Sex-determining region Y box-containing genes: regulators and biomarkers in gynecological cancers
Jiali Hu, Ke Li, Zhanghuan Li, Chao Gao, Fei Guo, Yingmei Wang, Fengxia Xue
Department of Gynecology and Obstetrics, Tianjin Medical University General Hospital, Tianjin 300052, China
ABSTRACT Sex-determining region Y box-containing genes are transcription factors with roles in multiple biological processes, including cell differentiation, proliferation, and apoptosis. Sex-determining region Y box-containing genes have also been shown to act as regulators and biomarkers in the progression of many different cancers, including gynecological cancers such as ovarian, cervical,and endometrial cancer. In this review, we summarize the contrasting regulatory roles of Sex-determining region Y box-containing genes in different gynecological cancers, as promotors with high expression levels or as suppressors with low expression levels.Expression levels of Sex-determining region Y box-containing genes were also identified as biomarkers of clinical features, including International Federation of Gynecology and Obstetrics stage, histopathologic grade together with disease-free survival, and treatment efficacy in patients with gynecological cancers. An understanding of the mechanisms whereby Sex-determining region Y box-containing genes regulate the progression of gynecological cancers will aid in the development of novel diagnostic and therapeutic strategies, while analysis of Sex-determining region Y box-containing expression levels will help to predict the prognosis of patients with gynecological cancers.
KEYWORDS Sex-determining region Y box-containing gene; gynecological cancer; regulator; biomarker; clinical feature; progression
Introduction
Sex-determining region Y box-containing(SOX) genes encode regulatory transcription factors that can act as tumor suppressors or promoters in carcinogenesis.SOXgenes were shown to be involved in the development of various cancers,including breast1, lung2, hepatocellular3, and gastrointestinal cancers4. Due to the increased morbidity and mortality of gynecological cancers (GCs) year by year, the roles ofSOXgenes in GCs, including ovarian (OC), cervical (CC), and endometrial cancer (EC) become a recent focus of researches5. Studies investigating the relationship between aberrant expression ofSOXgenes and the development of GCs found that someSOXgenes with high expression level were expected to act as oncogenic regulators which promoting the progression of GCs, while those with low expression level were regarded as suppressors with the opposite effects6-11. Furthermore, regulating expression level ofSOXgenes in certain cancer cell lines could influence cell proliferation and apoptosisin vitrobut without known mechanisms12,13. Additionally, analysis of abnormalSOXgenes expression in patient samples contributed to the detection of early GC lesions14-16. Expression level ofSOXgenes was also considered as biomarkers associated with clinical features in patients with GCs, including International Federation of Gynecology and Obstetrics (FIGO) stage,histopathologic grade, and disease-free survival (DFS), as well as treatment response10,15,17-19. Understanding the mechanisms wherebySOXgenes regulate the progression of GCs is therefore valuable and may facilitate the development of novel diagnostic, therapeutic, and prognostic strategies which aimed at improving the prognosis of women with GCs. This review provides a systematic summary of theSOXgenes’ roles in the progression of GCs, and highlights future directions for research.
Classification and functions of SOX genes
Classification of SOX genes
SOXgenes encode a conserved group of regulatory transcription factors comprising about 414-amino-acid polypeptides with a highly conserved high-mobility group(HMG) box20,21. This box encodes around 79-amino-acid DNA-binding domain, with two L-shaped arms that can bind to ATTGTT or related DNA sequence motifs in the minor groove by recognizing the sequence 5′-(A/T)(A/T)CAA(A/T)-3′, resulting in widening of the minor groove, unwinding of the DNA helix, and DNA bending22. This domain was first identified in SRY, as a crucial factor involved in determining male sex of mammals. Genes that encode proteins containing an HMG domain with at least 50% amino acid similarity to the SRY HMG domain are considered asSOXgenes23. To date, mammalian genomes have been found to include approximately 30 differentSOXgenes, which can be classified into 10 subgroups (A-J) based on the degree of homology of the amino acid sequence inside the HMG domain, the presence of conserved motifs outside the HMG domain, and their full-length structures (Figure 1)24-26. In this review, we summarize the roles of some GC-relatedSOXgenes, includingSOX1,SOX2,SOX3,SOX4,SOX7,SOX8,SOX9,SOX11,SOX14,SOX15,SOX17, andSOX18.
