木薯PYL13基因克隆及表达分析
赵思涵 颜彦 陈志晟 商桑 田丽波 胡伟
摘要:【目的】解析木薯脫落酸(ABA)受体PYR/PYL/RCARs家族基因(MePYL13)在木薯块根采后生理性变质(PPD)过程中的功能作用,为后续探索ABA信号通路在木薯PPD中的功能及作物抗逆育种打下基础。【方法】从木薯品种SC8中克隆MePYL13基因,应用生物信息学分析方法对其编码蛋白的理化性质、亲疏水性、保守结构域及二、三级结构等进行预测,对基因上游启动子进行元件分析,通过亚细胞定位观察MePYL13蛋白在植物细胞中的定位情况,并利用实时荧光定量PCR检测MePYL13基因在木薯PPD过程中的相对表达量。【结果】克隆获得的MePYL13基因编码区(CDS)长度663 bp,编码220个氨基酸,蛋白分子量23.957 kD,理论等电点(Ip)为6.74。MePYL13蛋白与巴西橡胶树(XP_021654464.1)PYL家族蛋白的氨基酸序列同源性最高,为91.55%,表明MePYL13蛋白氨基酸序列具有高度保守性。从MePYL13基因启动子鉴定获得与非生物胁迫逆境相关的响应元件,包括厌氧诱导元件(ARE,AAACCA)、光响应元件(ATCT-motif,AATCTAATCC)、干旱MYB元件(MBS,TAACTG)及ABA应答元件(ABRE,AAACAGA)和分生组织表达相关元件(CAT-box,GCCACT)等;MePYL13蛋白在木薯细胞的细胞核和细胞质上均有表达。随着PPD进程的推移,MePYL13基因相对表达量整体上呈上升趋势,至PPD后期(48 h)其相对表达量是0 h时(PPD前)的16倍,即MePYL13基因受木薯块根PPD的显著诱导。【结论】MePYL13基因是PYR/PYL/RCARs家族成员,可能在木薯块根PPD过程中发挥正向调控作用,为培育木薯PPD改良品种提供了潜在基因资源。
关键词: 木薯;PYR/PYL/RCARs家族;MePYL13基因;生理性变质现象(PPD);表达特征
中图分类号: S533.01 文献标志码: A 文章编号:2095-1191(2019)12-2629-09
Cloning and expression analysis of MePYL13 gene in cassava
ZHAO Si-han1,2, YAN Yan2, CHEN Zhi-sheng1,2, SHANG Sang1, TIAN Li-bo1*, HU Wei2*
(1 College of Horticulture, Hainan University, Haikou 570228, China; 2Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Science, Haikou 571101, China)
Abstract:【Objective】In this paper, abscisic acid(ABA) receptor PYR/PYL/RCARs family member(MePYL13) was cloned from cassava genome and its biological information and the expression characteristics in the process of post-harvest physiological deterioration(PPD) in cassava were analyzed, which provided a theoretical basis for further exploring the regulatory mechanism of ABA signaling pathway in cassava PPD and crop breeding for stress tolerance. 【Method】MePYL13 was cloned from cassava variety SC8. The physicochemical properties, hydrophilic/hydrophobic, conserved domain, secondary and tertiary structures of the protein encoded by the gene were predicted, the elements in MePYL13 gene promoter were analyzed through bioinformatics analysis. Subcellular localization of MePYL13 protein was also identified. Besides, the expression pattern of MePYL13 in cassava PPD process was investigated by real-time fluorescence quantitative PCR. 【Result】The coding regions(CDS) of this gene was 663 bp in length and encoded 220 amino acids, the protein molecular weight was 23.957 kD, and the theoretical isoelectric point(Ip) was 6.74. Multiple sequence alignment indicated that the similarity of MePYL13 protein with Hevea brasiliensis(XP_021654464.1) was the highest(91.55%). Amino acid sequence of MePYL13 protein was highly conservative. Several elements related to abiotic stress response were identified from the promoter of MePYL13, including anaerobic induction elements(ARE, AAACCA), light response elements(ATCT-motif,AATCTAATCC), drought MYB element(MBS, TAACTG), ABA response elements(ABRE, AAACAGA) and meristem expression-related elements(CAT-box, GCCACT), and so on. Subcellular location study indicated that MePYL13 protein was distributed in the nucleus and cytoplasm. Totally, MePYL13 was up-regulated at all the three time points in storage roots after harvested, and its relative expression level at 48 h was 16 times of that at 0 h(before PPD). Thus, MePYL13 was significantly increased in PPD process. 【Conclusion】 It is inferred that MePYL13 is a member of the PYR/PYL/RCARs family and may play a positive role in regulating PPD of cassava storage roots. This study is beneficial in providing potential genetic resources for cultivating improved cassava PPD varieties.
