum tuberosum AOS2 plays a role in resistance to late blight (Pajerowska-Mukhtar et al. 2008).
In this work, the possibility and efficiency of employing BSMV-VIGS to silence genes in Triticeae, including wheat,wild emmer wheat, and Aegilops tauschii, was tested with phytoene desaturase gene TaPDS, which is required for the biosynthesis of carotenoid pigments that protect chlorophyll from photobleaching (Holzberg et al. 2002).BSMV-VIGS application was explored in wheat spikes in different development stages and inoculation positions.Suitable F. graminearum strains were selected to be used for gene function studies by VIGS. Then the effects of TaAOC, TaAOS, and TaOPR3 genes on FHB resistance were analyzed by BSMV-VIGS system in wheat.
2. Materials and methods
2.1. Plant materials and growth conditions
The wheat cultivar Sumai 3 was used for gene cloning. The wild type N. benthamiana plants were grown in a controlled environmental chamber at 24°C/20°C with a 14 h/10 h day/night photoperiod. The wheat cultivar (Sumai 3), emmer wheat varieties (Triticum turgidum ssp. durum (Desf.)MacKey) cultivar Langdon (LDN hereafter), wild emmer wheat ES7 and Ae. tauschii accessions (2115, AL8/78 and WIR1405) at seedling stage used for VIGS experiments and TaAOS RANi plants were grown in a climate chamber at 22°C/18°C, under a 16 h/8 h light/dark regimen. The FHB resistant wheat lines (Sumai 3 and Ning 7840) were grown in the greenhouse at 20°C/15°C under a 16 h/8 h light/dark photoperiod by supplying of supplementary lights,and watered as needed.
2.2. Application of BSMV-VlGS in different genera of Triticeae
To discover new disease resistance genes rapidly, the feasibility and efficiency of employing BSMV-VIGS system to silence genes were tested in different genera of Triticeae mentioned above. The initial silencing experiment was performed with the BSMV vectors carrying a 200-bp fragment of wheat PDS gene (Yuan et al. 2011). PDS genes were cloned from the cDNA of all the six lines with the primer pair of PDS-F/R (Appendix A) designing based on wheat PDS gene sequence from NCBI (https://blast.ncbi.nlm.nih.gov/). Then all the amplified cDNA fragments were constructed to T-Vector pMD19 (Simple) (TaKaRa, Dalian,China), and confirmed by sequencing (BioSune, Jinan,China). Multiple alignments were conducted by DNAMAN.The TaPDS gene was cloned into pCa-γbLIC to generate pCa-γb:TaPDS. Then the N. benthamiana at the four-leaf stage were inoculated with Agrobacterium mixtures containing pCaBS-α, pCaBS-β, and pCa-γb:TaPDS. After 8 days, the leaves with mosaic trait were harvested and ground in PBS(pH 7.2) containing 1% celite, then the sap was mechanically inoculated onto the the first leaf of Triticeae at the two-leaf stage by rubbing. The number of plants with photobleaching phenotype was recorded to calculate the rate of inoculation and VIGS-silencing efficiency.
2.3. BSMV-VlGS on wheat spike
The BSMV vectors were obtained from Dr. Li Dawei at China Agricultural University (Yuan et al. 2011). TaPDS was used to verify the feasibility of BSMV-VIGS in Sumai 3 spike and to study the optimal inoculation period and position of wheat spike silencing. At flagging stage, top 2nd leaf and flag leaf were infiltrated with sap containing BSMV:TaPDS from inoculated wheat leaves, respectively (Fig. 1). The number of photobleached spikes was recorded to calculate the rate of infection. Top 2nd leaf, flag leaf and spike were infiltrated with sap containing BSMV:TaPDS at heading stage, respectively.
“Now listen,” said the robber-girl; “all our men are gone away,— only mother is here, and here she will stay; but at noon she always drinks out of a great bottle, and afterwards sleeps for a little while; and then, I’ll do something for you.” Then she jumped out of bed, clasped her mother round the neck, and pulled her by the beard, crying, “My own little nanny goat, good morning.” Then her mother filliped her nose till it was quite red; yet she did it all for love.