Functions of SOX genes
The activities of the various subgroups ofSOXgenes are multi-faceted. Fundamentally,SOXgenes are involved in sex determination and the development of the testis, prostate,endothelial cells, and the vascular, lymphatic, and nervous systems during vertebrate embryonic development24,27.However, the multiple functions ofSOXgenes in the development of these various systems alerted researchers to their potential roles in the development of diseases, especially cancers12. Most recent studies ofSOXgenes focused on their involvement in gastric cancer, lung cancer, hepatocellular carcinoma, and prostate cancer2-4,28,29. Studies indicated that mostSOXgenes played their roles in these cancers through the Wnt/β-catenin signaling pathway, as the so-called‘canonical’ Wnt pathway mediated by β-catenin12,13. Activation of the Wnt/β-catenin signaling pathway decreases phosphorylation of β-catenin in the cytoplasm and increases β-catenin transfer into the nucleus. Consequently, it activates the nuclear complex of β-catenin/T cell factor/lymphoid enhancer factors, and enhances expression level of cell cyclerelated molecules such as cyclin-D1 and c-Myc12,30. Thus, a discussion of howSOXgenes act in the progression of GCsviadifferent signaling pathways, especially the Wnt/β-catenin signaling pathway is presented below.
SOX genes in GCs
Numerous studies currently focus on the roles ofSOXgenes in GCs. In this review, OC, CC, and EC are selected to be representative GCs due to their high incidence and mortality.In general,SOXgenes have been identified as regulators influencing the progression of GCs, as well as biomarkers of clinical features. Clinical, cellular, and animal experiments have shown that someSOXgenes act as oncogenes while others act as tumor suppressor genes in these three cancers(Figure 2). General depiction of the expression level ofSOXgenes in GCs and mechanisms of how they perform effectively are shown in Figure 3.

Figure 1 Classifications and structures of SOX families. (A) SOX genes contain approximately 30 different SOX genes which are classified into ten subgroups (A-J). These various groups are highlighted by different colors. Origins and connection between branches are based on the structures and function of SOX protein encoded by SOX genes. (B) Schematic representation of the structures of some known SOX protein. The HMG box, transactivation/repression domain and other functional domains are indicated along with the length of SOX proteins. Groups and representative protein members are indicated to the left. N-terminal and C-terminal domains of SRY are depicted at the top. The sizes in amino acids (aa) of the various SOX proteins are shown to the right.
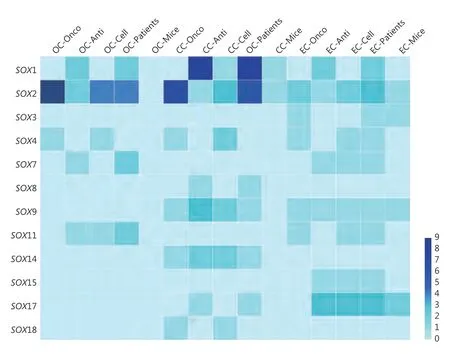
Figure 2 General summary of the researches on SOX genes in GCs. Darker blue coloration means more researches in the corresponding area as indicated in the legend on the right side of the heatmap. The number represents the corresponding number of researches. Onco:SOX that plays as an oncogene. Anti: SOX that plays as a tumor suppressor gene. Cell: researches based on cell lines. Patients: researches based on patients’ samples. Mice: researches based on nude mice.
SOX genes in OC
OC is one of the three most common cancers in women, with an estimated 22,240 new diagnoses and 14,070 deaths in the United States in 20185. Due to lack of specific symptoms and reliable screening methods, approximately 70% of patients with OC are diagnosed at advanced stage with metastasis beyond the ovary, which contributes to its high mortality31,32.Thus, there is an urgent need to find novel diagnostic biomarkers for detecting OC at a premalignant stage33.SOXgenes are identified as such biomarkers that can contribute to early screening and the prediction of clinical features in patients with OC, and regulation ofSOXgene expression could influence cell proliferation and treatment efficacy at the cellular level9,10,16,18,31,34.
