Precision surgery for primary liver cancer
Takeshi Takamoto, Masatoshi Makuuchi
1Hepatobiliary and Pancreatic Surgery Division, National Cancer Center Hospital, Tokyo 104-0045, Japan; 2President Emeritus, Towa Hospital, Adachi-ku 120-0003, Japan
ABSTRACT Liver resection remains the best curative option for primary liver cancer, such as hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma. In particular, in liver resection for HCC, anatomical resection of the tumor-bearing segments is highly recommended to eradicate the intrahepatic metastases spreading through portal venous branches. Anatomical liver resection,including anatomical segmentectomy and subsegmentectomy using the dye-injection method, is technically demanding and requires experience for completion of a precise procedure. The recent development of imaging studies and new computer technologies has allowed for the preoperative design of the operative procedure, intraoperative navigation, and postoperative quality evaluation of the anatomical liver resection. Although these new technologies are related to the progress of artificial intelligence, the actual operative procedure is still performed as human-hand work. A precise anatomical liver resection still requires meticulous exposure of the boundary of hepatic venous tributaries with deep knowledge of liver anatomy and utilization of intraoperative ultrasonography.
KEYWORDS Preoperative ultrasonic examination; intraoperative ultrasonography; anatomical liver resection; dye injection method;preoperative imaging
Introduction
More than 95% of primary liver cancer (PLC) cases consist of hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC)1,2. According to a global investigation in 20153, there were over 850,000 incident cases and over 800,000 deaths in the world per year. The similar incidence and mortality rates indicate that the treatments for PLC remain challenging. HCC, accounting for over 90% of PLC, is the second leading cause of death in men and the sixth in women4,5. ICC ranks the second most common PLC type and shows increasing incidence worldwide6-8. Early detection and early treatment of PLC are undoubtfully key to improving the prognosis of PLC, and prevention of tumor development in HCC is another important factor since 80%of HCC patients have hepatitis virus infection, which is a predisposition to cancer.
In East Asian countries (the area with the highest prevalence of PLC in the world), recent effective vaccination for hepatitis B virus campaigns9have allowed some countries to succeed in reducing the age-adjusted mortality rate of HCC9-11. However, an increasing incidence of HCC has been observed in North America. Recently, the incidence of HCC has increased in those who do not have hepatitis virus infection or alcohol abuse history12. Non-alcoholic fatty liver disease13,14and the consequent development of HCC have also become an issue. As the situation does not allow us to be optimistic about the reduction of PLC incidence, early precise diagnosis and treatment are required for improving the prognosis of PLC. The best treatment for HCC and ICC is surgical treatment, namely, hepatectomy. Radiofrequency ablation (RFA) is another treatment choice for HCC that has a comparable prognostic impact when small (< 3 cm)nodular tumors are found to arise in the cirrhotic liver15-17.However, ablation therapies for HCC, such as RFA,percutaneous ethanol injection18, and microwave ablation19,are theoretically insufficient treatments for HCC because non-anatomical, spherical shape ablation cannot deal with intrahepatic metastases that require territorial removal of tumor-bearing liver segment. The superiority or noninferiority of ablation therapies compared with hepatectomy is still controversial15,16,20, and the results of randomized control trials with higher quality are awaited21.Liver transplantation is another curative treatment for HCC that provides the best survival in selected patients22,23. This treatment allows for removal not only of the tumor but also of the underlying cirrhosis. However, due to the scarcity of liver grafts, this option is not always available. Several multikinase inhibitor chemotherapeutic agents (sorafenib24,regorafenib25, and lenvatinib26) have recently become available and only showed a few months of life-prolonging effects for unresectable HCC. The additional benefit of adjuvant chemotherapy for HCC compared to hepatectomy has not been clarified25, and the synergistic effect of chemotherapy and surgery is still being explored.Transcatheter arterial chemoembolization is considered the first-line palliative treatment for asymptomatic patients with limited unresectable multinodular HCC27,28. The chemotherapeutic options for ICC are more limited since only a few chemotherapies have been suggested to have prognostic effects in metastatic ICC29, such as gemcitabine pule cisplatin30or S131. Consequently, our main focus is on“precision liver surgery” for improving the prognosis of PLC.
Two aspects of hepatectomy for precision liver surgery
Two important aspects should be kept in mind when performing precision liver surgery for PLC: sufficient remnant liver volume and curative operative procedure. Our preoperative preparation and decision-making procedures are summarized in the flowchart shown in Figure 1.