Key words: cassava; PYR/PYL/RCARs family; MePYL13 gene; post-harvest physiological deterioration(PPD); expression characters
0 引言
【研究意义】木薯(Manihot esculenta)作为世界七大作物之一(方佳等,2010),全球现有90多个国家栽培种植,热带地区近8亿人口将木薯块根作为主要粮食(Liu et al.,2017)。在我国,木薯主要种植在华南地区,因具有产量高、适应性强、耐贫瘠和干旱土壤、少有病虫害等优点,其产业发展潜力巨大,目前在食品、饲料及工业等领域均有开发利用(Fermont et al.,2009;Burns et al.,2010;El-Sharkawy,2012;Xu et al.,2013);但木薯块根极不耐储藏,采后2~3 d即出现生理性变质现象(Post-harvest physiological deterioration,PPD)而造成巨大经济损失,严重制约木薯产业的可持续发展(赵平娟等,2013;Uarrota and Maraschin,2015)。活性氧积累是PPD发生的根本原因之一,有效激活活性氧清除系统,控制木薯采后块根活性氧水平,是延缓木薯PPD的有效手段(Reilly et al.,2004,2007;Owiti et al.,2011;Vanderschuren et al.,2014)。脱落酸(Abscisic acid,ABA)除了参与种子萌发(Feng et al.,2015)、植株发育(Jia et al.,2016)、果实成熟(Mou et al.,2016;Tijero et al.,2016)及其他生长发育过程的调控(Li et al.,2016;颜彦等,2018)外,还广泛参与植物应答多种非生物胁迫的信号转导途径(Umezawa et al.,2010;Boneh et al.,2012;Chen et al.,2018;Kuromori et al.,2018),如干旱、低温、高盐和氧化胁迫等。PYR/PYL/RCARs蛋白为植物中功能性ABA受体,属于配体结合蛋白的START(Star-related lipid-transfer)超家族,在ABA介导的多种非生物胁迫应答中发挥重要作用(Ma et al.,2009;Park et al.,2009)。因此,克隆木薯PYL13基因(MePYL13)并分析其表达特征,可为阐明MePYL13基因参与调控木薯PPD的分子机制提供参考依据。【前人研究进展】至今,已有多种植物鉴定获得PYR/PYL/RCARs家族基因,如从拟南芥中鉴定出14个(Park et al.,2009;Gonzalez-Guzman et al.,2012),從水稻中鉴定出13个(Kim et al.,2014),从番茄中鉴定出15个(González-Guzmán et al.,2014),从棉花中鉴定出22个(Liang et al.,2017);并研究证实PYR/PYL/RCAR基因可增强番茄(González-Guzmán et al.,2014)、水稻(Kim et al.,2014)和拟南芥(Zhao et al.,2016)的耐旱性。在拟南芥中,有研究报道AtPYL8和AtPYL9基因可影响根部的发育(Antoni et al.,2013;Xing et al.,2016),AtPYL9基因还参与调节植物叶片衰老,当拟南芥中过表达AtPYL5和AtPYL9基因时能增强植株对ABA的敏感性和耐旱性(Santiago et al.,2009;Zhao et al.,2016)。也有研究表明,当植物经受干旱胁迫时其内源ABA水平增加,进而诱导H2O2产生并激活Ca2+通道,引起气孔关闭;而pyr1/pyl1/pyl2/pyl4突变体会抑制ABA诱导的活性氧产生和气孔关闭(Wang et al.,2013)。Kim等(2014)、颜彦等(2018)研究表明,水稻中OsPYL5基因过表达可增强转基因植物的抗旱性,且在种子萌发过程中对ABA较敏感。Liang等(2017)研究发现,在干旱胁迫下耐旱棉花品种中GhPYL9-11A基因的表达水平高于不耐旱棉花品种,将该基因在拟南芥中过表达能增强转基因植株在种子萌发和幼苗早期对ABA的敏感性。