SOX genes as clinical biomarkers for OC
Regarding their roles as clinical biomarkers, multiple studies analyzed the relationships betweenSOXgene expression level in OC samples and clinical features, including FIGO stage,histopathologic grade, and DFS (Table 1). Researchers identifiedSOX1,SOX7, andSOX11as tumor suppressor genes, with low expression level in patient samples due to aberrant CpG island hyper-methylation or unclear mechanisms17,18. Low expression level of these genes in cancerous tissues or serum was detected more frequently in patients with more advanced stage, higher grade, more aggressive tumor behavior, and shorter recurrence-free survival (RFS), while higher level was associated with the opposite clinical features10,17,18,35,40. These results suggested that analyzingSOXgene expression might be a good biomarker for predicting the prognosis of patients.
However, in addition to tumor suppressor role ofSOXgenes,SOX2was shown to play dual roles in OC, with high expression level identified as a poor prognostic biomarker in some cases, but as a favorable factor in other cases. On one hand, highSOXexpression level in fallopian tube epithelium was exploited as a biomarker for OC screening, especially inBRCA1orBRCA2mutation carriers or in women with serous OC in high grade16. High expression level ofSOX2in patient samples was also shown to be related to high grade, advanced FIGO stage, and decreased DFS36,37. On the other hand,Pham et al.38demonstrated that high expression level ofSOX2was a favorable biomarker indicating longer DFS and overall survival (OS) in patients with stage II-IV high-grade serous OC among 215 cases of OC. Other researchers also affirmed the good prognostic effects ofSOX2in 570 samples from patients with ovarian serous cystadenocarcinoma39. The mechanisms responsible for these different effects ofSOX2may be due to various factors, such as feedback mechanisms ofSOX2expression, interactions betweenSOX2in the cytoplasm and nucleus, or differences between patient spectra and measuring methods. These flexible and bidirectional roles ofSOX2suggest thatSOX2could be a‘double-edged sword’ depending on how scientists choose to utilize it. However, analyzing expression level ofSOXgene in tissues is still generally considered to be a valuable approach for predicting clinical features in patients with OC.

Figure 3 Comprehensive depiction of the roles of SOX genes performed in GCs. SOX genes play different roles in different GCs. The black upward arrows indicate high expression level of SOX genes in the corresponding cancers, which play as promoters. The black down arrows indicate low expression of SOX genes the corresponding cancers, which play as inhibitors. The black circles indicate that the roles of SOX genes are controversial. The genes or molecules shown in the surrounding colored circles are among the pathways through which SOX genes play in GCs to promote cell proliferation, inhibit apoptosis, and enhance cell metastasis.
SOX genes as regulators in the progression of OC
In addition to their role as clinical biomarkers, accumulating evidence fromin vitrostudies indicated thatSOXgenes acted as vital regulators in the progression of OC, including in cell proliferation, apoptosis, and metastasis (Figure 4).SOX2was considered as an oncogene at the cellular level, and upregulation ofSOX2in OC cell lines promoted cell proliferation and tumor sphere formationviahypoxic treatment and overexpression of the intracellular domain of Notch9. Moreover, transduction ofSOX2into OC cell lines also enhanced resistance to cell apoptosis through overexpression of the anti-apoptotic geneBCL2and simultaneous down-regulation of the pro-apoptotic genesPUMA/BBC3andNOXA/PAMAIP134. Overexpression ofSOX2also accelerated cell migration by down-regulating Ecadherin and up-regulating vimentin expression31. These results at the cellular level were not as the same as the results based on clinical samples, which suggested thatin vitrostudies could not imitate the environment in the body completely. And more animal studies are needed to clarify the role ofSOX2in the progression of OC. In addition toSOX2, up-regulation ofSOX4in OC cell lines by the long non-coding RNA BRM promoted cell proliferation,migration, and invasionviaan unknown mechanism62. More researches are therefore also needed to investigate the mechanisms and potential ofSOX4in OC.
SOX7andSOX11, which are considered as tumor suppressor genes at both the clinical and molecular level,inhibited the progression of OC. Low level ofSOX7together with increased cyclooxygenase-2 and cyclin-D1 were detected in tissues from patients with epithelial OC, especially in patients with advanced serous cystadenocarcinoma10. This suggested thatSOX7might regulate cell proliferation by influencing the Wnt/β-catenin signaling pathway.Transduction ofSOX11into OC cell lines was also reported to inhibit cell proliferation, though the mechanism remained unclear18. These members of theSOXgene family all contribute to the progression of OC, and their various
mechanisms warrant more detailed investigation. These results also suggested that controlling the expression of certainSOXgenes may be a promising therapeutic target in the near further.

