中国望都地区夏季大气气溶胶挥发性特征
魏林通,曹礼明,魏 静,何凌燕,黄晓锋
中国望都地区夏季大气气溶胶挥发性特征
魏林通,曹礼明,魏 静,何凌燕,黄晓锋*
(北京大学深圳研究生院,城市人居环境科学与技术实验室,广东 深圳 518055)
采用热扩散管与气溶胶质谱联用系统对2014年夏季河北望都乡村点位亚微米级气溶胶进行在线测量,获取了两段污染过程的气溶胶化学组成及挥发性特征:相对低污染期气溶胶平均质量浓度为(23.3±15.1)µg/m3,有机物占主导,主要受偏北方向气团影响;重污染期平均浓度为(86.6±19.7)µg/m3,硫酸盐占主导,受偏南方向气团影响;主要化学组分挥发性顺序均为硝酸盐>氯盐>铵盐>有机物>硫酸盐;与相对低污染期相比,重污染期的硫酸盐对质量浓度贡献更高且挥发性降低,而硝酸盐表现出更高的挥发性;对有机气溶胶而言,重污染期有机物氧化态更高且挥发性更低,老化特征明显.气溶胶半挥发性特征反映了华北夏季高污染条件下区域传输的重要作用.
气溶胶挥发性;热扩散管;TD-AMS;有机气溶胶
大气气溶胶是我国大气污染的主要因素之一,对人体健康、大气能见度、大气化学和辐射平衡、全球气候变化等具有重要影响[1-3].目前国内研究多集中在利用离线、在线方法对气溶胶理化特性时空分布等进行观测[4-5].华北地区大气气溶胶污染也是严重的环境问题之一[4,6-7].半挥发性是大气气溶胶重要的理化性质之一,决定气溶胶中各化学组分在颗粒相和气相中的分配,并进一步影响气溶胶在大气环境中的浓度、反应速率和停留时间等[8-10],主要取决于颗粒物化学组成、粒径、浓度和混合态特征等[11-13].当气溶胶样品被加热或被干净空气稀释,易挥发性物质会从颗粒相进入气相,低挥发性物质则仍留在颗粒相中[14],导致浓度和粒径等性质发生变化[15].气溶胶挥发性也可反映大气演化过程,对研究二次气溶胶的形成机制有一定的参考作用[16-18],挥发特性间接可反映颗粒物对人体的健康效应[3].
近些年,有机气溶胶挥发性的测量得到了重视,展开了很多外场观测和实验室测量[19-21]. 目前挥发性测量常用方法是通过热扩散管对颗粒物进行热分馏,将其与不同仪器联用,如挥发性串联差分电迁移率分析仪(VTDMA),TD-AMS联用方法等获取粒径组成、化学组分及挥发性等信息[2,22-25],探究二次气溶胶生成和老化过程[16].目前我国相关观测结果较少,对于不同组分半挥发性特征尚未有较为清晰的研究结果[26-27].
本研究利用TD-AMS联用系统于2014年对望都夏季亚微米级气溶胶化学组分进行了高分辨率在线测量,对不同污染水平下化学组成特征及气溶胶半挥发性特征进行评估,结合气象条件等因素进一步探究我国华北地区大气污染水平及成因,为改善华北地区空气质量提供数据支撑,并可以为模型研究提供数据参考.
1 仪器与方法
1.1 观测点位与时间
望都点位(38.7°N, 115.2°E)设于中国河北保定望都县交通局绿化基地内,处于北京上风向,地处京津冀三角地带,周围无显著工业污染源,属于典型乡村点位,可反映华北地区大气污染区域性特征.PM2.5采样头距离集装箱顶部约2m,距离地面约10m.
本研究对2014年河北望都夏季亚微米级气溶胶进行了高分辨率在线测量,由于野外电力保障不足等,共获取了2014年望都夏季观测期间6月6日~6月9日(相对低污染期,ep1)和7月3日~ 7月8日(重污染期,ep2)两段时期的有效数据,表1给出了ep1和ep2的气象参数、气态污染物浓度的平均情况.

