Maintaining proton homeostasis is an essential role of the Warburg effect in proliferating cells
Chuangzhen Yang, Binghui Li,2,3
1Department of Biochemistry and Molecular Biology, Capital Medical University, Beijing 100069, China; 2Advanced Innovation Center for Human Brain Protection, Capital Medical University, Beijing 100069, China; 3Beijing Key Laboratory for Tumor Invasion and Metastasis, Capital Medical University, Beijing 100069, China
Non-proliferating cells efficiently generate adenosine 5’-triphosphate (ATP) through mitochondrial oxidative phosphorylation. By contrast, proliferating cells, including cancer cells, tend to rely on aerobic glycolysis, an inefficient way to generate energy, and this phenomenon is termed “the Warburg effect”1,2. However, the advantage of the Warburg effect provided for proliferating cells has been unclear3. Here we propose that aerobic glycolysis may maintain proton homeostasis to benefit proliferating cells.
A metabolic model of proton homeostasis
Proton homeostasis is one of the most important factors maintaining the microenvironment to enable metabolic reactions. Cells have several ways to maintain proton homeostasis mainly mediated by transporters alone or in combination, including antiporters, symporters and uniporters4(Figure 1A and 1B). Proton transport is usually coupled with ions or metabolic groups, otherwise it will result in membrane potential. When coupled with ions,proton transport simultaneously perturbs the involved ion homeostasis. In contrast to the actual re-distribution of protons in other ways, the carboxylic metabolites-associated protons can be chemically expanded or produced. In view of the obvious advantages, the metabolic pathway is naturally selected for cells to mainly maintain proton homeostasis during the evolution.
Cellular nutrients mainly include glucose and carboxylic metabolites, predominantly amino acids. Amino acids are centrally used to synthesize proteins or alternatively metabolized in mitochondria (Figure 1C). Glucose can be converted either to CO2and H2O in the mitochondria or to lactic acid that is excreted out of the cell, or used for thede novosynthesis of lipid (Figure 1C). In addition, glucoses and amino acids are also responsible for the synthesis of nucleic acids. These reactions are associated with redox couples, such as NADH/NAD+, FADH2/FAD and NADPH/NADP+, in addition to the generation or consumption of ATP.Intracellular redox couples have to keep balance overall,otherwise the metabolic reactions will break down. NADPH generated in the pentose phosphate pathway, which provides riboses for nucleic acid synthesis, needs balancing by fatty acid synthesis, the major intracellular consumer of NADPH.If the newly synthesized fatty acids exceed the requirement for lipid synthesis, they may enter mitochondria to undertake oxidation. The cycle of fatty acid synthesis and oxidation actually converts NADPH/NADP+to NADH/NAD+and FADH2/FAD, and the latter redox couples can be balanced by mitochondrial ATP generation. This explains why proliferating cancer cells have activede novofatty acid synthesis and sometimes simultaneously undertake fatty acid oxidation5,6. Carboxylic protons are converted to water mainly in the metabolism of amino acids or produced majorly in aerobic glycolysis. Proliferating cells have active anabolism and consume a great many amino acids, thus they have to require a compensation to maintain proton homeostasis. The limited solubility under the physiological condition disables CO2from mainly regulating proton homeostasis. Therefore, cells have no choice but to inescapably select lactic acid from aerobic glycolysis, the Warburg effect (Figure 1C). As the production of lactic acid is coupled with ATP/ADP cycle (Figure 1C), cells usually reduce the activity of oxidative phosphorylation7,8or straightforward hydrolyze ATP to favor aerobic glycolysis9.
