SIRT1在非小细胞肺癌TKIs获得性耐药中的调控作用
李桂芳 原翔 刘怡文 马名扬 孙江涛

[摘要] 目的 分析組蛋白去乙酰化酶SIRT1在非小细胞肺癌干细胞样特性中的调控作用。 方法 通过流式细胞术在PC-9及PC-9-GR细胞系中检测肿瘤干细胞比例;采用CCK8法检测IC50;采用qPCR检测SIRT1 mRNA表达;采用Western blot法检测SIRT1蛋白表达;通过细胞悬浮克隆成球实验检测不同组的细胞成球能力。 结果 成功建立了耐药细胞系PC-9-GR,PC-9-GR的IC50、ALDHbright肿瘤干细胞百分比、SIRT1蛋白表达以及SIRT1 mRNA表达均高于PC-9(P < 0.05);通过细胞悬浮克隆成球实验发现PC-9-GR成球能力显著高于PC-9(P < 0.05),应用吉非替尼后,其成球能力较对照组差异无统计学意义(P > 0.05);应用SIRT1特异性抑制剂TV6及双药联合后,两组细胞株的成球能力均显著降低(P < 0.05)。 结论 非小细胞肺癌-酪氨酸激酶抑制剂获得性耐药的产生与酪氨酸激酶抑制剂药物富集ALDH1+标记的肿瘤干细胞密切相关,而组蛋白去乙酰化酶SIRT1在维系这群肿瘤干细胞干性中发挥着重要作用,其抑制SIRT1对肿瘤干细胞的靶向,可能有助于延缓酪氨酸激酶抑制剂获得性耐药。
[关键词] 非小细胞肺癌;受体酪氨酸激酶抑制剂;乙醛脱氢酶-1;SIRT1;肿瘤干细胞
[中图分类号] R734.2 [文献标识码] A [文章编号] 1673-7210(2019)06(a)-0004-05
Regulation of SIRT1 in acquired resistance of TKIs in non-small cell lung cancer
LI Guifang1 YUAN Xiang2 LIU Yiwen2 MA Mingyang1 SUN Jiangtao3
1.First Affiliated Hospital, College of Clinical Medicine of He′nan University of Science and Technology, He′nan Province, Luoyang 471003, China; 2.He′nan Key Laboratory of Cancer Epigenetics Cancer Institute, the First Affiliated Hospital of He′nan University of Science and Technology, He′nan Province, Luoyang 471003, China; 3.The First Affiliated Hospital of He′nan University of Science and Technology, He′nan Province, Luoyang 471003, China
[Abscract] Objective To analyze the regulation of histone deacetylase SIRT1 in stem cell-like properties of non-small cell lung cancer. Methods The percentage of tumor stem cells was detected by flow cytometry in PC-9 and PC-9-GR cell lines; IC50 was detected by CCK8; SIRT1 mRNA expression was detected by qPCR; SIRT1 protein expression was detected by Western blot; the experiments of cell suspension cloning into spheres was used to detect the capability of sphere-forming of different groups. Results The drug-resistant cell line PC-9-GR was successfully established. The IC50, ALDHbright tumor stem cell percentage, SIRT1 protein expression and SIRT1 mRNA expression of PC-9-GR were higher than those of PC-9 (P < 0.05). The experiments of cell suspension cloning into spheres found that PC-9-GR was significantly higher than PC-9 (P < 0.05), and the effect of PC-9-GR on the capability of sphere-forming was not statistically significant after the application of Gefitinib (P > 0.05). After the combination of SIRT1 specific inhibitor TV6 and double drug combination, the capability of sphere-forming of the two cell lines was significantly lower than that of the control group (P < 0.05). Conclusions Acquired resistance to tyrosine kinase inhibitors in non-small cell lung cancer is closely related to the enrichment of ALDH1+ labeled cancer stem cells by tyrosine kinase inhibitors. Histone deacetylase SIRT1 plays an important role in maintaining the stem of these cancer stem cells. Its inhibition of SIRT1 targeting tumor stem cells may help delay the acquired resistance of tyrosine kinase inhibitors.