表1 观测期间气象参数与气态污染物浓度
1.2 TD-AMS联用技术及数据分析方法
本研究联用美国Aerodyne公司的热扩散管(TD)和高分辨率飞行时间气溶胶质谱仪(HR-ToF-AMS)获取亚微米级颗粒物的化学组成和挥发性特征.应用Magee公司黑碳仪(Aethalometer,AE-31)获取黑碳气溶胶质量浓度,黑碳仪采用PM2.5切割头,根据Lan等[28]研究PM1中BC质量浓度可能偏高约20%.
气溶胶经TD进AMS,TD内流量0.45L/min,加热管中停留时间约为27.9s,HR-ToF-AMS主要用来在线测量真空动力学粒径小于1μm的非难熔亚微米级颗粒物(NR- PM1),包括有机物、SO42-、NO3-、NH4+、Cl-等.在观测开始时,对AMS的进样流量、电离效率和粒径进行标定.通过SQUIRREL、PIKA软件分析,并经过流量、电离效率、采集效率校正,得到各化学组分的质量浓度,以及有机物的质谱信息[29],其中采集效率根据文献[30]中基于化学组成的方法进行计算.TD由加热管和扩散管组成,切换阀可在加热气路(TD)和旁路(BP)中交替,加热气路在50,100,150,200℃4个温度梯度中循环变化[27,31-32]. AMS的V模式灵敏度高,用于定量测量;W模式分辨率更高,获取高分辨率质谱数据,将AMS设为4个采样模式,分别为BPV、TDV、TDW、BPW,经传输效率校正分析气溶胶的化学组分及挥发性特征[31].挥发性特征以加热气路与旁路质量浓度之比的质量剩余分数(MFR)进行半定量描述[20,22], MFR越高,挥发性越低.
2 结果与讨论
2.1 环境气溶胶浓度水平及化学组成特征
图1显示ep1和ep2的PM1(此处表示NR-PM1与黑碳的总和,下同)平均浓度分别为(23.3±15.1) µg/m3和(86.6± 19.7) µg/m3,各化学组分占比及质量浓度如表2所示,ep1有机物占主导,占重构PM1总质量浓度的38.5%,ep2硫酸盐占主导,占38.1%.Hu[33]利用AMS观测北京夏季PM1平均质量浓度为(84.0±47.4)µg/m3,与本观测ep2结果相近.
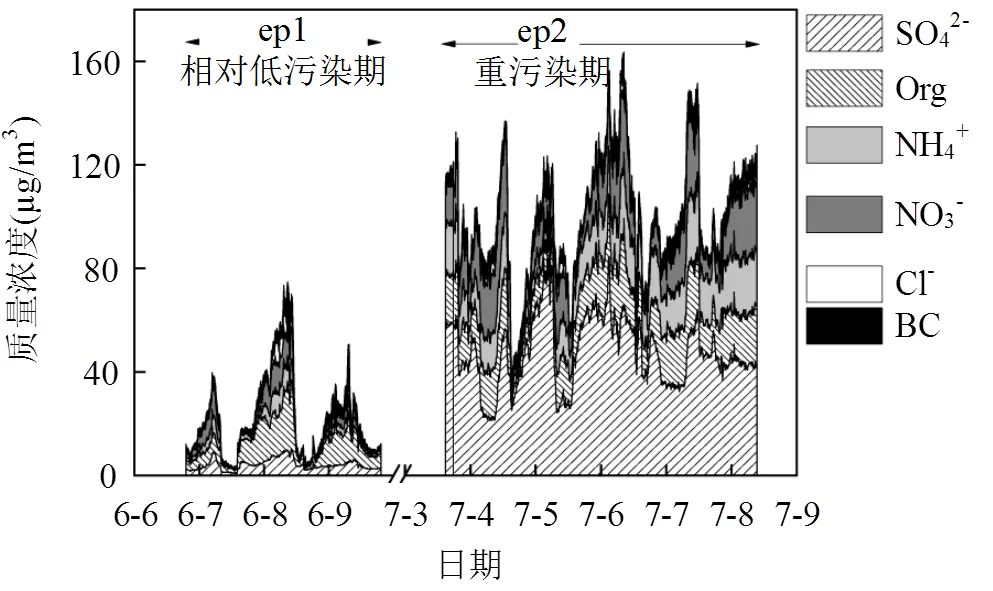
图1 2014年望都观测期间气溶胶化学组分时间序列
研究表明O/C值是反映有机物老化程度的重要参数[16,34].环境温度下ep1和ep2的O/C值分别为(0.51 ± 0.09)和(0.63 ± 0.05),与背景点洪泽湖春季结果0.53及北京夏季观测结果(0.56 ± 0.1)接近,高于北京冬季观测结果(0.32 ± 0.07)[33,35],说明望都夏季有机物整体氧化态较高,主要来自于老化的污染物;在污染程度更高的ep2,O/C值也更高,因此ep2有机物氧化程度更高,可以说明ep2老化程度较高的有机气溶胶占主导,体现了一定程度的区域传输特征,ep1受到相对较轻的影响.