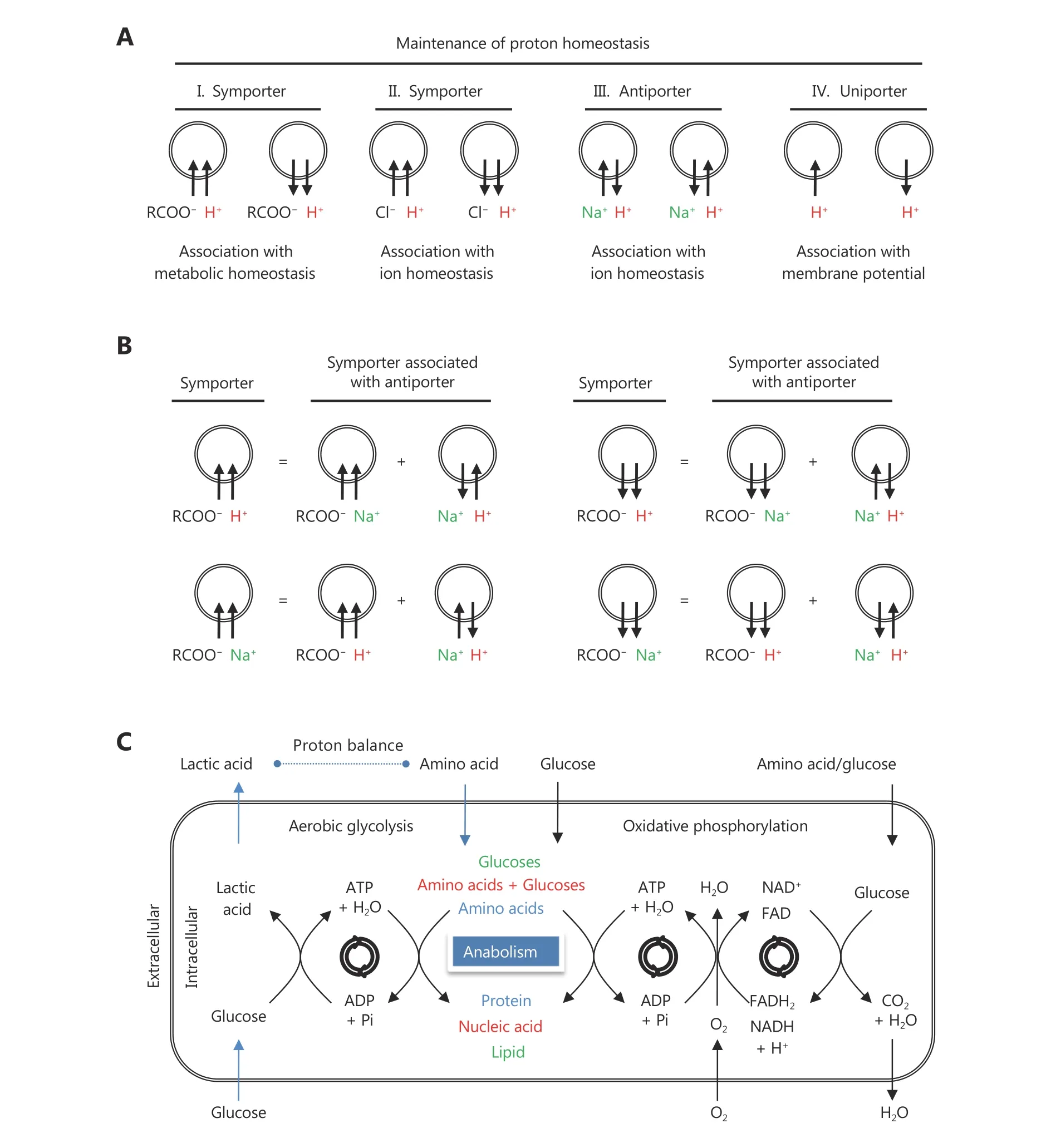
Figure 1 Maintenance of cellular proton homeostasis. (A) Proton transporters. RCOO-, carboxylic group; Cl- represents anion; Na+represents cation. (B) Combination of proton transporters. (C) Metabolic maintenance of cellular proton homeostasis. In the in vivo condition, the extracellular space is comparable to the intracellular volume, thus proton balance of aerobic glycolysis is required for consumption of amino acids.