The fragments of TaAOS, TaOPR3, and TaAOC were obtained by reverse transcription-PCR using the primer pairs TaAOS-F/TaAOS-R, TaOPR3-F/TaOPR3-R, and TaAOC-F/TaAOC-R, respectively (Appendix A), and VIGS in wheat leaves was performed as described previously (Campbell and Huang 2010; Yuan et al. 2011). After 8-10 days, the inoculated wheat leaves were harvested and ground in PBS (pH 7.2) containing 1% celite. Then the sap was mechanically rub-inoculated onto the flag leaves of Sumai 3.The transcript abundances of target gene were detected by semi-quantitative RT-PCR, and 18S rRNA was used as an internal control.
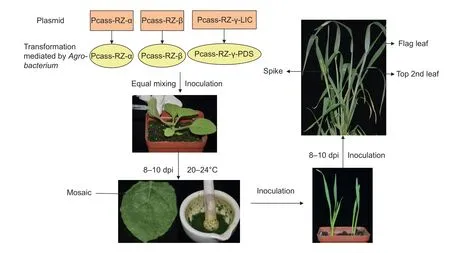
Fig. 1 Flow chart showing the protocol used for silencing the target gene in wheat spike. dpi, days post inoculation.
2.4. Total RNA extraction and cDNA synthesis
Total RNA was extracted with TransZol reagent as described by the manufacturer (TransGen Biotech, Beijing, China),and treated with DNase. The concentration of total RNA was determined by NanoDrop ND-1000 Spectrophotometer(Nano Drop Technologies, Wilmington, DE, USA), while the quality was tested by agarose gel electrophoresis. Firststrand cDNA synthesis was carried out with TransScipt First-Strand cDNA Synthesis Super Mix Kit using 4 μg of total RNA and oligo (dT) primer (TransGen Biotech, Beijing, China).
2.5. Measurements of transcript abundances by qRT-PCR
To determine the efficiency of VIGS, the seedling leaves of Sumai 3 inoculated with BSMV:TaPDS were collected at 8 days post inoculation (dpi) and frozen immediately in liquid nitrogen, and stored at -80°C. Quantitative real-time PCR analysis was performed using a Roche LightCycler®480(Roche Diagnostics GmbH, Mannheim, Germany) as described previously (Hou et al. 2015). The housekeeping gene β-actin was used as an internal standard (Ma et al.2015). The threshold cycle (CT) values were used to calculate the relative expression of TaPDS with the formula 2-ΔΔCT. All quantitative real-time (qRT)-PCR reactions were performed on three biological replicates, and three technical replicates were conducted for each sample. All primers used in this study are listed in Appendix A.
2.6. F. graminearum strain selection
F. graminearum strains (SF01-2, SF04-4, SF06-1, and SF07-2) with different pathogenicities, kindly provided by Prof. Liang Yuancun at College of Plant Protection,Shandong Agricultural University, were selected to check the influence of BSMV-VIGS on FHB resistance in Sumai 3 and Ning7840. BSMV:00 was mechanically rub-inoculated onto flag leaves at flagging stage. The infected wheat were divided into four groups and the untreated wheat was used as control. Next, the spikes were inoculated with different F. graminearum spores at anthesis stage. The method of F. graminearum inoculation was conducted as described previously (Kong et al. 2007). The inoculated spikes were covered with transparent plastic bags to keep moisture for 3 days. The diseased rachises after inoculation of 7 and 14 days were recorded to calculate the infection rate.
2.7. RNAi-induced gene-silencing
An RNAi construct was made using the Gateway System.The 120 bp-length fragment for TaAOS was amplified using gene-specific primers and cloned into a plant transformation vector PANDA-β containing the ZmUbi promoter. The RNAi construct contained the cDNA fragment derived from TaAOS oriented in the antisense and sense directions at the 5´ and 3´ ends of the construct, respectively. The recombinant plasmid was used to transform the target region into transgenic acceptor JW1 (a wheat line from Shandong Academy of Agriculture Sciences) via Agrobacterium-mediated transformation (Liu et al. 2014).Three independent transgenic lines, in which TaAOS was confirmed to be silenced, were employed for FHB resistance analysis using the method mentioned previously. FHB resistance was evaluated by calculating the average number of diseased spikelets (NDS) at 12 dpi.