此外,利用Hysplit软件分别对两段时间内的气团轨迹进行分析,两段时期的反向轨迹如图2所示,ep1的气团主要来自于望都附近地区(反向轨迹路径较短)以及西北方向即河北北部及远处的内蒙地区,ep2的气团主要来自于望都偏南向的河北南部、山东.结合化学组成特征,ep2的二次组分占比更高(ep1和ep2硫酸盐占PM1分别为17.8%和38.1%),且污染程度显著加剧,说明来自于南部的区域传输对ep2的污染过程有决定性影响.

表2 望都夏季观测期间PM1化学组分质量浓度
2.2 大气气溶胶挥发性特征分析
如图3所示,ep1和ep2各化学组分经过加热后都表现出不同程度的挥发性特征,但MFR随温度变化特征却表现出一定的相似性.其中,NO3-、Cl-挥发性最高,从环境温度加热至50℃时,MFR值显著降低,ep1和ep2的NO3-的MFR值分别为0.45和0.27,Cl-的MFR值分别为0.58和0.30,约50%的NO3-、Cl-受热挥发进入气相.
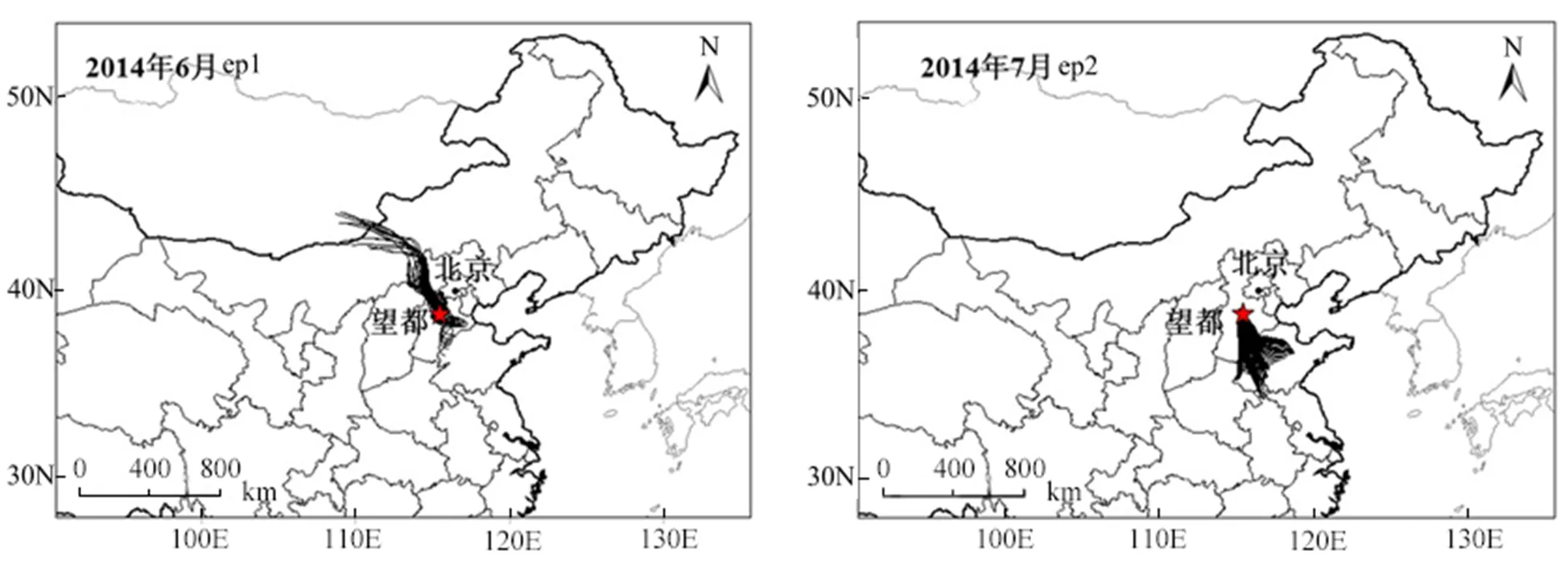
图2 观测期间两段污染过程的反向轨迹图
随温度增加,MFR继续下降并渐趋平缓,加热到200℃时,ep1和ep2NO3-的MFR值分别为0.21和0.09,Cl-的MFR值分别为0.19和0.11.SO42-挥发性最低,加热到50℃时MFR仍高于0.8,从100℃升高到150℃后,SO42-的MFR才显著降低,当加热至200℃时,MFR约为0.1~0.2.由于NH4+在颗粒物中主要是以(NH4)2SO4、NH4NO3和NH4Cl的形式存在,因此其MFR是SO42-和NO3-、Cl-综合作用的结果,介于其之间.ep1与ep2不同加热温度下SO42-的MFR较为接近,但与ep1相比,ep2的SO42-占比更高,因此在ep2中,NH4+与SO42-挥发性特征更为相似.此外,有机物的挥发性居中,MFR随温度升高而平稳降低,这是由于有机物化学组成较为复杂,由多种挥发性不同的有机物混合而成.