Assessment of liver functional reserve
The first step to obtain sufficient future remnant liver volume is the evaluation of liver functional reserve before hepatectomy. Most patients who develop PLC have a background of chronic hepatitis, and the degree of liver damage differs among patients. The individual evaluation requires the results of not only usual blood tests, such as Child-Turcotte-Pugh score32or model for end-stage liver disease score33,34, but also load tests, which assess the degree of liver tolerance under metabolic burden. One of the most popular liver load tests is the indocyanine green (ICG)retention test35,36. When discrepancy between the results of the ICG test and other static laboratory data is found, an additional examination (e.g., scintigraphy using 99mdiethylenetriaminepentaacetic acid-galactosyl human serum albumin37-39) is recommended. It is important to individually evaluate the minimum remnant liver volume required using the dynamic assessment of liver functional reserve, and then the operative procedures should be compared and selected.We always use Makuuchi’s criteria, the ICG retention rate at 15 minutes (ICGR15) based on the result of the ICG test,which can prescribe the maximum tolerable operative procedure (Figure 2)40,41. We observed Makuuchi’s criteria from the 1980s and achieved no mortality after hepatectomy over 1,000 consecutive cases at the beginning of the 2000s42.Before the 1980s, even large institutions in our country experienced approximately 20% mortality with over 1,500 mL average blood loss or over 5,000 mL blood loss in over 15% patients who underwent hepatectomy43,44. After 2007,our nation-wide surveillance showed that the average blood loss in hepatectomy was reduced to less than 700 mL and that mortality was less than 1%2.
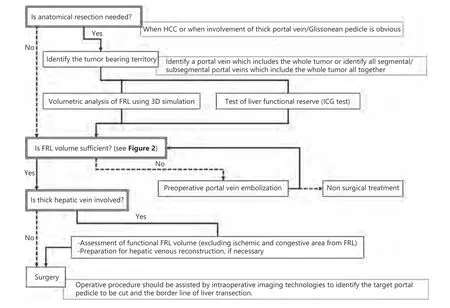
Figure 1 Flowchart of our preparation and decision-making for precision surgery for primary liver cancer. HCC, hepatocellular carcinoma;FRL, future remnant liver; ICG, indocyanine green.
Adequate operative procedure
Resection with sufficient surgical margins without leaving tumor cells behind is essential in the surgical treatment of solid cancers. Additionally, a surgical procedure eliminating micrometastases around the main lesion is ideal. The characteristics of HCC progression are microinvasion to the portal vein and intrahepatic metastases45,46. It has been revealed that microvascular invasion is observed in 20% of HCC cases less than 2 cm in diameter47and in more than half of HCC cases over 4 cm in diameter48,49. Positive microvascular invasion is linked to a higher incidence of intrahepatic metastases. Theoretically, removal of the tumor burden territory is necessary to achieve complete elimination of macroscopic and/or microscopic intrahepatic metastases(daughter lesions or satellite lesions)50,51. This evidence and theory are the rationale supporting anatomical liver resection for HCC (Figure 3)40. For ICC, the second major type of PLC, the benefit of anatomical liver resection is not clear.Limited liver resection with sufficient surgical margins avoiding tumor exposure52,53for a mass-forming type of ICC is generally selected; however, anatomical liver resection should be considered when invasion to the Glissonian sheath or intrahepatic bile ducts is observed. An extended hemihepatectomy and concurrent resection of the extrahepatic bile duct are required when the invasion is extended to the hepatic hilum. Since one-third of ICC cases are accompanied by lymph node metastases54, preoperative and intraoperative assessment of regional lymph node metastases is indispensable, and extended lymphadenectomy (resection of lymph nodes in the hepatoduodenal ligament and around the common hepatic artery) should be added when lymph node metastasis is pathologically diagnosed from frozen sections55.However, the prognostic effect of routine lymphadenectomy for ICC is still under debate55,56.
Recent topics of performing anatomical liver resection
Designing and planning the procedure

Figure 2 Decision tree for selection of operative procedures. There are three important factors, i.e. ascites, serum total bilirubin value and indocyanine green (ICG) retention rate at 15 minutes (ICGR15) or ICG-K values. For patients with uncontrollable ascites or having serum total bilirubin level over 2.0 mg/dL, hepatectomy will not be proceeded.

Figure 3 Schema of hepatocellular carcinoma’s extension.(A) Tumor invades into portal venous branches and tumor cells are carried to the distal part of the liver by portal venous flow. (B) The carried tumor cells will grow into microscopic tumor thrombus and then into daughter nodules or intrahepatic metastasis. (C) The tumor thrombus becomes a source of wider tumor spread.Reprinted with permission from Ref. 45.