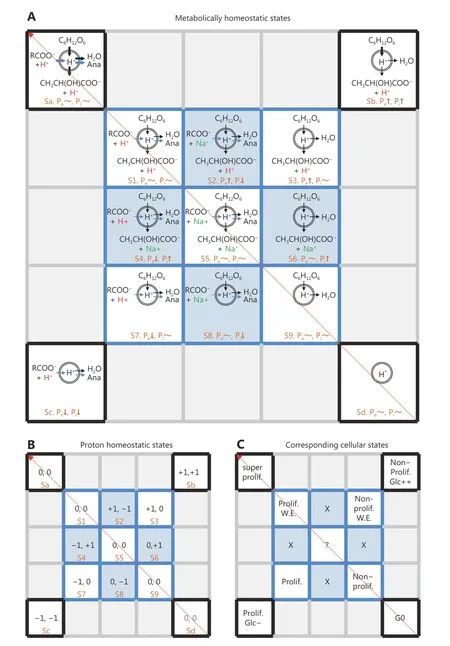
Figure 2 The theoretical model of cellular proton homeostasis. (A) States of cellular metabolic homeostasis based on metabolic flux.RCOO-, carboxylates, mainly amino acids; Na+ represents cation; Ana, anabolism. (B) States of cellular proton homeostasis based on metabolic states in (A). Pe, extracellular protons; Pi, intracellular protons. +1, increased protons; -1, decreased protons; 0, balanced proton flux. (C) The states of cell proliferation corresponding to (A) and (B). S1, proliferation with the Warburg effect; S3, non-proliferation with the Warburg effect; S7, proliferation without the Warburg effect; S9, non-proliferation. Sa, super-proliferation with the Warburg effect; Sb, nonproliferation with overactive glucose consumption, i.e. anaerobic glycolysis; Sc, proliferation without glucose; Sd, dormancy.
Notably, in the physiological condition, all carboxylic groups exist as the unprotonated forms, thus cells could absorb or excrete protonated carboxylic acids or unprotonated carboxylates directly by single symporters or indirectly by symporters associated with antiporters(Figure 1B). In contrast, carboxylic metabolites are always produced or consumed as the protonated forms, thus the protonated carboxylic acids are simply the best participators in the cellular metabolic flux. Here we listed all nine possible metabolic states based on the order of protonated carboxylic acids, unprotonated carboxylates or nothing in the metabolic flux (Figure 2A). We analyzed in theory the intracellular or extracellular proton homeostasis based on the production or consumption of protons. If extracellular protons are absorbed or intracellular protons are consumed by cells, the extracellular proton concentration (Pe) or intracellular proton concentration (Pi) will decrease, and -1 is assigned to this situation. On the contrary, +1 is assigned to the increased protons that are excreted from or produced by cells. 0 is assigned to the balanced proton flux. Among all states of metabolic homeostasis in a 3X3 matrix, S2, S4, S6 and S8 cells are not able to maintain their intracellular proton homeostasis (Figure 2A and 2B), thus they are doomedly eliminated during the evolution. S1, S5 and S9 cells have the balanced proton homeostasis (Figure 2B). S1 represents proliferating cells with the Warburg effect,including cancer cells, while S9 cells are corresponding to non-proliferating cells (Figure 2C). Up to date, no ioncoupled transporter responsible for excretion of metabolites is reported, which makes sure that lactic acid, not lactate, is carried out of cells by monocarboxylate transporters4to support proton homeostasis. This also means that S5 cells have to secrete carboxylates using symporters in combination with antiporters (Figure 1B). S5 having fussy metabolic flux may denote proliferating normal cells or a set of cells evolving to uncontrolled proliferating cancer cells (from S9 to S1). Interestingly, S3 and S7 cells could not keep alone their balanced extracellular environment but they do it together (Figure 2B). Upon co-survival with S3 cells, S7 cells can proliferate without the Warburg effect that is actually provided by non-proliferating S3 cells (Figure 2C). The reverse Warburg effect was recently frequently reported in cancer-associated fibroblasts10,11. Therefore, S7 cells could not survive well alonein vitro. They might represent a subset group ofin vivocancer cells that are beyond our current reach, and they are probably related to the clinical resistance and invalidation of anticancer drugs, most of which are developed based on the knowledge obtained from the studies on the cultured cancer cells, S1 cells.
There are totally nine different combinations for proton homeostasis based on the rule of product. According to the arrangement pattern of the S3-S7 diagonal, the missed{-1, -1} and {+1, +1} should be Sc and Sb at the corners of an expanded 5X5 matrix, while {0, 0} and {0, 0} are symmetrically filled in the two other corners, Sa and Sc(Figure 2B). This 5X5 matrix displays interesting complementary flanks of Sd-Sa (Figure 2B). Considering the increasing metabolic activities in cells at the Sd-Sa diagonal,Sa cells are expected to have an amplified metabolic flux while Sd should represent dormant cells with the lowest metabolic activity (Figure 2C). Sb cells actively produce lactic acids over their efflux (Figure 2B), and this usually happens upon anaerobic glycolysis (Figure 2A and 2C). Sc cells are deficient in proton provision (Figure 2B), meaning that they are proliferating in the absence of glucose (Figure 2A and 2C). Cancer cellsin vivohave much fewer available glucoses than normal cells due to their active glucose consumption and the poor tumor vasculature, and often suffer from temporary glucose deficiency8, thus Sc may represent a common situation of cancer cells.