这与其他挥发性观测结果相一致[20,31].因此,5种NR-PM1组分的挥发性顺序是硝酸盐>氯盐>铵盐>有机物>硫酸盐.较高组分的比例不断增加,说明具有半挥发性的有机物氧化程度较低,间接说明了有机物的挥发性随着老化程度升高而降低,这与VBS模型[36]及以往观测的结果相一致[16].
2.3 不同污染程度气溶胶化学组分挥发性特征
2个观测时段的亚微米级气溶胶化学组分的MFR值和质量浓度均存在一定相关性,因此进一步探究气溶胶化学组分挥发性特征与自身浓度之间的关系,可以对影响挥发性的相关因素进行判定.图4是50°C下气溶胶化学组分的挥发性与自身质量浓度间的关系.
ep1和ep2无机组分浓度有较大差距,尤其SO42-的质量浓度存在显著差异,图4(b)显示ep1中SO42-的MFR更低,且较离散,表现出更多新鲜生成的特征,ep2中SO42-挥发性有所降低,随着浓度升高MFR趋于稳定,但ep1与ep2的SO42-浓度水平差距较大,因此本地二次反应生成不足以贡献ep2的高SO42-质量浓度水平,反映出望都作为区域背景点,重污染时期受区域传输影响更为显著.图4(c)显示,随质量浓度增加,NO3-挥发性先升高后趋于稳定,可能是由于高污染时期,更多新鲜二次NO3-附在气溶胶表面,在加热时更易通过挥发进入气相中;NH4+的MFR结果与环境大气中的形式有关,Cl-本身浓度较低,因此NH4+、Cl-的结果较为分散.图4(a)显示,对有机物而言,ep1和ep2的质量浓度水平差异较小,ep1期间随有机物质量浓度增加,MFR变化较为平稳,相比之下ep2的MFR相对更高,在一定程度上说明ep2中老化程度较高的组分占主导,更不易挥发,这与SO42-的分析结果相一致.图4(e)为NR-PM1的MFR,体现了气溶胶各化学组分整体的挥发性特征,表明ep2颗粒物整体的挥发性低于ep1,根据上述分析,NO3-挥发性最高且随浓度升高而升高,因而在重污染期,可能是由于颗粒物的有机物和SO42-组分外层包裹了更多的NO3-,使得NR-PM1整体挥发性表现出随浓度升高而降低的特征.