Yu等(2017)将PtPYRL1和PtPYRL5基因分别在杨树中过表达,发现转基因植株具有更强的活性氧清除能力,在逆境胁迫下具有更好的耐受性,说明PtPYRL1或PtPYRL5基因在杨树ABA信号途径中发挥正向调控作用。可见,PYL基因响应多种非生物逆境胁迫,并参与调节植物的多个生长发育代谢过程。【本研究切入点】PYR/PYL/RCARs家族成员作为ABA受体,在ABA介导的非生物胁迫应答中发挥重要功能作用,但至今鲜见有关木薯PYL基因克隆及其分析表达特性的研究报道。【拟解决的关键问题】克隆木薯PYR/PYL/RCARs家族基因MePYL13全长序列,并进行生物信息学及表达分析,旨在解析MePYL13基因在木薯块根应答PPD信号通路中的功能作用,为后续探索ABA信号通路在木薯PPD中的功能及作物抗逆育种打下基础。
1 材料与方法
1. 1 试验材料
木薯品种SC8由中国热带农业科学院热带生物技术研究所保存提供。多糖多酚植物总RNA提取试剂盒购自天根生化科技(北京)有限公司,pMD18-T载体购自宝生物工程(大连)有限公司,TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix试剂盒购自北京全式金生物技术有限公司,大肠杆菌(Escherichia coli) TOP10、2×Taq Master Mix酶和ChamQ Universal SYBR qPCR Master Mix试剂盒购自南京诺唯赞生物科技有限公司,农杆菌GV3101(PSoup+P19)购自上海唯地生物技术有限公司,载体pNC-Green-SubC和Nimble Cloning试剂盒购自海南壹田生物科技有限公司,T4 DNA连接酶和限制性内切酶购自赛默飞世尔科技(中国)有限公司。
1. 2 材料处理
将木薯块根切下1~2片2 cm左右厚的薄片,放入干净托盘中并置于相对湿度75%,温度26 ℃,16 h光照/8 h黑暗光周期的恒温培养箱中,分别于第0、6、12、24和48 h时取样,用液氮冷冻后-80 ℃保存备用。
1. 3 RNA提取及cDNA合成
使用多糖多酚植物总RNA提取试剂盒提取木薯SC8块根RNA,再以反转录试剂盒合成cDNA。
1. 4 基因克隆及載体构建
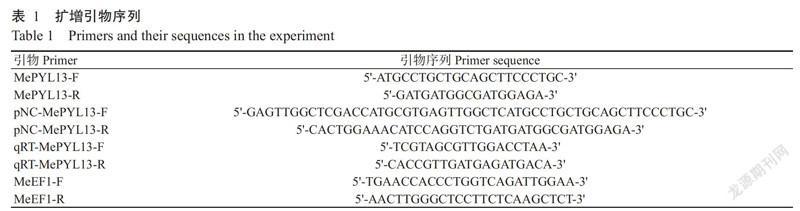
以木薯SC8块根的cDNA第一链为模板,利用NCBI设计MePYL13基因引物(表1)进行PCR扩增。PCR反应体系25.0 μL:2×Taq Master Mix 12.5 μL,上、下游引物(MePYL13-F和MePYL13-R)各1.0 μL,cDNA模板1.0 μL,ddH2O 9.5 μL。扩增程序:95 ℃预变性5 min;95 ℃ 30 s,55 ℃ 30 s,72 ℃ 1 min,进行30个循环;72 ℃延伸5 min。回收纯化PCR扩增产物连接至pMD18-T载体上,然后转化大肠杆菌TOP10,选择阳性克隆菌液送至生工生物工程(上海)股份有限公司测序,将测序正确菌液保存并提取质粒。
1. 5 生物信息学分析
利用NCBI链接的BLAST对MePYL13基因进行开放阅读框预测及查找同源序列,以DNAMAN进行翻译和同源性比对分析;运用NCBI-CDD数据库进行保守结构域预测分析;利用在线工具ProtParam预测MePYL13蛋白的亲/疏水性及等电点等理化性质,并以SOPMA和SWISS-MODEL进行MePYL13蛋白二、三级结构预测(颜彦等,2018);利用MEGA 5.0中的Neighbor-joining(NJ)法构建系统发育进化树;用Plant CARE进行启动子元件分析。
1. 6 亚细胞定位
设计MePYL13基因引物(pNC-MePYL13-F和pNC-MePYL13-R),以已有质粒为模板进行PCR扩增,PCR扩增产物经胶回收后与载体pNC-Green-SubC用Nimble Cloning试剂盒连接和转化,挑选阳性克隆送至生工生物工程(上海)股份有限公司测序,提取的质粒转化农杆菌GV3101(PSoup+P19)感受态细胞,通过瞬时表达法将转化的农杆菌注射至烟草叶片培养48 h。以空载体pNC-Green-SubC为对照,利用FluoViewTM FV1000激光扫描共聚焦显微镜观察荧光在细胞中的分布情况。
1. 7 基因表达分析