Table 1 Abnormal expression of SOX genes and their potential clinical implications in gynecological cancers
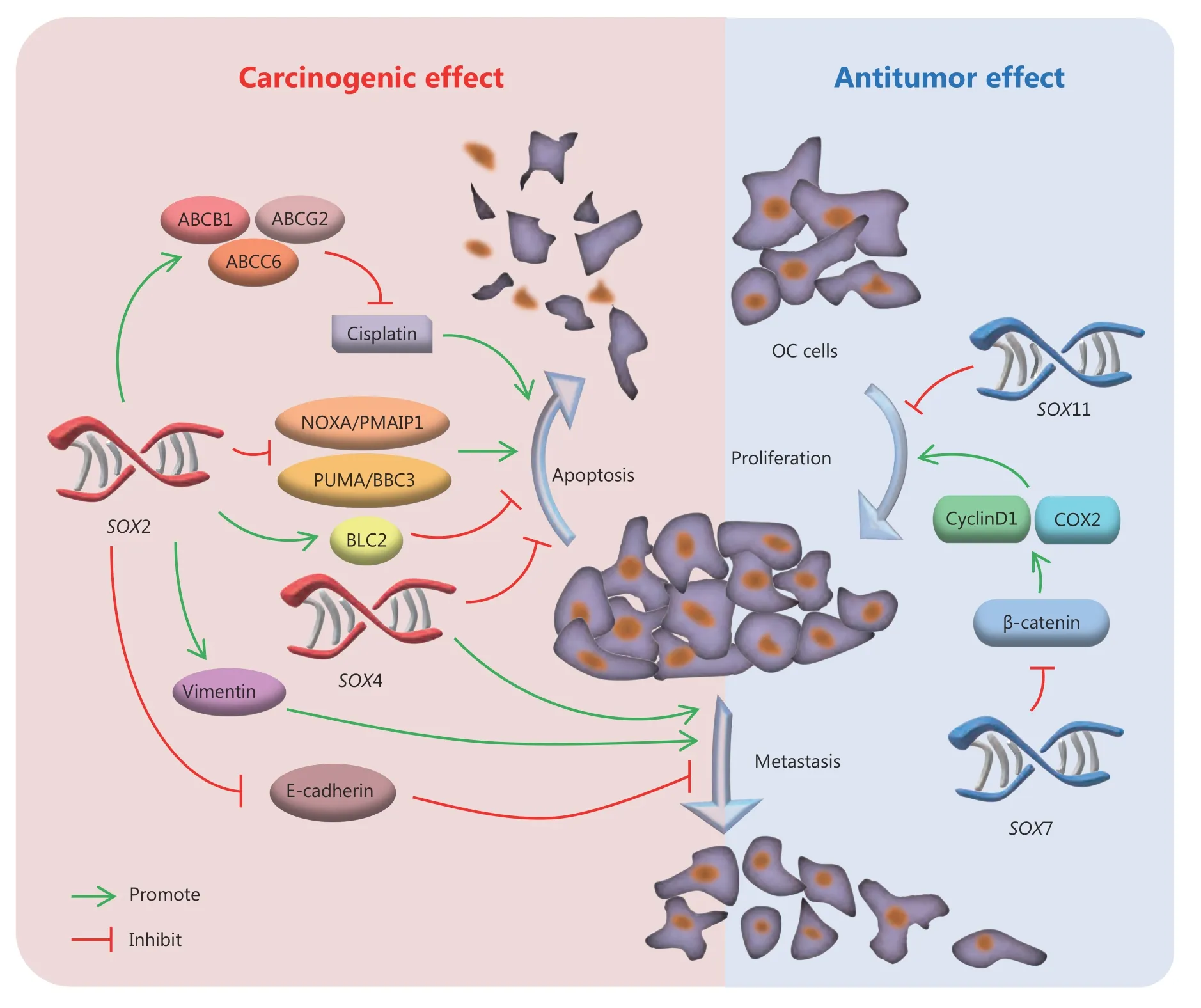
Figure 4 Roles of SOX genes (SOX2, SOX4, SOX7 and SOX11) in the progression of OC. SOX2 and SOX4 performed as oncogenes in red color. SOX7 and SOX11 acted as tumor suppressor genes in blue color. Small green arrows indicate promotion and small red arrows indicate inhibition. Cyclin D1 and Cyclooxygenase2 (COX2) are promoting factors in the cell cycle. E-cadherin is an intercellular adhesion molecule, and vimentin is a protein that helps epithelial cells maintain characteristics of fibroblasts such as weaken adhesion and enhance mobility.