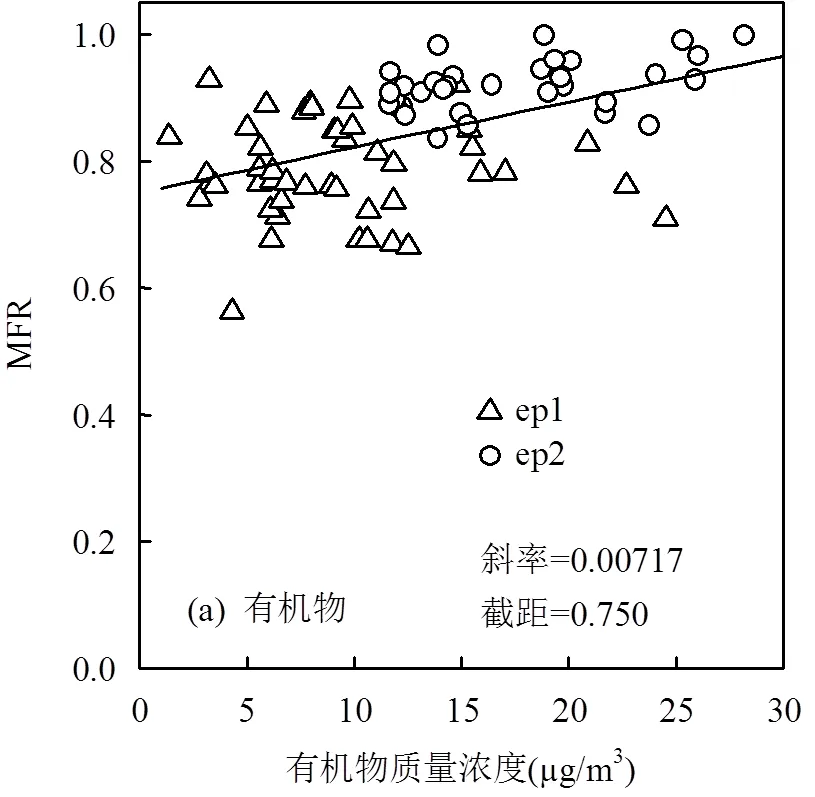

Fig.5Mass-based campaign-averaged size distributions of chemical components of NR-PM1during two episodes
图5显示环境温度与50℃加热条件下各组分粒径分布形状相近且峰值粒径基本一致,说明不同粒径段颗粒物半挥发性差异不大.与之前研究结果相同[20,32].ep2各化学组分峰值粒径偏大,有机物和硫酸盐尤为明显,从粒径角度验证两段污染过程可能具有不同来源,且ep2颗粒物老化程度相对更高.
2.4 大气亚微米级有机气溶胶挥发性水平分析
不同有机气溶胶挥发性也存在差异.由于本次观测获取的数据量有限无法进行气溶胶质谱正矩阵因子(PMF)解析,选取具有代表性的碎片进行讨论./43(主要离子为C3H7+和C2H3O+)与/44(主要离子为CO2+)可作为氧化态有机气溶胶的特征离子碎片,如图6所示,/44挥发性最低,/43挥发性居中;/57(C4H9+和C3H5O+,以C4H9+为主)和/60(主要离子为C2H4O2+)分别是还原态有机气溶胶和生物质燃烧特征碎片,表现出相对较高挥发性.有机物整体挥发性居中,与前人研究结果相似[20].污染程度更重的ep2/44的MFR更低,且有机物半挥发性更接近于/44,说明与ep1相比,ep2期间有机物中更多高氧化态、老化的有机气溶胶占主导,与2.2中对O/C值的分析结果一致,与香河夏季观测结果相似[32].表3表明,与ep1相比,ep2的/44增加倍数远高于/57,说明在有机物中,二次有机组分增加显著,而对无机组分而言,SNA(SO42-、NO3-和NH4+)增加近5倍,也显著高于代表一次污染的BC.总体而言,ep2二次有机和无机组分增加倍数均高于ep1,验证了二次污染在重污染期中占主导地位.