提取各时间点保存木薯块根样品的RNA,反转录合成cDNA,设计荧光定量引物qRT-MePYL13-F和qRT-MePYL13-R(表1)及MeEF1基因引物MeEF1-F和MeEF1-R(Xu et al.,2013),以MeEF1基因为内参基因,在Mx3005P荧光定量PCR仪(上海吉泰生物科技有限公司)进行实时荧光定量PCR扩增,反应体系20.0 μL:2×ChamQ Universal SYBR qPCR Master Mix 10.0 μL,上、下游引物各0.4 μL,cDNA模板2.0 μL,ddH2O 7.2 μL。扩增程序:95 ℃预变性30 s;95 ℃ 10 s,56 ℃ 30 s,72 ℃ 30 s,进行40个循环;95 ℃ 15 s,60 ℃ 1 min,95 ℃ 15 s。设3次重复,采用2-△△Ct计算基因的相对表达量(胡伟等,2015)。
1. 8 统计分析
使用Excel 2016对试验数据进行整理,以SPSS 20.0进行单因素方差分析(One-way ANOVA)(韩瑞才等,2017)。
2 结果与分析
2. 1 MePYL13基因PCR扩增结果
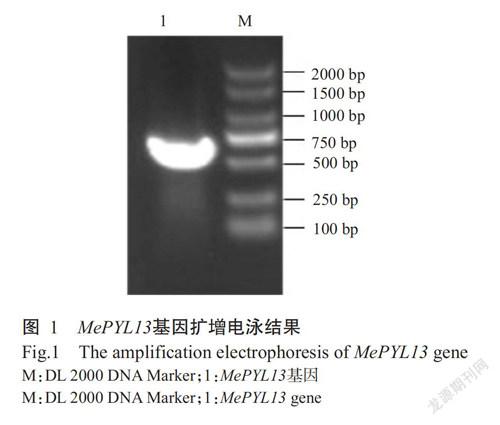
根据NCBI数据库中的木薯PYL基因(GenBank登录号LOC110618627)设计特异性扩增引物,以木薯SC8块根cDNA为模板,扩增获得大小约650 bp的特异条带(图1)。测序结果显示,目的基因编码区(CDS)长度663 bp,编码220个氨基酸,蛋白分子量23.957 kD,理论等电点(Ip)为6.74,命名为MePYL13基因。
2. 2 生物信息学分析结果
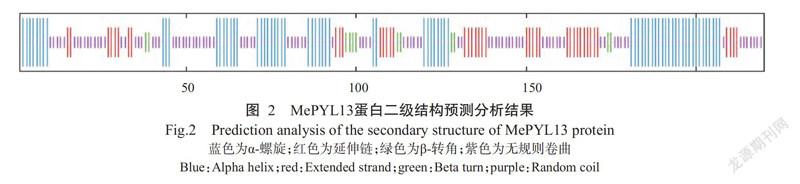
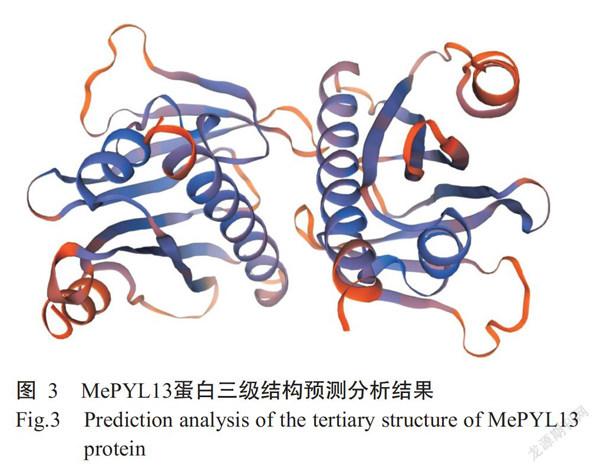
MePYL13蛋白不稳定系数为56.56,表明其为不稳定蛋白;亲水性平均值(GRAVY)为-0.183,属于亲水性蛋白。MePYL13蛋白二级结构预测结果(图2)显示,α-螺旋占32.27%,延伸链占19.09%,β-转角占5.45%,无规则卷曲占43.18%。应用SWISS-MODEL平台预测MePYL13蛋白三级结构,预测结果(图3)与其二级结构预测结果一致。
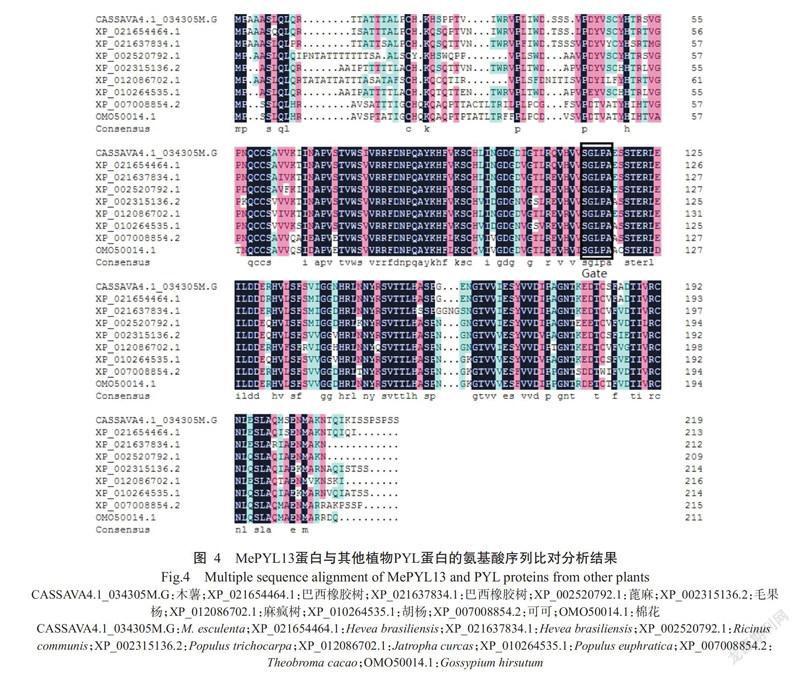
通过BLAST比对分析MePYL13蛋白与其他已知植物PYL家族蛋白的同源性,结果(图4)发现,MePYL13蛋白与巴西橡胶树(Hevea brasiliensis)(XP_021654464.1)PYL家族蛋白的氨基酸序列同源性最高,为91.55%。使用ClustalX进行多序列比对,结果表明MePYL13氨基酸序列具有控制ABA结合的关键区域Gate。综上所述,扩增获得的MePYL13基因即为PYR/PYL/RCARs家族成员,且可能具有该基因家族的生物学功能。
2. 3 系统发育进化分析结果
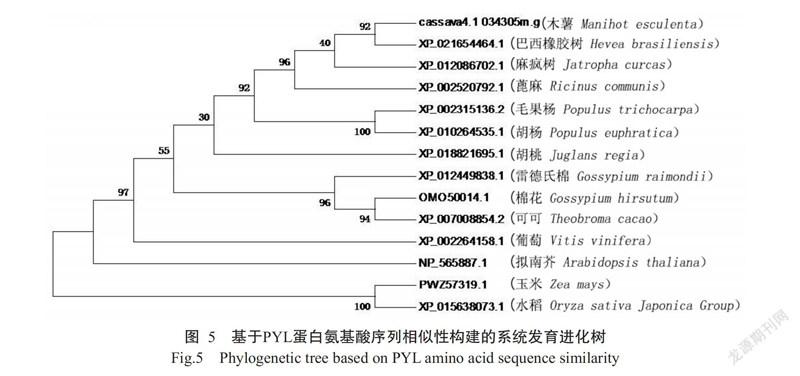
为进一步探究MePYL13蛋白与其他物种PYR/PYL/RCARs家族的进化关系,在NCBI数据库中将MePYL13蛋白氨基酸序列与其他物种进行比对分析,结果显示,MePYL13蛋白氨基酸序列与巴西橡胶树、蓖麻(Ricinus communis)、毛果杨(Populus trichocarpa)、麻疯树(Jatropha curcas)、胡杨(Populus euphratica)的PYL蛋白氨基酸序列相似性分别为91.55%、79.71%、77.88%、75.45%和70.44%。基于PYL蛋白氨基酸序列相似性构建的系统发育进化树(图5)也显示,MePYL13蛋白与巴西橡胶树、蓖麻和麻疯树聚类在同一分支上,均为大戟科植物,说明PYL蛋白具有较高的同源性;胡桃(Juglans regia)、雷德氏棉(Gossypium raimondii)、棉花(G. hirsutum)和可可(Theobroma cacao)聚类在另一分支上,其物种均起源于热带或亚热带地区。说明植物中PYL蛋白氨基酸序列高度保守,木薯MePYL13蛋白与巴西橡胶树PYL蛋白分子进化距离最小,亲缘关系最近,其基因功能可能最相似。
2. 4 MePYL13基因启动子元件分析结果
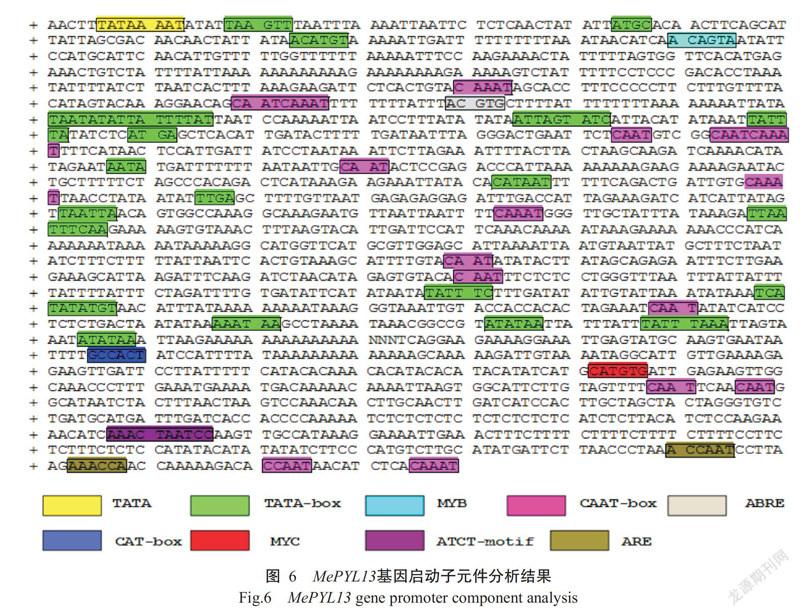