First, the location of anatomical resection should be determined by finding the main feeding artery to the HCC in the arterial phase of a dynamic CT scan or by finding the main feeding portal vein in the portal phase of a dynamic CT scan or other modalities. The feeding artery and portal vein are not always only one blood vessel. Basically, the standard surgical procedure for HCC is anatomical resection. The precise determination of the extent of resection of segments or subsegments is made by paying attention to the existence of intrahepatic metastases. When intrahepatic metastases are found around the main tumor, all the portal venous branches, which are assumed to be the paths of the intrahepatic metastases, should be immediately and comprehensively resected.
The recent development of three-dimensional (3D)simulation software57-59has enabled the instant display of this area and the precise calculation of the territorial volume of any selected portal branch. This software offers liver surgeons more precise and accurate data in terms of future remnant liver volume (FRLV), and practicing any types of liver resections can be performed on the computer, which is called virtual hepatectomy. The 3D simulation can also show the assumed drainage area of any hepatic venous tributary,which contributes more precise preoperative evaluation of the congestive area in the future remnant liver, when the concomitant resection of a hepatic vein is considered60. It is essential when deciding the extent of liver resection to consider the balance between the liver functional reserve and tumor size as well as disease extension. If the FRLV is insufficient for the liver functional reserve, portal embolization prior to hepatectomy should be considered41,61.
Recognition of the target segment
Before performing liver resection, the target portal venous branch should be detected using intraoperative ultrasonography (IOUS). It is not easy for nonexpert liver surgeons to identify the target portal venous branch, which have been noted during the preoperative planning for using of 3D simulation software. Generally, detection of the target portal venous branch requires deep knowledge of intrahepatic vascular anatomy and practical experience of IOUS. This is the first obstacle for performing anatomical segmentectomy. Recent developments in the intraoperative navigation system have the potential to support elimination of this first obstacle. Several ultrasound navigation systems have been developed, and this proves the synchronization between preoperative CT images and real-time IOUS images.Once an ultrasonography image is matched to the corresponding slice image of the preoperative CT scan, the preoperative CT image synchronizes with the real-time images of IOUS. In the IOUS navigation system, it is essential to detect the spacial position and direction of the ultrasonic probe, and there are several methods. First, the visual tracking system uses a camera for visualization of the operative field. The camera detects the location and features of the uniquely shaped attachment that is fixed on the ultrasound probe62. Second, the electromagnetic system creates an invisible magnetic field in the operating area using a magnetic field generator. The generator is attached on the operative table, and a wired small magnetic field sensor is fixed on the ultrasound probe63. The spatial position and direction of the sensor in the magnetic field can be detected.The procedure of matching the images of two different modalities, preoperative CT and real-time IOUS, is called registration. However, the registration is performed manually by marking several anatomical landmarks, such as the bifurcation of the left/right portal veins, the large tributary of the middle hepatic vein (MHV), or the bifurcation of the anterior and posterior portal veins. This manual registration procedure can only be used by users who are familiar with liver anatomy and IOUS images. This manual registration results in the need for a person outside the operative field to input the anatomical landmarks into the navigation system in cooperation with the surgeon in the operative field.Currently, the next generation of software has been developed, which uses an electromagnetic IOUS navigation system with machine learning and artificial intelligence to perform an automatic registration procedure, aiming at more convenient and smaller positional error64.