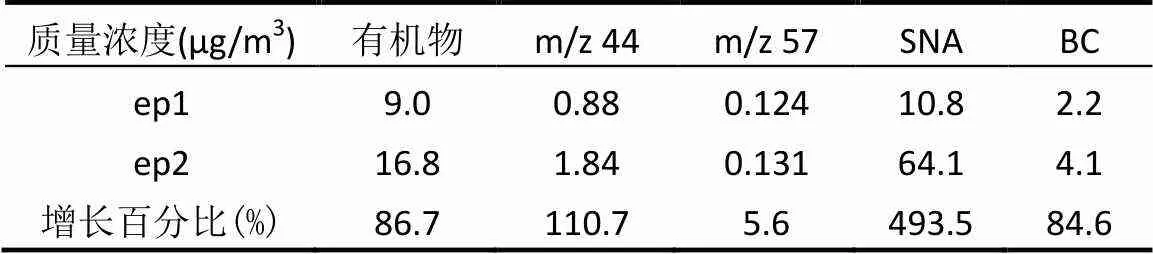
表3 两段污染过程部分有机碎片及各组分质量浓度
3 结论
3.1 观测期间,相对低污染期气溶胶平均浓度为(23.3±15.1) µg/m3,有机物占主导,反向轨迹表明主要受偏北方向气团影响,重污染期平均浓度为(86.6± 19.7)µg/m3,硫酸盐占主导,受偏南方向气团影响.
3.2 亚微米级气溶胶主要化学组分中,挥发性顺序为硝酸盐>氯盐>铵盐>有机物>硫酸盐.与相对低污染期相比,重污染期的硫酸盐对PM1质量浓度的贡献高且具有更低的挥发性,而硝酸盐在高污染下表现出更高的挥发性和更为新鲜的生成特征.
3.3 对有机气溶胶而言,随温度增加,O/C升高,意味着大部分具有半挥发性的有机物氧化态较低.而在重污染期,有机物氧化态更高,且挥发性更低,表明重污染期区域传输的影响显著加大.
[1] Stocker T F, Qin D, Plattner G K, et al. IPCC. Climate change: The physical science basis – contribution of Working Group I to the fifth assessment report of the intergovernmental panel on climate change [R]. 2013,18(2):95-123.
[2] An W J, Pathak R K, Lee B, et al. Aerosol volatility measurement using an improved thermodenuder: Application to secondary organic aerosol [J]. Journal of Aerosol Science, 2007,38(3):305-314.
[3] Biswas S, Verma V, Schauer J J, et al. Oxidative Potential of Semi- Volatile and Non Volatile Particulate Matter (PM) from Heavy-Duty Vehicles Retrofitted with Emission Control Technologies [J]. Environmental Science & Technology, 2009,43(10):3905-3912.
[4] Li M, Li X, Li Y J, et al. Real-time chemical characterization of atmospheric particulate matter in China: A review [J]. Atmospheric Environment, 2017,158:270-304.
[5] 周 甜,闫才青,李小滢,等.华北平原城乡夏季PM2.5组成特征及来源研究[J]. 中国环境科学, 2017,37(9):3227-3236. Zhou T, Yan C Q, Li X Y, et al. Chemical characteristics and sources of PM2.5in urban and rural sites in the North China Plain during summer [J]. China Environmental Science, 2017,37(9):3227-3236.
[6] Huang R, Zhang Y, Bozzetti C, et al. High secondary aerosol contribution to particulate pollution during haze events in China [J]. Nature, 2014,7521(514):218-222.
[7] Lang J, Li S, Cheng S, et al. Chemical Characteristics and Sources of Submicron Particles in a City with Heavy Pollution in China [J]. Atmosphere, 2018,9(10):388.
[8] Donahue N M, Robinson A L, Trump E R, et al. Volatility and Aging of Atmospheric Organic Aerosol [M]. Atmospheric and Aerosol Chemistry, Mcneill V F, Ariya P A, Berlin, Heidelberg: Springer Berlin Heidelberg, 2012.
[9] Pankow J. An absorption model of the gas/aerosol partitioning involved in the formation of secondary organic aerosol [J]. Atmospheric Environment, 2007,2(41):75-79.
[10] Donahue N M, Kroll J H, Pandis S N, et al. A two-dimensional volatility basis set – Part 2: Diagnostics of organic-aerosol evolution [J]. Atmospheric Chemistry and Physics, 2012,12(2):615-634.