通过PlantCARE预测分析MePYL13基因转录起始位点上游2000 bp序列的顺式作用元件,结果(图6)发现多个常见的顺式作用元件,如TATA-box和CAAT-box,并鉴定获得与非生物胁迫逆境相关的响应元件,包括厌氧诱导(ARE,AAACCA)、光响应(ATCT-motif,AATCTAATCC)、干旱MYB元件(MBS,TAACTG)等,以及ABA应答元件(ABRE,AAACAGA)和分生组织表达相关元件(CAT-box,GCCACT),说明MePYL13基因的表达可能受多重信号系统调控。
2. 5 MePYL13蛋白亚细胞定位分析结果
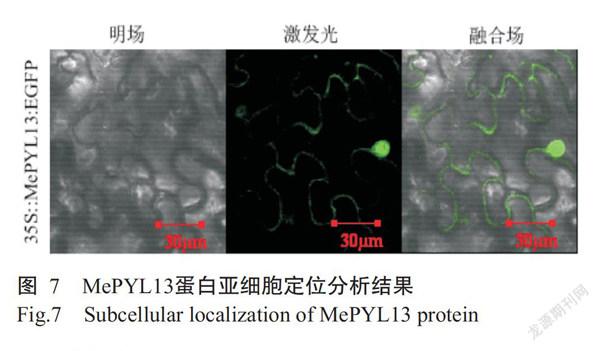
将MePYL13基因与载体pNC-Green-SubC连接获得的质粒转化至农杆菌GV3101(PSoup+P19)感受态细胞中,再通过瞬时表达法将菌液注射至烟草叶片中,经激光扫描共聚焦显微镜观察到有荧光分布在细胞核和细胞质上(图7),说明MePYL13蛋白在木薯细胞的细胞核和细胞质上均有表达。
2. 6 MePYL13基因表达分析结果
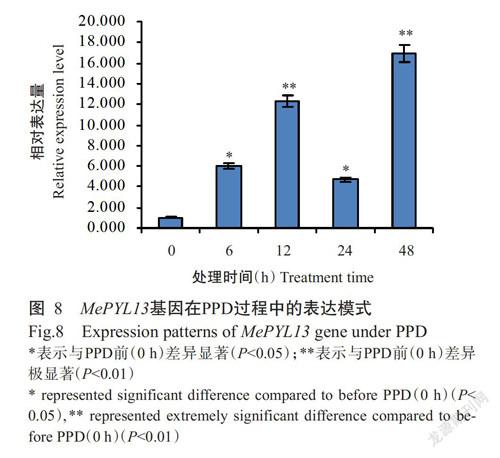
为解析MePYL13基因在木薯PPD过程中的功能,利用实时荧光定量PCR检测MePYL13基因在PPD不同时段的表达情况。由图8可知,在木薯块根PPD早期(6和12 h),MePYL13基因表达急剧上升,其相对表达量分别是0 h时(PPD前)的6倍和12 倍;随后MePYL13基因的相对表达量有所回落,至24 h时其相对表达量是0 h时的4倍;在PPD后期(48 h),MePYL13基因表达继续上升,其相对表达量是0 h时的16倍。受PPD诱导,MePYL13基因在木薯块根中的表达整体上呈上调趋势,推测其在木薯PPD过程中发挥重要作用。
3 讨论
木薯是我國热带和亚热带地区最重要的经济作物之一(张鹏等,2014),但PPD缩短了木薯的贮存期,严重影响其商品价值。已有研究表明,ABA可能是果实成熟衰老的上游调控信号,进而影响采后生理特性及贮藏品质(杨方威等,2016)。PYL基因作为ABA信号通路的核心组成部分(Ma et al.,2009;Park et al.,2009),在ABA介导的非生物胁迫过程中发挥重要作用,如AtPYL9基因过表达拟南芥植株在干旱条件下可显著提高其抗氧化酶活性,而减轻细胞膜损伤(Zhao et al.,2016);将PtPYRL1或PtPYRL5基因在杨树中过表达,其转基因植株具有更强的活性氧清除能力(Yu et al.,2017)。木薯块根的PPD过程主要与活性氧积累有关(Reilly et al.,2004,2007;Owiti et al.,2011;Vanderschuren et al.,2014)。为解析PYL家族基因在木薯块根PPD过程中是否具有功能作用,本研究对MePYL13基因进行克隆及表达特征分析,结果表明,MePYL13基因CDS为663 bp,编码220个氨基酸,MePYL13蛋白与巴西橡胶树、蓖麻、毛果杨和麻疯树的PYL蛋白氨基酸序列同源性均在75.00%以上,具有控制ABA结合的关键区域Gate,且在MePYL13基因启动子上发现有响应ABA诱导的核心元件ABRE。由于不同植物中PYL蛋白高度保守,故推测PYL蛋白在不同植物中可能发挥相似的功能(韩瑞才等,2017;邓小敏等,2018)。
蛋白质修饰和含量变化及蛋白间的相互作用是细胞行使功能的关键,基因编码蛋白产物因在植物细胞中发挥作用的位置不同,其功能也各不相同(邢浩然等,2006)。本研究通过亚细胞定位分析,发现MePYL13蛋白定位于细胞核和细胞质,与在拟南芥(Ma et al.,2009)、大豆(Bai et al.,2013)和水稻(Tian et al.,2015)上报道的PYL家族基因位置一致,说明PYL家族基因的功能在不同植物物种中高度保守。此外,MePYL13基因在木薯块根PPD过程中的表达谱显示,随着PPD进程的推移MePYL13基因相对表达量呈先升高后降低再升高的变化趋势,至PPD后期(48 h)其相对表达量是0 h时的16倍,即MePYL13基因受木薯块根PPD的显著诱导,由此推测MePYL13基因在木薯块根PPD过程中发挥正向调控作用,与PYL基因受非生物逆境胁迫诱导(Antoni et al.,2013;Wang et al.,2013;Xing et al.,2016;Zhao et al.,2016;颜彦等,2018)的结论一致,今后可通过在木薯中过表达MePYL13基因进一步研究其在木薯块根采后PPD过程的调控机制。