3. Results
3.1. BSMV-inducing gene silence in different genera of Triticeae
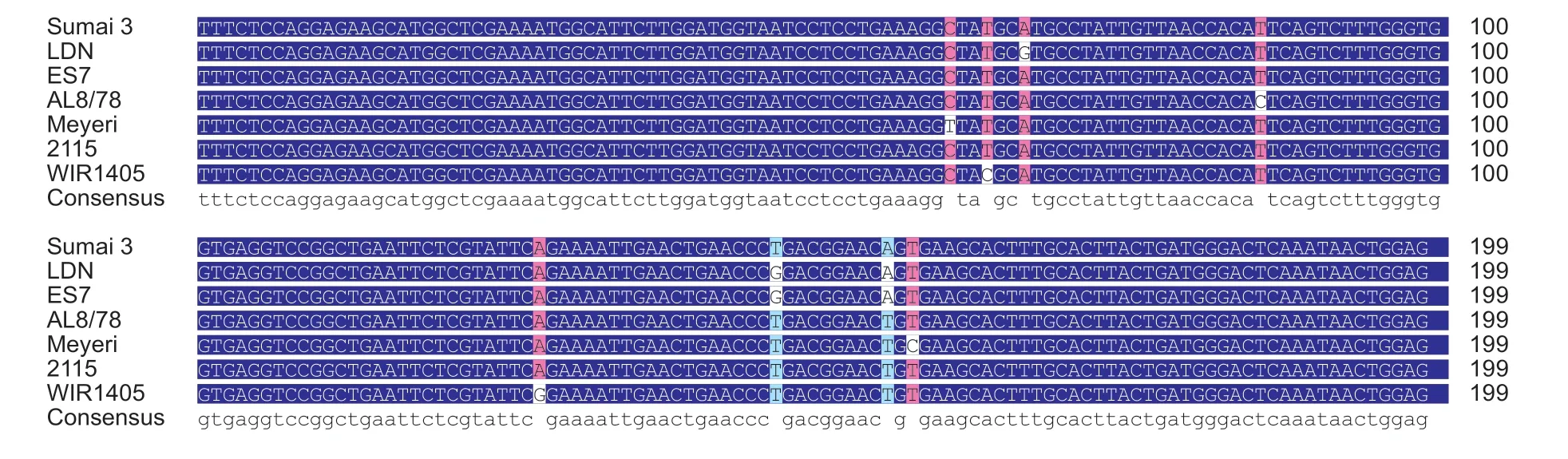
Fig. 2 Multiple alignments of partial cDNA sequenece of PDS gene used for virus induced gene silencing (VIGS) from Sumai 3,LDN, ES7, AL878, Meyeri, 2115, and WIR1405. LDN, Langdon; ES7, one wild emmer wheat accession.

Fig. 3 PDS gene silencing in wheat and its related species at seedling stage. A, the photobleaching phenotype of TaPDS-silenced leaves. Barley stripe mosaic virus (BSMV):00, BSMV containing empty vectors; BSMV:TaPDS, BSMV containing TaPDS. B,efficiency of BSMV inoculation and PDS gene silencing. LDN, Langdon. C, verification of silence of TaPDS in BSMV-infected leaves by qRT-PCR. The cDNA from 8 dpi leaves was amplified by primers amplifying the total amount of TaPDS transcripts for A, B, and D copies. Data are mean±SD (n=8).
To confirm the timeliness of gene silencing at the molecular level, quantitative real-time PCR (qRT-PCR) was conducted to measure the PDS transcript abundances in the seedlings inoculated with BSMV:TaPDS in Sumai 3.Surprisingly, about 60% reduction in PDS transcript abundance was detected as early as 18 dpi compared with control (Fig. 3-C).