[11] Wehner B, Berghof M, Cheng Y F, et al. Mixing state of nonvolatile aerosol particle fractions and comparison with light absorption in the polluted Beijing region [J]. Journal of Geophysical Research- Atmospheres, 2009,114(D00g17).
[12] Zhang S L, Ma N, Kecorius S, et al. Mixing state of atmospheric particles over the North China Plain [J]. Atmospheric Environment, 2016,125(A):152-164.
[13] Faulhaber A E, Thomas B M, Jimenez J L, et al. Characterization of a thermodenuder-particle beam mass spectrometer system for the study of organic aerosol volatility and composition [J]. Atmospheric Measurement Techniques, 2009,2(1):15-31.
[14] Louvaris E E, Karnezi E, Kostenidou E, et al. Estimation of the volatility distribution of organic aerosol combining thermodenuder and isothermal dilution measurements [J]. Atmospheric Measurement Techniques, 2017,10(10):3909-3918.
[15] Saha P K, Khlystov A, Grieshop A P. Downwind evolution of the volatility and mixing state of near-road aerosols near a US interstate highway [J]. Atmospheric Chemistry and Physics, 2018,18(3):2139- 2154.
[16] Jimenez J L, Canagaratna M R, Donahue N M, et al. Evolution of Organic Aerosols in the Atmosphere [J]. Science, 2009,326(5959): 1525-1529.
[17] Kroll J H, Seinfeld J H. Chemistry of secondary organic aerosol: Formation and evolution of low-volatility organics in the atmosphere [J]. Atmospheric Environment, 2008,42(16):3593-3624.
[18] Cappa C D, Jimenez J L. Quantitative estimates of the volatility of ambient organic aerosol [J]. Atmospheric Chemistry and Physics, 2010,10(12):5409-5424.
[19] Lee B H, Kostenidou E, Hildebrandt L, et al. Measurement of the ambient organic aerosol volatility distribution: application during the Finokalia Aerosol Measurement Experiment (FAME-2008) [J]. Atmospheric Chemistry and Physics, 2010,10(24):12149-12160.
[20] Huffman J A, Docherty K S, Aiken A C, et al. Chemically-resolved aerosol volatility measurements from two megacity field studies [J]. Atmospheric Chemistry and Physics, 2009,9(18):7161-7182.
[21] Wu Z, Poulain L, Wehner B, et al. Characterization of the volatile fraction of laboratory-generated aerosol particles by thermodenuder-aerosol mass spectrometer coupling experiments [J]. Journal of Aerosol Science, 2009,40(7):603-612.
[22] Huffman J A, Ziemann P J, Jayne J T, et al. Development and characterization of a fast-stepping/scanning thermodenuder for chemically-resolved aerosol volatility measurements [J]. Aerosol Science and Technology, 2008,42(5):395-407.
[23] Burtscher H, Baltensperger U, Bukowiecki N, et al. Separation of volatile and non-volatile aerosol fractions by thermodesorption: instrumental development and applications [J]. Journal of Aerosol Science, 2001,32(4):427-442.
[24] Fierz M, Vernooij M G C, Burtscher H. An improved low-flow thermodenuder [J]. Journal of Aerosol Science, 2007,38(11):1163- 1168.
[25] Wehner B, Philippin S, Wiedensohler A. Design and calibration of a thermodenuder with an improved heatingunit to measure the size-dependent volatile fraction of aerosol particles [J]. Journal of Aerosol Science, 2002,(33):1087-1093.
[26] 戴守辉,毕新慧,黄 欢,等.单颗粒气溶胶质谱仪串联热稀释器在线测量单个气溶胶颗粒的挥发性[J]. 分析化学, 2014,42(8):1155- 1160. Dai S H, Bi X H, Huang H, et al. Measurement of particle volatility using single particle aerosol mass spectrometry tandem thermodiluter [J]. Chinese Journal of Analytical Chemistry, 2014,42(8):1155-1160.
[27] 李园园,黄晓锋,曾立武,等.基于热扩散管的深圳大气气溶胶半挥发性分析[J]. 中国环境科学, 2015,35(5):1281-1287. Li Y Y, Huang X F, Zeng L W, et al. Characterization of atmospheric aerosol semi-volatility in Shenzhen using the thermal denuder [J]. China Environmental Science, 2015,35(5):1281-1287.