4 结论
MePYL13基因是PYR/PYL/RCARs家族成员,可能在木薯块根PPD过程中发挥正向调控作用,为培育木薯PPD改良品种提供了潜在基因资源。
参考文献:
邓小敏,于洁,陈莹,晁金泉,张世鑫,田维敏. 2018. 橡胶树乳管细胞GPPS基因的克隆与表达分析[J]. 南方农业学报,49(11):2117-2128. [Deng X M,Yu J,Chen Y,Chao J Q,Zhang S X,Tian W M. 2018. Cloning and expression analysis of GPPS genes from the laticifer cells of Hevea brasiliensis[J]. Journal of Southern Agriculture,49(11):2117-2128.]
方佳,濮文辉,张慧坚. 2010. 国内外木薯产业发展近况[J]. 中国农学通报,26(16):353-361. [Fang J,Pu W H,Zhang H J. 2010. The development status of cassava industry at home and abroad[J]. Chinese Agricultural Science Bulletin,26(16):353-361.]
胡伟,颜彦,韦运谢,王文泉,夏志强,卢诚,侯晓婉,彭明. 2015. 木薯MeASR基因克隆及表达分析[J]. 生物技术通报,31(10):125-130. [Hu W,Yan Y,Wei Y X,Wang W Q,Xia Z Q,Lu C,Hou X W,Peng M. 2015. Clone and expression of MeASR gene in cassava[J]. Biotechnology Bulletin,31(10):125-130.]
韩瑞才,武志峰,唐双勤,潘晓华,石庆华,王根发,吴自明. 2017. 水稻黄嘌呤脱氢酶基因OsXDH克隆及表达分析[J]. 南方农业学报,48(12):2113-2121. [Han R C,Wu Z F,Tang S Q,Pan X H,Shi Q H,Wang G F,Wu Z M. 2017. Cloning and expression analysis for xanthine dehydrogenase gene OsXDH in rice[J]. Journal of Southern Agriculture,48(12):2113-2121.]
邢浩然,刘丽娟,刘国振. 2006. 植物蛋白质的亚细胞定位研究进展[J]. 华北农学报,21(S2):1-6. [Xing H R,Liu L J,Liu G Z. 2006. Advancement of protein subcellular localization in plants[J]. Acta Agriculturae Boreali-Sinica,21(S2):1-6.]
顏彦,铁韦韦,丁泽红,吴春来,胡伟. 2018. 木薯MePYL8基因克隆及表达分析[J]. 分子植物学报,16(14):4498-4504. [Yan Y,Tie W W,Ding Z H,Wu C L,Hu W. 2018. Cloning and expression analysis of MePYL8 gene in cassava[J]. Molecular Plant Breeding,16(14):4498-4504.]
杨方威,段懿菲,冯叙桥. 2016. 脱落酸的生物合成及对水果成熟的调控研究进展[J]. 食品科学,37(3):266-272. [Yang F W,Duan Y F,Feng X Q. 2016. Advances in biosynthesis of abscisic acid and its roles in regulation of fruit ripening[J]. Food Science,37(3):266-272.]