SOX genes influence treatment efficacy in OC
In terms of treatment, overexpression ofSOX2was shown to enhance chemoresistance of OC cell lines to cisplatin through activating the expression of the drug efflux transporter genesABCB1andABCG29. Knockdown ofSOX2in cell lines accordingly decreased chemoresistance to cisplatinviadownregulating expression ofABCB1,ABCG2, andABCC631.These results were accordance with those of researches on the roles ofSOX2based on OC cell lines, but not with researches based on patients’ samples in other cases9,31,34,38,39. Further simultaneousin vitroandin vivoresearches intoSOX2are therefore needed to develop its role as a promising new therapeutic target for patients with OC.
SOX genes in CC
CC is a common GCs considered to be an accidental endpoint of persistent infections with certain types of human papillomavirus (HPV), especially HPV16 and HPV185,63.Some investigators found thatSOX2regulated HPV16 transcription by inhibiting activity of its long control region and finally decreasing expression of the E6 and E7 oncogenes in CC carcinogenesis64. Furthermore, mounting evidence implicated other members of theSOXgenes family in the regulation of CC progression (Figure 5) and identified their roles as biomarkers for predicting the prognosis of patients with CC (Table 1).
SOX genes as screening biomarkers and prognostic factors in CC
Analysis of the aberrant expression level ofSOX1,SOX8,SOX9,SOX14, andSOX17were identified as a novel early screening method for distinguishing between early CC lesions and normal tissues by methylated-CpG island recovery assay (Table 1). Higher methylation level of these genes was accompanied by more severe cervical squamous cell lesions14,41-46,55. Moreover, the sensitivity and specificity of this early screening method was increased by the combined detection of the methylation level of more than oneSOXgene, such asSOX1andSOX14, orSOX8andSOX1753,65.Further studies are therefore needed to determine if analyzing the expression level of allSOXgenes combined might increase their sensitivity as screening biomarkers for CC.
In terms of prognostic biomarkers,SOX2was identified as a dual-effect gene, with its expression level having different implications for clinical features in different studies. Four studies detected high level ofSOX2in tissues from CC patients and concluded that highSOX2expression was associated with higher grade, poorer differentiation,advanced stage, and poorer survival7,48-50(Table 1).However, the opposite effects were observed in other studies.For example, Kim et al.47detectedSOX2expression in tissue samples from normal cervical epithelium, cervical intraepithelial neoplasia, and CC by immunohistochemistry,and found that high expression level ofSOX2were correlated with favorable DFS and OS. The unknown mechanisms behind this phenomenon may help to account for the roles ofSOX2in OC at the clinical level. Nevertheless, more researches are needed to elucidate the precise mechanisms responsible for the effects ofSOX2.

Figure 5 Roles of SOX genes (SOX1, SOX2, SOX4, SOX9, SOX14 and SOX18) in the progression of CC. Oncogenes included SOX4, SOX2 and SOX18 in red color. SOX1 was regarded as suppressor gene in blue color. SOX9 and SOX14 played bidirectional roles in purple color. Small green arrows indicate promotion and small red arrows indicate inhibition. There were few studies of SOX4 and SOX18 yet. P21WAF1/CIP1 is a main kind of cyclin-dependent kinase inhibitor in cell cycle.
SOX genes regulate progression of CC
Similar to their roles in OC, numerous studies investigated the involvement ofSOXgenes in regulating the progression of CC (Figure 5). Consistent with an oncogenic role, some studies found that transduction of CC cell lines withSOX2,SOX4, andSOX18promoted cell proliferation, metastasis,and invasion52,66-68. Specifically, endogenous overexpression ofSOX2orSOX4in CC cell lines drove the cell cycle from G0/G1 to S stage by promoting expression of cell cycle promoters, such as cyclinE2, minichromosome maintenance protein 10 (MCM10), and weel protein kinase52,66.Overexpression ofSOX2in CC cell lines also promoted metastasis and invasion by augmenting the expression of epithelial-mesenchymal transition-promoted molecules,such as vimentin, β-catenin, and Snail67. However, the action ofSOX2at the cellular level did not necessarily reflect the same behavior in clinical samples because of the absence of the body’s internal environment.SOX18promoted the development of CC without clear mechanisms, so further studies are needed to clarify the mechanisms responsible for the oncogenic role ofSOX1868.