[28] Lan Z J, Chen D L, Li X, et al. Modal characteristics of carbonaceous aerosol size distribution in an urban atmosphere of South China [J]. Atmospheric Research, 2011,100(1):0-60.
[29] Canagaratna M R, Jayne J T, Jimenez J L, et al. Chemical and microphysical characterization of ambient aerosols with the aerodyne aerosol mass spectrometer [J]. Mass Spectrometry Reviews, 2007, 26(2):185-222.
[30] Middlebrook A M, Bahreini R, Jimenez J L, et al. Evaluation of composition-dependent collection efficiencies for the aerodyne aerosol mass spectrometer using field data [J]. Aerosol Science and Technology, 2012,46(3):258-271.
[31] Cao L, Huang X, Li Y, et al. Volatility measurement of atmospheric submicron aerosols in an urban atmosphere in southern China [J]. Atmospheric Chemistry and Physics, 2018,18(3):1729-1743.
[32] 黄聪妮,黄晓锋,王少霞,等.华北地区夏季大气亚微米颗粒物的半挥发性特征[J]. 环境科学研究, 2015,28(9):1345-1352. Huang C N, Huang X F, Wang S X, et al. Characterization of semi-volatility of atmospheric submicron particles at a regional site in Northern China during summer [J]. Research of Environmental Science, 2015,28(9):1345-1352.
[33] Hu W, Hu M, Hu W, et al. Chemical composition, sources, and aging process of submicron aerosols in Beijing: Contrast between summer and winter [J]. Journal of Geophysical Research: Atmospheres, 2016,121(4):1955-1977.
[34] Aiken A C, Decarlo P F, Kroll J H, et al. O/C and OM/OC ratios of primary, secondary, and ambient organic aerosols with high-resolution time-of-flight aerosol mass spectrometry [J]. Environmental Science & Technology, 2008,42(12):4478-4485.
[35] Zhu Q, He L, Huang X, et al. Atmospheric aerosol compositions and sources at two national backgroundsites in northern and southern China [J]. Atmospheric Chemistry and Physics, 2016,16(15):10283- 10297.
[36] Donahue N M, Robinson A L, Stanier C O, et al. Coupled partitioning, dilution, and chemical aging of semivolatile organics [J]. Environmental Science & Technology, 2006,40(8):2635-2643.
Characterization of atmospheric aerosol volatility in North China during summertime.
WEI Lin-tong, CAO Li-ming, WEI Jing, HE Ling-yan,HUANG Xiao-feng*
(Key Laboratory of Urban Human Residential Environmental Science and Technology, Shenzhen Graduate School, Peking University, Shenzhen 518055, China)., 2019,39(9):3647~3654
Thermal denuder-aerosol mass spectrometer (TD-AMS) system was utilized to measure the aerosol chemical composition and volatility in the summer of 2014 in Wangdu, a rural site in North China Plain, and data of two different episodes were collected: average PM1mass concentration of relatively low pollution episodes (ep1) was (23.3 ± 15.1) µg/m3, dominated by sulfate, mainly influenced by air masses from the north; while (86.6 ± 19.7) µg/m3during high pollution episodes (ep2), dominated by organics aerosol (OA), influenced by air masses from the south; the volatility sequence of chemical species was nitrate>chloride>ammonia> organic matter>sulfate; when compared with ep1, during ep2, sulfate had a higher contribution for PM1and was less volatile, and nitrate was more volatile; OA during ep2 was less oxidized and showed lower volatility, indicating it was more aged. The semi-volatility of atmospheric aerosol suggested that regional transport played a key role under high pollution conditions in North China during summer.
aerosol volatility;thermal denuder;TD-AMS;organic aerosol
X513
A
1000-6923(2019)09-3647-08
魏林通(1995-),女,江西吉安人,硕士,主要从事大气气溶胶挥发性特征研究.发表论文1篇.
2019-01-28
国家重点研发计划项目(2017YFC0210004);国家自然科学基金资助项目(41622304;91544215)
* 责任作者, 教授, huangxf@pku.edu.cn