3.2. BSMV-VlGS application in wheat spikes

Fig. 4 Effect of inoculation stage and inoculation site on bleaching efficiency. A, photobleaching after PDS gene silencing in spikes with different inoculation periods and different inoculation sites. dpi, days post inoculation. B, inoculation efficiency of BSMV:TaPDS at different inoculation sites in different periods. Data are mean±SD (n=6). C, silencing efficiency of PDS gene at different inoculation sites in different periods. Barley stripe mosaic virus (BSMV):00, BSMV containing empty vectors; BSMV:TaPDS,BSMV containing TaPDS.
The optimal inoculation period and position for gene silencing in wheat spike were studied. Plants inoculated with BSMV:TaPDS or BSMV:00 showed yellow-green virus spot on the flag leaves at 8 to 10 dpi, indicating that BSMV had been successfully inoculated on wheat. A mottled photobleaching phenotype of BSMV:TaPDS silencing was elicited at 19 dpi in different development periods and inoculation positions, but not in BSMV:00 plants. At 25 dpi, the degree of spike bleaching of BSMV:TaPDS plants reached a maximum, but the degrees of white photobleaching were not consistent in different development periods and inoculation positions (Fig. 4-A). The results showed that 78% was the highest photobleaching efficiency when flag leaf was inoculated at flagging stage, compared with the efficiency of 51.4% when flag leaf was inoculated at heading stage. The efficiency of top 2nd leaf inoculating at flagging stage, top 2nd leaf inoculating and spike inoculating at heading stage were 42.3, 20.6, and 31.2%, respectively,and the transcripts of the TaPDS were successfully silenced(Fig. 4-B and C). Therefore, BSMV-VIGS can be used to evaluate gene functions in wheat spike by inoculating flag leaf at flagging stage.
3.3. Pathogenicity of F. graminearum strains
Sumai 3 showed higher FHB resistance than Ning 7840 after inoculation with wild type F. graminearum (Table 1).However, the rate of spikes with infected rachis increased obviously after BSMV:00 treatment compared with the control in both Sumai 3 and Ning 7840, which indicated that BSMV infection could reduce FHB resistance of wheat to some extent (Table 1). In order to find an optimal F. graminearum strain for gene function studies using BSMVVIGS system in wheat, several F. graminearum strains that have different pathogenicities were selected to analyze the effect of BSMV on FHB resistance. F. graminearum strain SF06-1 with weaker pathogenicity showed smaller influence on FHB resistance than the other strains after BSMV:00 treatment. Based on the above results, the strain SF06-1 could be chosen for minimizing the impact of BSMV on resistance to FHB.
3.4. Effect of JA biosynthesis related genes on FHB resistance
TaAOC, TaAOS, and TaOPR3 involved in JA signal pathway were selected to assess the potential of BSMV-VIGS for gene function analysis on FHB resistance. To determine the effects of these genes on wheat FHB resistance,the target fragments were amplified and inserted into pCa-γbLIC. The sap from the infected leaves of wheat seedings was inoculated to the flag leaf of Sumai 3 at flagging stage. Next, the infected plants were inoculated by F. graminearum strain SF06-1 by a way of singlefloret inoculation. After 16 dpi, the plants treated with BSMV:TaAOC, BSMV:TaAOS or BSMV:TaOPR3 showed more susceptible to FHB compared with BSMV:00 and WT (Fig. 5-A). The transcript levels of TaAOC, TaAOS,and TaOPR3 in infected plants were evaluated via semiquantitative RT-PCR at 18 dpi. The results showed that the transcripts of the TaAOC, TaAOS, and TaOPR3 were successfully silenced (Fig. 5-B). The extend efficiency of FHB diseased spikelets of TaAOS, TaAOC, and TaOPR3-silenced spikes were calculated. The results showed thatthe silenced plants were significantly more susceptible to FHB than those infected with BSMV:00. The rate of spikes with infected rachis is separately 54.72, 40.62,18.39, 13.98, and 9.37% at 30 dpi under BSMV:TaAOC,BSMV:TaAOS, BSMV:TaOPR3, BSMV:00 treatments and mock, respectively (Fig.5-C). Taken together, the data presented above indicated that the TaAOC, TaAOS,and TaOPR3-silenced plants showed increased disease spikelets compared with control, implying their positive regulatory role in FHB resistance.