In contrast,SOX1is considered as a tumor suppressor gene, in line with its activity in clinical samples as mentioned above. Up-regulation ofSOX1inhibited the growth of CC cells bothin vitroandin vivoby impeding the transcriptional activity of T cell factor in the Wnt/β-catenin signaling pathway. It also promoted metastasis by up-regulating the cancer metastasis suppressor genecadherin 1(CDH1) and simultaneously down-regulatingSnail2, the inhibitor of Ecadherin transcription6. Future researches should focus on exploring howSOX1influence cell apoptosis and treatment efficacy.
Intriguingly, althoughSOX9andSOX14were regarded as tumor suppressor genes with low expression level detected in patients’ samples due to methylation, these twoSOXgenes presented dual functions based on research on cell lines. As oncogenes, some researchers reported that overexpression ofSOX9in CC cell lines promoted cell proliferation by downregulating the expression ofPTEN, which prevents cells from growing too quickly54. And overexpression ofSOX14were proved to boost cell proliferation and invasion by activating the Wnt/β-catenin signaling pathway along with high level of β-catenin69. In contrast, as tumor suppressor genes, upregulation ofSOX9orSOX14could block the cell cycle transition by activating the expression of p21WAF1/CIP1and p53, resulting in suppression of cell growth and tumor formationin vitro. Overexpression ofSOX14also induced apoptosis by promoting the expression of Bax and cleavedpoly ADP-ribose polymerase8,70. These interesting results indicated the need for more thorough investigations to compare and combine the effects of different experimental conditions on outcomes.
Effects of SOX genes on treatment efficacy in CC
In terms of treatment,SOX2andSOX4are considered as oncogenes accordance with their roles in the progression of CC. Superficially, expression level ofSOX2was higher in tissues from patients with radiation-resistance compared with those with radiation-sensitivity, suggesting thatSOX2was a biomarker of unfavorable therapeutic reactivity51.Overexpression ofSOXgenes, such asSOX4, in Caski cell lines also decreased the treatment efficacy of cisplatin by upregulating the drug efflux transporter geneABCG252. And more investigations are required to explore howSOXgenes influence treatment efficacy in patients with CC. In addition toSOX2andSOX4, one study implied that high expression ofSOX9in CC cell lines enhanced cell resistance to cisplatin through combining with the promoter region of miR-130a and down-regulating expression ofcopper transporter protein 1(CTR1), which is a significant factor affecting the activity of cisplatin54. However, this result was in contrast to the action ofSOX9in the progression of CC, and the function ofSOX9in the treatment of CC still needs clarification.
In conclusion,SOXgenes play significant roles in CC, andSOXmembers may act as promising biomarkers or therapeutic targets in clinical practice in the near future.
SOX genes in EC
EC, with an estimated 63,230 new cases and approximately 11,350 deaths, was regarded as the most common female reproductive system malignancy in the United States in 20185. Despite extensive research focusing on exploring the genetic and epigenetic characteristics of EC, its pathogenesis and progression remain unclear. Recently, some studies indicated the important roles forSOXgenes in regulating the progression of EC (Figure 6) and in indicating clinical features and treatment efficacy of patients with EC(Table 1)19,21,58,71,72.
SOX genes as biomarkers of clinical features in EC

Figure 6 Mechanisms of SOX genes (SOX2, SOX3, SOX4, SOX7, SOX9, SOX11, SOX15 and SOX17) in the development of EC. Oncogenes included SOX3, SOX4 and SOX11 in red color. Tumor suppressor genes consisted of SOX7, SOX15 and SOX17 in blue color. SOX2 and SOX9 played bidirectional roles in purple color. Small green arrows indicate promotion and small red arrows indicate inhibition. The mechanisms of SOX4, SOX11 and SOX15 have only been mentioned in a few researches yet. Akt promote growth factor-mediated proliferation and survival of cells both directly and indirectly. NF-κB is a protein complex that controlled transcription of DNA, cytokine production and cell survival. BCL2-associated X protein functions as an apoptotic activator. Cleaved caspase-3 is an executioner of apoptosis. Caspase-9 is an initiator of apoptosis. Survivin is an inhibitor of apoptosis. Mastermind like3 (MAML3) is a co-activator of β-catenin-mediated transcription.