Visualization and recognition of the borderline between segments
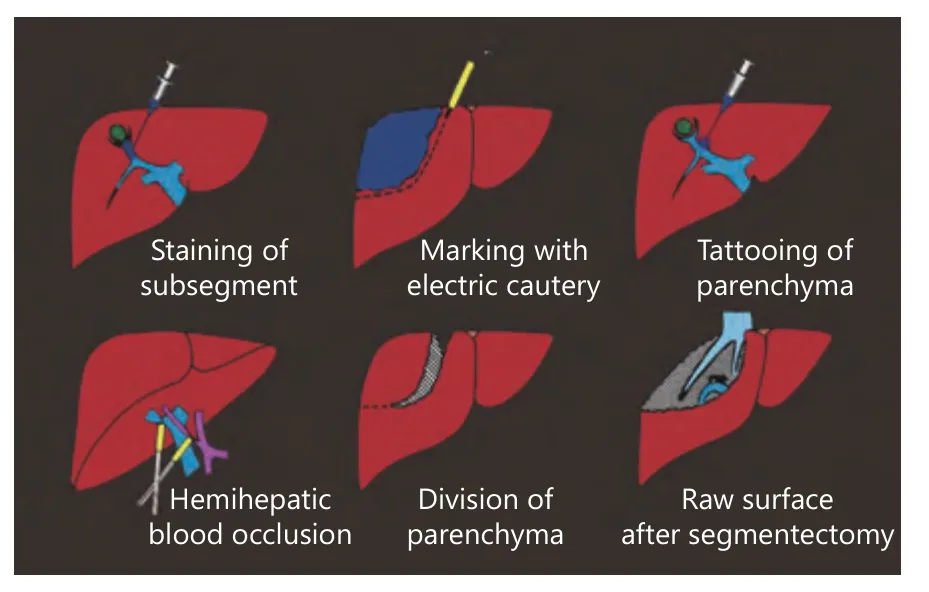
Figure 4 Procedure of anatomical resection with dye-injection method. Tumor-bearing segment or subsegment is stained by injecting dye under the guide of ultrasound, then the stained area is marked. Under intermittent inflow occlusion, liver parenchyma is divided. And the root of portal pedicle and landmark veins are fully exposed on the surface of the resection plane. Anatomical resection is only one choice of treatment for obtaining radicality.Reprinted with permission from Ref. 45.
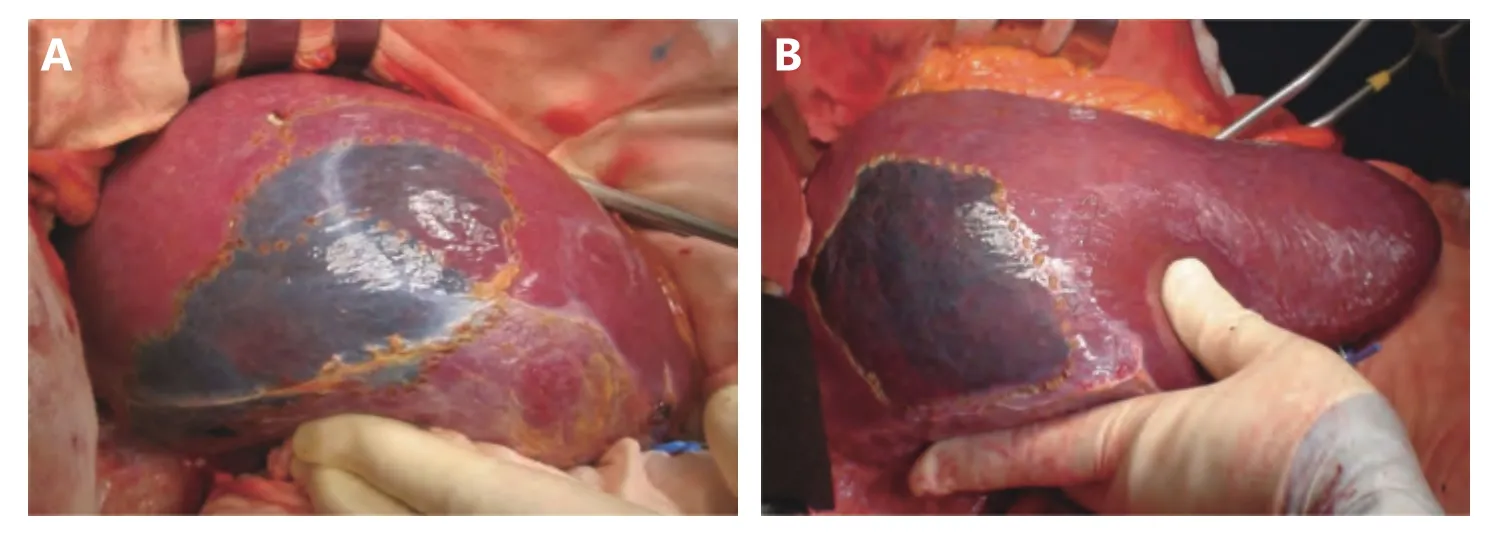
Figure 5 Individual differences of segments (S8dor). The stained area of S8dor was shown in two cases (A/B). Naturally, the shape and volume of each liver segments differs case by case.