Table 1 The effect of different pathogenicity Fusarium graminearum on the spreading of scab

Fig. 5 Identification of genes function on the resistance to Fusarium head blight (FHB) with virus induced gene silencing (VIGS).A, phenotype of Fusarium graminearum inoculation after gene silencing in wheat spike. B, semi-quantatative RT-PCR of genes in VIGS treated wheat spikes. The wheat 18S rRNA gene was as the endogenous reference for normalization. C, the extend efficiency FHB diseased spikelets of TaAOS, TaAOC, and TaOPR3-silenced spikes compared with wild type plant of Sumai 3.BSMV:00, Barley stripe mosaic virus containing empty vectors; BSMV:TaAOC, BSMV containing TaAOC; BSMW:TaOPR3, BSMV containing TaOPR3. Data are mean±SD (n=8). Asterisks indicate significant differences (*, P<0.05; **, P<0.01).
To further verify the gene function on FHB resistance, the gene of TaAOS was silenced in JW1 by RNAi. It was shown that the disease symptom on spike of transgenic plants was more severe than that of the wild plant (Fig. 6-A), which evenly increased about 1.5 infected rachis internodes and 0.8 infected spikelets compared with the control (Fig. 6-B).
4. Discussion
VIGS is a useful research tool for rapid creation of gene silencing phenotypes that can be used to assess plant gene function (Kumagai et al. 1995; Ratcliff et al. 1997;Baulcombe 1999). Pre-researches have shown that BSMV-VIGS can be used for down regulation of genes in monocot plant, such as culinary ginger (Renner et al. 2009),as well as more distantly related Brachypodium distachyon(Demircan et al. 2010; Pacak et al. 2010) and Dasyprum villosum (Wang et al. 2010). More recently, VIGS system was developed for maize, rice, hexaploid oat, and diploid oat (Ding et al. 2006; Pacak et al. 2010). What is more,BSMV ND18 infected barley sap can be used to establish systemic infections of millet, several inbred maize lines,and other Triticum species (Yuan et al. 2011). Hence, it is likely that BSMV-VIGS can be applied more widely to other crop species. With this regard, the expanded application of the BSMV-VIGS system was explored using TaPDS as a marker gene. About 8 dpi, viral speckles were observed at the new leaf base of different wheat wild relatives, indicated that wheat wild relatives could be infected with BSMV.Approximately 12 dpi inoculation, PDS silencing, a mottled photobleaching phenotype was observed at the bottom of the 3rd leaf of wheat wild relatives. The results showed that wheat PDS gene fragment can successfully induced silencing in wheat wild relatives. The infection rate of wild emmer and Ae. tauschii Coss were lower than Sumai 3 at 28 dpi. However, the efficiency of white photobleaching was approximately similar across different wheat wild relatives once they were infected by the recombinant BSMV carrying TaPDS fragment (Fig. 3-B). Taking together, BSMV-VIGS can be applied to assist in evaluating gene functions in Triticum species.
BSMV-VIGS, as a transient expression vector, can be applied to silence genes in seedings and wheat spikes.Ma et al. (2012) first reported the successful application of BSMV-VIGS for gene silencing in grains and wheat spikes, which indicated that inoculation onto wheat spikes from heading to flowering stages was optimal for efficient silencing of PDS in wheat spikes. The efficiency of VIGS depends on the ability of host plants to sustain virus infection(Bennypaul et al. 2012; Panwar et al. 2013). Ruiz (1989)showed that VIGS of the GFP, carried by potato virus X(PVX) vector, was initiated in all green tissues of PVX infected N. benthamiana, as occurred with PDS VIGS.However, after 30 days of infection, the GFP VIGS was no longer initiated in newly emerging leaves (Ruiz et al.1989). When evaluating gene function on FHB resistance by VIGS, the target gene in spike should be silenced at flowering stage. The FHB resistance can be assessed at flowering stage while the gene is silenced to maximum extent in spikes at 25 days after inoculating the flag leaves at flagging stage (Fig. 4). What is more, the results showed that the inoculation in flag leaves was more effective than that in spikes.