Many researchers explored the roles ofSOXgenes in indicating the clinical features of patients with EC (Table 1).Low expression level of tumor suppressorSOXgenes, such asSOX1,SOX7,SOX9, andSOX17, due to methylation and other mechanisms, could be considered as novel prognostic biomarkers of EC. For example, low expression level ofSOX1,SOX7, andSOX17was shown to be potential biomarkers for detecting EC masked by atypical hyperplasia,and indicated advanced stage, higher grade, and shorter RFS19,21,56,57,60. Additionally, high expression level ofSOX17in tissues indicated increased toxicity of cisplatin and high therapeutic sensitivity of patients61. However,SOX9expression showed a significant stepwise increase from normal tissues through grade 1 to grade 2/3 cancer tissues,probably due to a hidden feedback system59. This study suggested that detecting the detailed mechanisms ofSOX9will provide valuable information.
Interestingly,SOX2is identified as a bi-functional gene in EC, as in OC and CC. Pityński et al.15analyzed expression level ofSOX2in samples from EC patients and found higher expression level of it in high-grade (G3) compared with moderate-grade (G2) and low-grade (G1) of EC. High expression level ofSOX2in tissues was also associated with poorer outcomes of patients with advanced-stage EC11. In contrast, Wong et al.58proposed thatSOX2was a tumor suppressor gene inhibiting the progression of EC, and low level of it was identified as an indicator of type II serous, clear cell adenocarcinoma as well as shorter survival. These phenomena suggested that further exploration of the molecular mechanisms ofSOX2should be carried out in relation to clinical management of EC.
SOX genes regulate progression of EC
In relation to the progression of EC, researchers regulated the expression ofSOXgene in EC cell lines by transduction with the correspondingSOXgenes. Through this method, they found that overexpression ofSOX3,SOX4, andSOX11promoted cell proliferation while overexpression ofSOX7,SOX15, andSOX17inhibited cell growth and accelerated apoptosis. Meanwhile,SOX2andSOX9were regarded as dual-function genes in the progression of EC (Figure 6).
As oncogenes, up-regulation ofSOX3,SOX4, andSOX11in EC cell lines was associated with accelerated cell proliferationviaunknown mechanisms, while silencing of these genes had the inverse effects72-74. Moreover,SOX3also promoted EC cell metastasisin vitroby down-regulating the epithelial marker E-cadherin and up-regulating the mesenchymal marker vimentin72. However, the roles ofSOX4andSOX11in promoting cell metastasis and invasion remain unclear.
SOX7,SOX15, andSOX17, regarded as tumor suppressor genes, inhibited cell proliferation through different signaling pathways. Enforced expression ofSOX7andSOX17, both belonging toSOXsubgroup F, played inhibitory roles in the growth and colony formation of EC cellin vitroby suppressing the accumulation of β-catenin in the Wnt/βcatenin signaling pathway19,21.SOX7also inhibited the downstream factors of β-catenin, such as cyclinD1, c-Myc and fibroblast growth factor 9 (FGF9), in the Wnt/β-catenin signaling pathway19. Cell lines with elevatedSOX17expression also demonstrated high apoptosis and low proliferation rates through up-regulating wild-type p53,Bcl2-associated X protein, and cleaved caspase-3 and caspase-9, while simultaneously down-regulating the level of survivin and mastermind like-321,61. Conversely, down-regulation ofSOX17increased the rate of cell proliferation in cell lines, and suggested that the low expression level ofSOX17may be due to frequent mutations, including missense, frameshift, and hotspot missense changes. They also detected a moderate increase in β-catenin, as a key regulator of EC, following transfection of EC cell lines with mutatedSOX1721,60. These results suggest that the regulation ofSOX17may vary in the progression of EC. In addition to the above genes,SOX15is a novel and vital gene in EC. And the expression level of it was at significantly lower level in EC tissues compared with adjacent uninvolved tissues from the same patient75. And upregulation ofSOX15in EC cell lines suppressed cell proliferation and viability by inducing cell-cycle arrest in G0/G1 stage, promoting cell apoptosis, and weakening cell migration, whereas knockout ofSOX15had the opposite effects. More researches are therefore needed to clarify the detailed mechanisms ofSOX1575.