After recognition of the portal branch supplying the segment to be resected, the next step is the visualization of the territory. The most popular method to visualize the segment and subsegments is the dye-injection method, which involves injecting indigo carmine solution into the target portal branch with the assistance of IOUS (Figure 4)45. As shown in Figure 5, the shape and volume of a segment (i.e., S8dor)differ among cases. The liver raw surface view after anatomical segmentectomy (S7 resection in Figure 6) differs among cases. There is individual variation in the location of the portal pedicle’s stump and hepatic venous tributaries that are exposed on the raw surface of the liver. For example, in the anatomical resection of segment 7, the inferior right hepatic vein (IRHV) will be exposed when a patient has an IRHV. The dye injection method is also technically demanding since delicate control of the injection speed of the dye into the portal vein using IOUS is required. Several surgeons have proposed a modified dye injection method,mixing a small amount of ICG solution into indigo carmine solution and observing the stained territory using a nearinfrared fluorescence (NIR) camera65-67. This modified method, the NIR/ICG method, has the potential to more clearly show the stained territory with better contrast and to visualize the borderline between segments inside the liver that cannot be seen with the naked eye. After the recognition of the borderline between segments on the liver surface, liver transection is stared along the borderline. The largest issue during liver transection is the difficulty of visualizing the border of segments inside the liver. A precise anatomical segmentectomy could be theoretically completed by removal of the ICG-stained area or transecting the liver following the borderline of the stained area. However, it is quite troublesome and unrealistic to proceed with liver resection when switching the operating astral lights and the NIR camera on and off. Practically, in anatomical liver resection using the NIR/ICG method during open surgery, the NIR camera is used to confirm that the liver transection line is accurate during the interval of liver transection. A unique device for the NIR/ICG method using the principle of projection mapping has been presented. A projector overlays the ICG-stained area of the liver for instant capture with a mounted NIR camera68. This method will be more accessible and accepted widely when the inconvenience of performing liver resection under a non-shadowless lamp is overcome and when its safety is more evident. For a precise anatomical hepatectomy, the elemental but surest method is to follow the minute tributaries of the hepatic vein. Anatomically,hepatic venous tributaries run along the border between any segments and subsegments of the liver. During anatomical hepatectomy, it is important to find small tributaries starting from a depth of a few millimeters under the liver surface and to follow them along the central side without damaging them. Not a single tributary but many hepatic veins should be exposed on the liver transection plane. With the assistance of ultrasound modalities, such as color Doppler, power Doppler or directional eFLOW modes (Hitachi-Aloka Co.,Ltd., Tokyo, Japan), which enable the detection of minute blood vessels in more detail, small hepatic venous tributaries can be examined near the liver surface, and it can be confirmed that they flow into a large hepatic vein, which will be exposed on the liver transection surface. It is necessary for the surgeon and assistants to have excellent experience with this liver surgery technique in order to perform this meticulous surgical procedure.
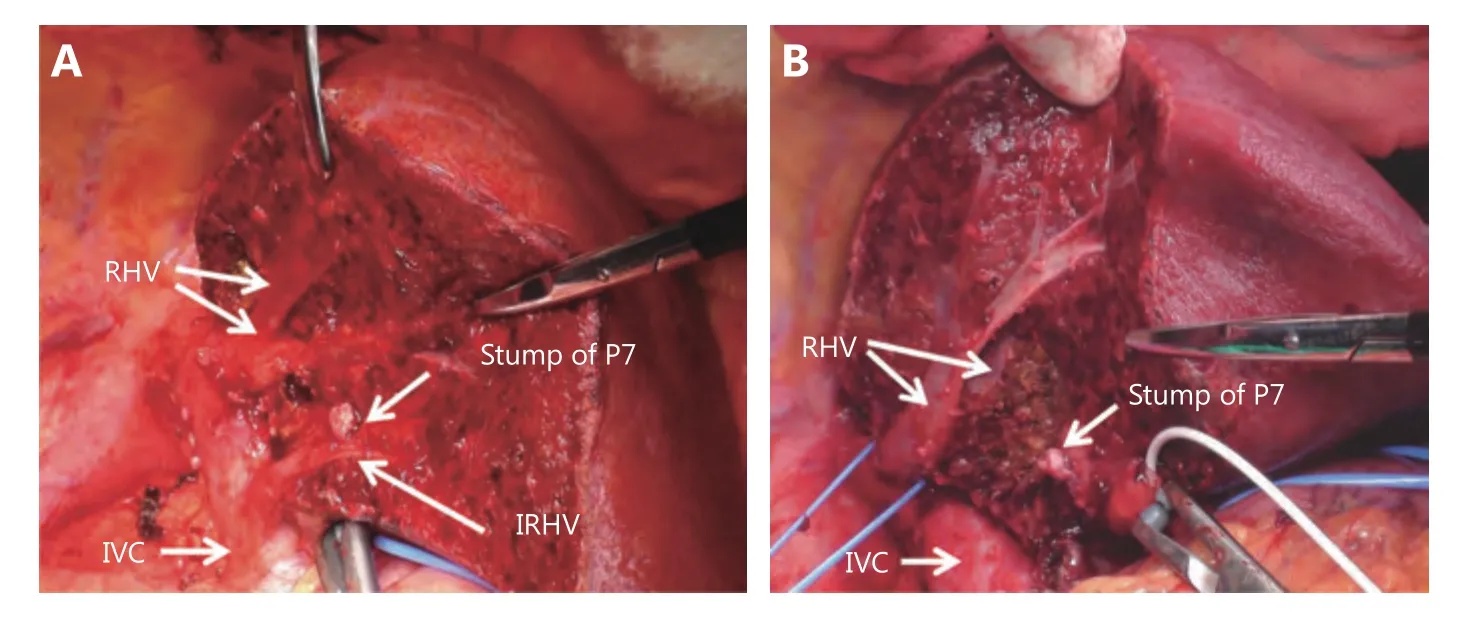