The survival strategies of cancer cells in the absence of glucose
According to the proton homeostasis law, Sc cells at least have three strategies to overwhelm proton insufficiency resulting from glucose deficiency. Firstly, for the long-term adaption, cells have to open up the proton source by genetically increasing glucose uptake, which is achieved through over-expressing glucose transporters, especially GLUT38, or by utilizing other substitutive carbohydrates,such as fructose, a sugar widely existing in our diet. Secondly,cells may cut down the proton consumption by reducing anabolic activity, because active anabolism in proliferating cells needs to consume a large number of protons (Figure 1C).Hence, in such a condition, cell growth inhibition might help cancer cells survive upon glucose insufficiency. On the contrary, promotion of cell growth could inducein vivocell death. Indeed, it has been reported that activating cellular activities can kill some subsets of cancer cells by inducing massive metabolic stress, such as ROS and ER stress, and this cell death depended on increased anabolism12,13. However, it was already observed that once cancer cells adapted to such treatments, they evolved to have highly active metabolism and a 10-fold increase in GLUT312. This means that Sc cells have been converted to Sa cells by genetically improving glucose availability (Figure 2A). Therefore, inhibition of GLUT3 may validate this kind of treatment. Thirdly, Sc cells may directly survive upon proton supplies in store around them. Our previous report showed that acidic medium blocked glucose deprivation-induced cell death in various cell lines14,15. This is consistent with a fact thatin vivotumors often survive in an acidic environment16, which thus seems to be helpful to the survival of cancer cells against glucose deficiency. We also observed that cells grew well in relatively alkaline conditions in the presence of glucose14,15. This most likely resulted from the compensation by stimulated lactate production in alkaline conditions. These results further confirm that aerobic glycolysis exerts a critical role in maintaining proton homeostasis.
Conclusions
Proliferating cells must comply with the proton homeostasis law, which majorly relies on aerobic glycolysis. Once the nutritional condition is not ready, proliferating normal cells usually stop growing by checkpoints in different stages,whereas proliferating cancer cells keep going due to loss of checkpoints. Uncontrolledly proliferating cancer cells could evolutionarily accommodate any gene mutation that favors proton homeostasis. Therefore, dissection of how cancer cells maintain proton homeostasis to support their proliferation may expose their common Achilles’ heel. It should be noted that the Warburg effect, as the most famous hallmark of metabolic reprogramming, may sustain cell proliferation in many ways in addition to maintaining proton homeostasis.
Acknowledgements
We would like to thank Dr. Wei Du (University of Chicago,USA) for constructive discussions and suggestions. This work is supported by National Natural Science Foundation of China (Grant No. 81622037 and 81672762), Beijing Municipal Natural Science Foundation (Grant No. 5194023)and Support Project of High-level Teachers in Beijing Municipal Universities in the Period of 13th Five-year Plan(Grant No. CIT&TCD20190333).
Conflict of interest statement
No potential conflicts of interest are disclosed.
 Cancer Biology & Medicine2019年3期
Cancer Biology & Medicine2019年3期
- Cancer Biology & Medicine的其它文章
- TNFα inhibitor C87 sensitizes EGFRvIII transfected glioblastoma cells to gefitinib by a concurrent blockade of TNFα signaling
- A four-gene signature-derived risk score for glioblastoma:prospects for prognostic and response predictive analyses
- Prediction of cervical lymph node metastases in papillary thyroid microcarcinoma by sonographic features of the primary site
- Decrease in the Ki67 index during neoadjuvant chemotherapy predicts favorable relapse-free survival in patients with locally advanced breast cancer
- Incidence, distribution of histological subtypes and primary sites of soft tissue sarcoma in China
- Prevalence and clinical significance of pathogenic germline BRCA1/2 mutations in Chinese non-small cell lung cancer patients