Apart from these oncogenes above,SOX2andSOX9are considered as bi-directionally regulated genes in EC.Overexpression ofSOX2in cells promoted cell proliferation by inhibiting expression of p2111, while low level ofSOX2in tissues caused by promoter hyper-methylation was conversely accompanied by initiation of EC58. These phenomena reflecting the role ofSOX2as a biomarker of clinical features in patients with EC was possibly due to different locations ofSOX2and its currently unclear mechanisms. RegardingSOX9, Behringer et al.71found that overexpression ofSOX9in uterine epithelial cells in a progesterone receptor-Cre mouse model promoted the formation of more simple and complex cystic glandular structures. However, the results differed at the cellular level.Stable overexpression ofSOX9in EC cell lines served as a negative regulator of cell proliferation, particularly in the exponential growth phase, through activation of the p14ARF/p53/p21WAF1pathway and interactions with nuclear factor-κB and Akt59. These results revealed thatSOX9behaved differentlyin vitroandin vivo, waiting for further investigations. The roles ofSOX2andSOX9in EC thus remain largely uncertain.
Effects of SOX genes on treatment efficacy in EC
In relation to treatment of EC, onlySOX17was demonstrated an association with the chemosensitivity of EC cells to cisplatin. Zhang et al.61overexpressedSOX17in HEC-1B cells and found that cells with elevated expression ofSOX17had higher sensitivity to cisplatin, lower cell viability,and higher cell apoptosis rate when treated with cisplatin.These results suggest thatSOX17may be a promising target for gene treatment of EC.
Similarities of SOX genes in GC and prospective challenges
In this review, we classifiedSOXgenes and emphasized their critical roles as regulators in the progression of GCs. AndSOXgenes were also considered as biomarkers of clinical features, including FIGO stage, histological grade, treatment efficacy, and prognosis of patients with GCs.SOXgenes owned the potential to assist gynecologists in making precise clinical decisions. There were some similarities inSOXgenes among GCs. 1) Methylation analysis of the tumor suppressorSOX1gene in patients’ samples was regarded as a novel method for early screening and as a biomarker of clinical features. 2) Expression level of the dual-actingSOX2gene revealed low level in some cases and high level in other cases,indicating different prognostic values in different patients,and highlighting the need for more research to clarify its role.3) At a cellular level, transduction of the oncogeneSOX4in cell lines promoted the progression of GCs by enhancing cell proliferation and metastasis. 4)SOX7andSOX17, both belong to subgroup FSOXgenes, acted as tumor suppressor genes which were associated with decreased rate of progression. Given thatSOX3belongs to subgroup B withSOX1andSOX2, meanwhile,SOX18belongs to subgroup F withSOX7andSOX17. Researchers could investigate the roles ofSOX3andSOX18in GCs to see if they play similar roles to other members from the same subgroups.
The current review was limited to studies of the three most common GCs and certain histological subtypes, such as cervical squamous cell carcinoma, epithelial OC, and unexplained EC. Therefore, larger population-based studies of other kinds and subtypes of GCs, such as uterine myoma,uterine sarcoma, tumors of the fallopian tube, and vulvar squamous cell carcinoma, are warranted to further our understanding of the associations betweenSOXgenes and the progression of GCs. Although studies to date only scratched the surface in terms of understanding the biological and clinical functions ofSOXgenes in GCs, these pre-clinical studies held promise and provided the basis for future studies aiming at elucidating the detailed functions of these genes.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81572568 and 81272863).
Conflicts of interest statement
No potential conflicts of interest are disclosed.
 Cancer Biology & Medicine2019年3期
Cancer Biology & Medicine2019年3期
- Cancer Biology & Medicine的其它文章
- TNFα inhibitor C87 sensitizes EGFRvIII transfected glioblastoma cells to gefitinib by a concurrent blockade of TNFα signaling
- A four-gene signature-derived risk score for glioblastoma:prospects for prognostic and response predictive analyses
- Prediction of cervical lymph node metastases in papillary thyroid microcarcinoma by sonographic features of the primary site
- Decrease in the Ki67 index during neoadjuvant chemotherapy predicts favorable relapse-free survival in patients with locally advanced breast cancer
- Incidence, distribution of histological subtypes and primary sites of soft tissue sarcoma in China
- Prevalence and clinical significance of pathogenic germline BRCA1/2 mutations in Chinese non-small cell lung cancer patients