Figure 6 Individual differences of liver raw surface after segmentectomy (S7). The appearances of liver raw surface after S7 segmentectomy of two cases were compared. There are difference in the shape of liver resection surface and positions of each landmarks(A/B). Additionally, when a patient has inferior right hepatic vein, it is be exposed on the caudal boundary surface (A).
Evaluation of anatomical liver resection
There are two methods for evaluating the precision of anatomical liver resection. First, whether the hepatic veins on the transection plane of the liver are clearly exposed should be evaluated (Figure 7). Strictly speaking, multiple small hepatic venous tributaries should be visualized from the liver surface to large major hepatic veins. This is similarly true in typical cases, such as hemi-hepatectomy, right paramedian sectionectomy, and right lateral sectionectomy, as in anatomical segmentectomy using dye injection methods(Figure 8). Because a completion picture after liver resection can be processed using 3D simulation software, it is also helpful to compare the real view and the completion picture of the liver cutting surface. Although the definition of CT images is not fine enough to show the millimeter-sized hepatic venous tributaries, the gross view is comparable,especially the positions of the portal pedicle stump and major hepatic vein, which is half-circumferentially exposed, on the liver raw surface. Furthermore, the volume of a segment that is planned for resection can be calculated using 3D simulation software. The concordance between the calculated volume of the segment and the weight of the resected specimen can be evidence of a precise anatomical liver resection. In fact, the calculated segmental volume in the 3D simulation software and the weight of the specimen show good correlation59. The specific gravity of the liver parenchyma is regarded as 1.0 in most articles; however,Lemke et al.69measured liver grafts of living-related liver transplantation by the Archimedes method and showed that the physical density of transplanted liver lobes was approximately 1.12 g/mL.
Practice of a precise anatomical liver resection with the dye injection method
A portion of the right hepatectomy procedure will be presented as follows as an example of a precise anatomical liver resection. In particular, the techniques and tips used to expose the MHV from small tributaries to the main trunk are mentioned (Figure 9).
1) First, the right liver is mobilized from the retroperitoneum, the right adrenal grand and the inferior vena cava (IVC). The right hepatic vein can be encircled extrahepatically after dividing the IVC ligament70. Then, the procedure moves toward the detachment of the porta hepatis. After cholecystectomy, the right hepatic artery(RHA) and right portal vein (RPV) are isolated. After confirming that the hepatic arterial flow and portal venous flow in the remnant left liver are preserved after clamping the RHA and RPV using the IOUS color doppler mode, the RHA and RPV are ligated and divided. The right hepatic plate,including the right hepatic duct, is then dissected. Then, the right liver becomes ischemic, and the demarcation line will appear on the liver surface. After marking the liver transection line on the surface using an electric cautery, liver transection can be initiated with the intermittent Pringle maneuver.

Figure 7 A case who received anatomical segmentectomy of S6 with dye-injection method. Indigocarmine solution was injected into portal branch of segment six and S6 was stained (A). On the liver resection surface after S6 anatomical resection, two tributaries of right hepatic veins are clearly exposed (B). Resections of peripheral liver such as S6 are considered to have good indication for laparoscopic hepatectomy, but it is quite difficult to expose the hepatic veins so clearly in laparoscopic hepatectomy. There is little possibility that the laparoscopic hepatectomy shows stronger impact on prognosis than open hepatectomy for the surgical treatment of HCC.