张鹏,杨俊,周文智,王红霞,马秋香. 2014. 能源木薯高淀粉抗逆分子育种研究进展与展望[J]. 生命科学,26(5):465-473. [Zhang P,Yang J,Zhou W Z,Wang H X,Ma Q X. 2014. Progress and perspective of cassava molecular breeding forbioenergy development[J]. Chinese Bulletin of Life Sciences,26(5):465-473.]
赵平娟,孙海彦,黎娟华,卢诚,彭明. 2013. 木薯采后生理性变质的研究进展[J]. 热带农业科学,33(1):35-41. [Zhao P J,Sun H Y,Li J H,Lu C,Peng M. 2013. Review of cassava post-harvest physiological deterioration research[J]. Chinese Journal of Tropical Agriculture,33(1):35-41.]
Antoni R,Gonzalez-Guzman M,Rodriguez L,Peirats-Llobet M,Pizzio G A,Fernandez M A,de Winne N,de Jaeger G,Dietrich D,Bennett M J,Rodriguez P L. 2013. PYRABACTIN RESISTANCE1-LIKE8 plays an important role for the regulation of abscisic acid signaling in root[J]. Plant Physiology,161(2):931-941.
Bai G,Yang D H,Zhao Y,Ha S,Yang F,Ma J,Gao X S,Wang Z M,Zhu J K. 2013. Interactions between soybean ABA receptors and type 2C protein phosphatases[J]. Plant Molecular Biology,83(6):651-664.
Boneh U,Biton I,Schwartz A,Ben-Ari G. 2012. Characterization of the ABA signal transduction pathway in Vitis vinifera[J]. Plant Science,187:89-96.
Burns A,Gleadow R,Cliff J,Zacarias A,Cavagnaro T. 2010. Cassava:The drought,war and famine crop in a changing world[J]. Sustainability,2(11):3572-3607.
Chen Z F,Wang Z,Yang Y G,Li M,Xu B C. 2018. Abscisic acid and brassinolide combined application synergistically enhances drought tolerance and photosynthesis of tall fescue under water stress[J]. Scientia Horticulturae,228:1-9.
El-Sharkawy M A. 2012. Stress-tolerant cassava:The role of integrative ecophysiology-breeding research in crop improvement[J]. Open Journal of Soil Science,2(2):162-186.
Feng C Z,Chen Y,Wang C,Kong Y H,Wu W H,Chen Y F. 2015. Arabidopsis RAV1 transcription factor,phosphorylated by SnRK2 kinases,regulates the expression of ABI3,ABI4,and ABI5 during seed germination and early seedling development[J]. The Plant Journal,80(4):654-668.
Fermont A M,van Asten P J A,Tittonell P,van Wijk M T,Giller K E. 2009. Closing the cassava yield gap:An ana-lysis from small holder farms in East Africa[J]. Field Crops Research,112(1):24-36.
Gonzalez-Guzman M,Pizzio G A,Antoni R,Vera-Sirera F,Merilo E,Bassel G W,Fernandez M A,Holdsworth M J,Perez-Amador M A,Kollist H,Rodriguez P L. 2012. Arabidopsis PYR/PYL/RCAR receptors play a major role in quantitative regulation of stomatal aperture and transcriptional response to abscisic acid[J]. The Plant Cell,24(6):2483-2496.
González-Guzmán M,Rodríguez L,Lorenzo-Orts L,Pons C,Sarrión-Perdigones A,Fernández M A,Peirats-Llobet M,Forment J,Moreno-Alvero M,Cutler S R,Albert A,Granell A,Rodríguez P L. 2014. Tomato PYR/PYL/RCAR abscisic acid receptors show high expression in root,differential sensitivity to the abscisic acid agonist quinabactin,and the capability to enhance plant drought resistance[J]. Journal of Experimental Botany,65(15):4451-4464.
Jia H F,Jiu S T,Zhang C,Wang C,Tariq P,Liu Z J,Wang B J,Cui L W,Fang J G. 2016. Abscisic acid and sucrose regulate tomato and strawberry fruit ripening through the abscisic acid-stress-ripening transcription factor[J]. Plant Biotechnology Journal,14(10):2045-2065.
Kim H,Lee K,Hwang H,Bhatnagar N,Kim D Y,Yoon I S,Byun M O,Kim S T,Jung K H,Kim B G. 2014. Overexpression of PYL5 in rice enhances drought tolerance,inhibits growth,and modulates gene expression[J]. Journal of Experimental Botany,65(2):453-464.
Kuromori T,Seo M,Shinozaki K. 2018. ABA transport and plant water stress responses[J]. Trends in Plant Science,23(6):513-522.
Li D,Mou W,Luo Z,Li L,Limwachiranon J,Mao L,Ying T. 2016. Developmental and stress regulation on expression of a novel miRNA,Fan-miR73,and its target ABI5 in strawberry[J]. Scientific Reports,6:28385. doi: 10.1038/srep28385.
Liang C Z,Liu Y,Li Y Y,Meng Z G,Yan R,Zhu T,Wang Y,Kang S J,Abid M A,Malik W,Sun G,Guo S,Zhang R. 2017. Activation of ABA receptors gene GhPYL9-11A is positively correlated with cotton drought tolerance in transgenic Arabidopsis[J]. Frontiers in Plant Science,8:1453. doi: 10.3389/fpls.2017.01453.