Fig. 6 Identification of the function of TaAOS on Fusarium head blight (FHB) resistance by RNAi. A, spike phenotypes of wild type (WT) and TaAOS RNAi transgenic lines. B, the statistical analysis of the number of diseased spikelets (NDS) and rachises(NDR) in WT and TaAOS RNAi transgenic wheat at 12 days post inoculation (dpi). Data are mean±SD (n=20).
The plant may reduce its own immunity when it is inoculated by BSMV. In this work, the results showed that the BSMV:00 treated plant is more susceptible to F. graminearum than the control. Previous studies showed that various F. graminearum strains present different pathogenicities (Bottalico et al. 2002; Starkey et al. 2007). In order to minimize the impact that BSMV on FHB resistance in wheat, F. graminearum strains with weaker pathogenicity should be used. The pathogenic species isolated from wheat spikes with FHB disease in Shandong Province showed composition and pathogenicity differentiation (Zhang et al. 2013). F. graminearum strain SF06-1, having smaller influence on FHB resistance after BSMV:00 treatment, can be used in determination of gene function on FHB resistance in wheat by BSMV-VIGS.
FHB, a devastating disease world wide, affecting wheat and other small grains. Previous studies have made some contributions to understand the signal pathways involving in FHB resistance (Yang et al. 2003; Ragiba et al. 2010; Ding et al. 2011). JAs are plant signaling molecules that play important roles in defense against insects and necrotrophic pathogens. JA signaling pathway may mediate FHB resistance in wheat (Li and Yang 2008; Gottwald et al. 2012).To determine the effects of JA signal pathway on wheat FHB resistance, TaAOC, TaAOS, and TaOPR3 involved in JA signal pathway were silenced using BSMV-VIGS. The silenced plants showed severer FHB symptom than those infected with BSMV:00. The result indicated that it is feasible to unearth more genes that are positive on FHB resistance by BSMV-VIGS (Fig. 6).
5. Conclusion
The application of BSMV-VIGS was expanded to Triticeae in this study. The suitable BSMV inoculation period was explored to satisfy FHB evaluation at wheat flowering phase. Then, the BSMV-VIGS system was applied to assess TaAOC, TaAOS, and TaOPR3 functions on FHB resistance.TaAOS function on FHB resistance was confirmed by RNAi transgenic wheat. In addition, the success application of BSMV-VIGS on Triticeae leaves is also of great significance which can be applied to study gene functions on leaf diseases, such as powdery mildew and stripe rust in different genera of Triticeae.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (3315203911 and 31471488), the National Key Research and Development Program of China(2016YFD0100602), the Transgenic Special Item, China(2016ZX08002003-002 and 2016ZX08009-003), and the Bohai Granary Science and Technology Demonstration Project of Shandong Province, China (2017BHLC020).
Appendixassociated with this paper can be available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
 Journal of Integrative Agriculture2019年10期
Journal of Integrative Agriculture2019年10期
- Journal of Integrative Agriculture的其它文章
- Dynamic changes of root proteome reveal diverse responsive proteins in maize subjected to cadmium stress
- Strategies to enhance cottonseed oil contents and reshape fatty acid profile employing different breeding and genetic engineering approaches
- Maize/peanut intercropping increases photosynthetic characteristics,13C-photosynthate distribution, and grain yield of summer maize
- Rhizosphere soil bacterial community composition in soybean genotypes and feedback to soil P availability
- Effect of biochar on grain yield and leaf photosynthetic physiology of soybean cultivars with different phosphorus efficiencies
- lnheritance of steroidal glycoalkaloids in potato tuber flesh