Figure 8 Clear exposure of major hepatic veins is the evidence of complete anatomical resection. Every major hepatic vein was exposed with half circumference in right paramedian sectorectomy (A), right lateral sectorectomy (B), left hepatectomy (C), and right hepatectomy(D). The rough liver resection plane is the evidence that the intersegmental plane of the liver is not flat.
2) Before beginning liver transection, the IOUS probe is placed on the demarcation line, which appears along the Rex-Cantlie line, and small hepatic venous tributaries at a depth of 0.5 to 1 cm from the liver surface can be detected and can be followed as they run into the main trunk of the MHV and the IVC using IOUS.
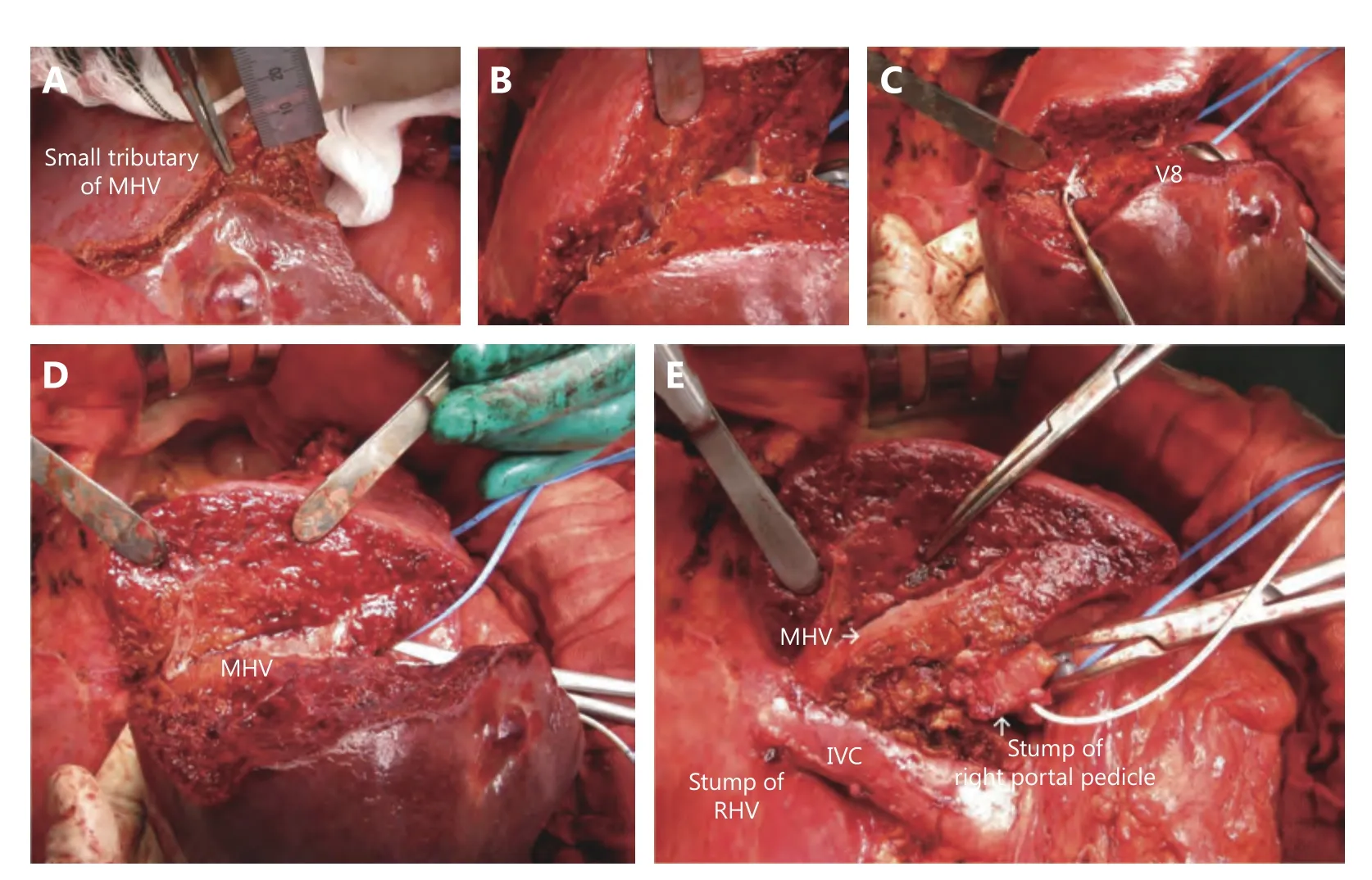
Figure 9 Procedure of right hepatectomy with exposure of minute peripheral hepatic veins. From 0.5 to 1 cm from the liver surface, a small hepatic venous tributary is found (A). Liver transection is proceeded following the tributary, then thicker hepatic veins appear (B). Several hepatic venous tributaries drainaging right liver were carefully ligated and divided (D), then the liver transection plane consistently meets the main trunk of middle hepatic vein (E). On the liver resection plane, the middle hepatic vein and its tributaries were clearly exposed from the liver surface to the confluence of inferior vena cava.