Liu S,Zainuddin I M,Vanderschuren H,Doughty J,Beeching J R. 2017. RNAi inhibition of feruloyl CoA 6’-hydroxylase reduces scopoletin biosynthesis and post-harvest physiological deterioration in cassava(Manihot esculenta Crantz) storage roots[J]. Plant Molecular Biology,94(1-2):185-195.
Ma Y,Szostkiewicz I,Korte A,Moes D,Yang Y,Christmann A,Grill E. 2009. Regulators of PP2C phosphatase activity function as abscisic acid sensors[J]. Science,324(5930):1064-1068.
Mou W,Li D,Bu J,Jiang Y,Khan Z U,Luo Z,Mao L,Ying T. 2016. Comprehensive analysis of ABA effects on ethy-lene biosynthesis and signaling during tomato fruit ripe-ning[J]. PLoS One,11(4):e0154072.
Owiti J,Grossmann J,Gehrig P,Dessimoz C,Laloi C,Hansen M B,Gruissem W,Vanderschuren H. 2011. iTRAQ-based analysis of changes in the cassava root proteome reveals pathways associated with post-harvest physiological deterioration[J]. The Plant Journal,67(1):145-156.
Park S Y,Fung P,Nishimura N,Jensen D R,Fujii H,Zhao Y,Lumba S,Santiago J,Rodrigues A,Chow T F,Alfred S E,Bonetta D,Finkelstein R,Provart N J,Desveaux D,Rodriquez P L,McCourt P,Zhu J K,Schroeder J I,Volkman B F,Cutler S R. 2009. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins[J]. Science,324(5930):1068-1071.
Reilly K,Bernal D,Cortés D F,Gómez-Vásquez R,Tohme J,Beeching J R. 2007. Towards identifying the full set of genes expressed during cassava post-harvest physiological deterioration[J]. Plant Molecular Biology,64(1-2):187-203.
Reilly K,Gómez-Vásquez R,Buschmann H,Tohme J,Bee-ching J R. 2004. Oxidative stress responses during cassava post-harvest physiological deterioration[J]. Plant Molecular Biology,56(4):625-641.
Santiago J,Rodrigues A,Saez A,Rubio S,Antoni R,Dupeux F,Park S Y,Márquez J A,Cutler S R,Rodriguez P L. 2009. Modulation of drought resistance by the abscisic acid receptor PYL5 through inhibition of clade A PP2Cs[J]. The Plant Journal,60(4):575-588.
Tian X,Wang Z,Li X,Lv T,Liu H,Wang L,Niu H,Bu Q. 2015. Characterization and functional analysis of pyrabactin resistance-like abscisic acid receptor family in rice[J]. Rice,8(1):28.
Tijero V,Teribia N,Muñoz P,Munné-Bosch S. 2016. Implication of abscisic acid on ripening and quality in sweet cherries:Differential effects during pre-and post-harvest[J]. Frontiers in Plant Science,7:602. doi:10.3389/fpls. 2016.00602.
Uarrota V G,Maraschin M. 2015. Metabolomic,enzymatic,and histochemical analyzes of cassava roots during postharvest physiological deterioration[J]. BMC Research Notes,8:648. doi: 10.1186/s13104-015-1580-3.
Umezawa T,Nakashima K,Miyakawa T,Kuromori T,Tanokura M,Shinozaki K,Yamaguchi-Shinozaki K. 2010. Molecular basis of the core regulatory network in ABA Responses:Sensing,signaling and transport[J]. Plant and Cell Physiology,51(11):1821-1839.
Vanderschuren H,Nyaboga E,Poon J S,Baerenfaller K,Grossmann J,Hirsch-Hoffmann M,Kirchgessner N,Nanni P,Gruissem W. 2014. Large-scale proteomics of the cassava storage root and identification of a target gene to reduce postharvest deterioration[J]. The Plant Cell,26(5):1913-1924.
Wang Y Z,Chen Z H,Zhang B,Hills A,Blatt M R. 2013. PYR/PYL/RCAR abscisic acid receptors regulate K+ and Cl- channels through reactive oxygen species-mediated activation of Ca2+ channels at the plasma membrane of intact arabidopsis guard cells[J]. Plant Physiology,163(2):566-577.
Xing L,Zhao Y,Gao J,Xiang C,Zhu J K. 2016. The ABA receptor PYL9 together with PYL8 plays an important role in regulating lateral root growth[J]. Scientific Reports,6:27177. doi: 10.1038/srep27177.
Xu J,Duan X G,Yang J,Beeching J R,Zhang P. 2013. Enhanced reactive oxygen species scavenging by overproduction of superoxide dismutase and catalase delays postharvest physiological deterioration of cassava storage roots[J]. Plant Physiology,161(3):1517-1528.
Yu J L,Ge H M,Wang X K,Tang R J,Wang Y,Zhao F G,Lan W Z,Luan S,Yang L. 2017. Overexpression of pyrabactin resistance-like abscisic acid receptors enhances drought,osmotic,and cold tolerance in transgenic poplars[J]. Frontiers in Plant Science,8:1752. doi:10.3389/fpls. 2017.01752.
Zhao Y,Chan Z L,Gao J H,Xing L,Cao M J,Yu C M,Hu Y L,You J,Shi H T,Zhu Y F,Gong Y H,Mu Z X,Wang H Q,Deng X,Wang P C,Bressan R,Zhu J K. 2016. ABA receptor PYL9 promotes drought resistance and leaf senescence[J]. Proceedings of the National Academy of Sciences of the United States of America,113(7):1949-1954.
(責任编辑 兰宗宝)