3) Liver transection is initiated with the Kelly clamp crush method, and a small tributary found with IOUS is detected.Since energy devices easily burn away small hepatic venous tributaries, meticulous ligation using 4-0 threads is preferred.This procedure is executed in multiple locations on the Rex-Cantlie line to expose multiple small tributaries, and further liver transection to a depth of 3 to 5 cm will expose the points where these tributaries run into a relatively large hepatic vein or into the main trunk of the MHV. Half the circumference of the MHV should be exposed. During liver transection, tiny hepatic venous tributaries approximately one millimeter in diameter that drain the right liver can be found. Instead of ligating with tiny threads, it is useful to pull these small tributaries off with surgical forceps from the liver parenchyma to the MHV, holding them at the side of the liver and pulling them off in the direction of the MHV.
4) Bleeding from the MHV is troublesome. In particular,an injury of a hepatic venous tributary at the confluence of the MHV or a pinhole injury on the MHV leads to massive blood loss. With equanimity, suturing hemostasis should be quickly performed after gently pressing the pinhole with the finger. Basically, the operator’s left hand should be used to elevate the right liver and to compress the sidewall of the MHV or the confluence into the IVC for the purpose of reducing hepatic venous pressure. This technique using the surgeon’s left hand is a great advantage of right thoracolaparotomy for hepatectomy.
5) It is ideal when the operator remembers and confirms the number and location of the large hepatic venous tributaries of the MHV, draining S5 and S8, using 3D simulation software and IOUS. It is important to prevent splitting the hepatic venous wall at the confluence.Furthermore, it is also important to pay attention to the motion of the suction tube used by the assistants. When a suction tube is moved in a caudal to cranial direction, a hepatic venous tributary running along the liver transection plane can be easily torn and split at the confluence of its tributaries. Hence, the suction tube should be moved in one direction, from cranial to caudal, when an assistant tries to clean the liver transection plane.
6) A meticulous and accurate liver transection following hepatic venous tributaries naturally leads to the exposure of the major hepatic vein and the root of the portal pedicle to be resected. For right hepatectomy, after following the main trunk of the MHV and arriving at the confluence of the IVC,the right hepatectomy is finished.
Perspective of precision liver surgery
The prognostic impact of anatomical liver resection for HCC was once under hot discussion. Some authors have insisted that resection has no advantage over non-anatomical resection71-74; however, several reports from high-volume hepatectomy centers50,75,76, nation-wide surveys77, multinational examinations78, and meta-analyses79-81have revealed the benefit of anatomical liver resection to non-anatomical resection in terms of recurrence-free and overall survival. The standard surgical treatment for HCC is unquestionably anatomical liver resection.
The next focus in liver surgery is how to maintain quality assurance during anatomical liver resection. Technical transfer to the next generation is a major issue. Currently,operative planning is assisted by 3D simulation software,suggesting the completion pictures after liver resection as an answer, and various intraoperative imaging tools are used to navigate the surgical procedure in the operation room.Precision liver surgery, as the best treatment for PLC, will continue and prevail with the assistance of these new technologies.
Conflict of interest statement
No potential conflicts of interest are disclosed.
 Cancer Biology & Medicine2019年3期
Cancer Biology & Medicine2019年3期
- Cancer Biology & Medicine的其它文章
- TNFα inhibitor C87 sensitizes EGFRvIII transfected glioblastoma cells to gefitinib by a concurrent blockade of TNFα signaling
- A four-gene signature-derived risk score for glioblastoma:prospects for prognostic and response predictive analyses
- Prediction of cervical lymph node metastases in papillary thyroid microcarcinoma by sonographic features of the primary site
- Decrease in the Ki67 index during neoadjuvant chemotherapy predicts favorable relapse-free survival in patients with locally advanced breast cancer
- Incidence, distribution of histological subtypes and primary sites of soft tissue sarcoma in China
- Prevalence and clinical significance of pathogenic germline BRCA1/2 mutations in Chinese non-small cell lung cancer patients