[Key words] Non-small cell lung cancer; Receptor tyrosine kinase inhibitor; Acetaldehyde dehydrogenase-1; SIRT1; Cancer stem cells
肺癌是發病率和死亡率最高的恶性肿瘤之一。非小细胞肺癌(NSCLC)约占肺癌的80.4%[1]。以表皮生长因子受体酪氨酸酶抑制剂(EGFR-TKIs)为主的分子靶向治疗为晚期NSCLC患者开启了“希望之门”,但耐药的产生使后续治疗步履维艰。关于EGFR-TKIs获得性耐药机制的研究报道百花齐放,其中以EGFR T790M突变为主,但仍有30%的机制至今尚未被阐明。
肿瘤干细胞(cancer stem cells,CSCs)是存在于肿瘤中、具有高度自我更新和异常分化潜能的一小部分细胞亚群[2]。近年来,CSCs领域的研究处于蓬勃的上升态势,并在肺癌成功分离、富集出了CSCs。乙醛脱氢酶-1(ALDH1)已被多个研究团队证实为可作为NSCLC优选的干细胞标记物[3-9]。沉默信息调节因子1(SIRT1)是一种组蛋白去乙酰化酶,可以去乙酰化一系列组蛋白及非组蛋白底物。研究[10]表明,SRIT1在调节各种生物学功能,如衰老、代谢、DNA损伤以及肿瘤发生发展过程中发挥重要作用。
本研究应用肺癌细胞系,通过流式细胞术、Western blot、细胞悬浮克隆成球试验等方法检测SIRT1在NSCLC中的表达,并分析SIRT1异常表达对NSCLC干细胞样特性的调控作用及意义。
1 材料与方法
1.1 细胞系的培养
细胞培养:选择肺癌细胞系PC-9购自上海博古生物细胞所,用含有10%FBS(购自Gibco)的RPMI-1640培养基(购自Gibco)培养,放置37℃ 5%CO2的培养箱;2~3 d换液,传代一次。
耐药细胞系的建立:采用逐步诱导法。Gefitinib(购自selleck)浓度从5 nmol/L逐步增加至2 μmol/L,共培养6个月。将诱导成功的耐药细胞命名为PC-9-GR。
1.2 CCK8法检测IC50
将细胞接种到96孔培养板内,37℃ 5%CO2培养箱中过夜,再用不同浓度吉非替尼处理,根据说明书进行检测。每个实验至少独立重复3次。
1.3 流式细胞术检测
细胞计数后取适量细胞于流式管中,每个检测管中加入适量Aldefluor缓冲液,对照管中加入维拉帕米(DEAB)溶液作为阴性对照,流式细胞仪上机检测。所有样品至少重复3次。
1.4 Western blot方法检测SIRT1蛋白表达
将细胞收集后加入RIPA细胞裂解液(购自北京康为生物科技有限公司),冰上裂解,得到的上清即为细胞总蛋白。然后根据BCA试剂盒(购自北京康为生物科技有限公司)说明书进行检测。其后进行电泳、转膜,封闭,然后SIRT1(购自Abcam)一抗(1∶2000)及内参GAPDH抗体(购自北京康为生物科技有限公司)(1∶2000)4℃过夜,二抗(1∶2000)室温孵育2 h,ECL显影剂(购自北京康为生物科技有限公司)处理后于凝胶成像系统检测目的蛋白条带,利用Image J软件检测条带灰度值。
1.5 QPCR检测SIRT1 mRNA表达
采用Trizol法提取细胞的总RNA,并进行反转录合成cDNA。利用PubMed数据库设计引物序列,SIRT1的上游引物为:5′-GCCTCATCTGCATTTTGATG-3′,下游引物为:5′-TCTGGCATGTCCCACTATCA-3′;GAPDH引物序列为:上游引物:5′-GCCACATCGCT-CAGACACC-3′,下游引物:5′-GATGGCAACAATAT-CCACTTTACC-3′。取反转录合成的cDNA进行qPCR,退火温度为60℃,经40个循环扩增,计算2-ΔΔCt值。
1.6 细胞悬浮培养克隆成球能力检测
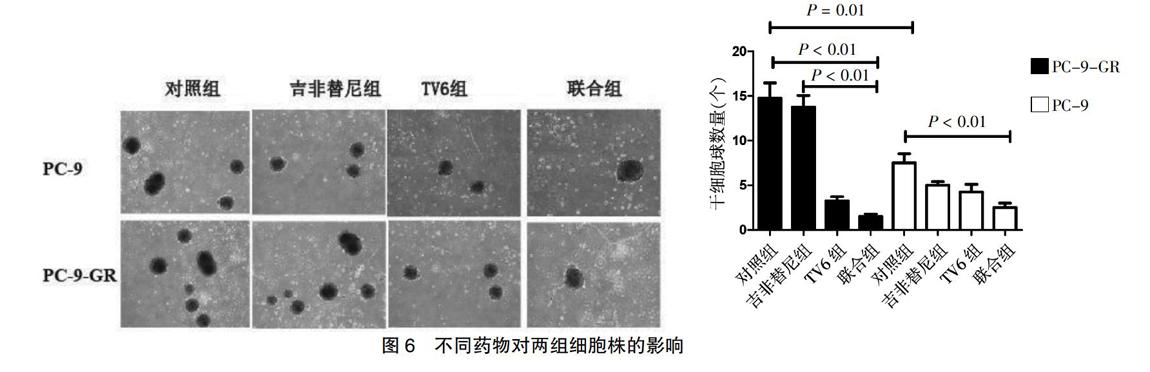
将细胞分成不同组别,在不同组中加入一定量浓度的抑制剂及药物,在培养的第3天更换培养基,持续培养约7 d,照相,对直径>40 μm的次级细胞球进行计数。
1.7 统计学方法
采用SPSS 19.0统计学软件进行数据分析,计量资料用均数±标准差(x±s)表示,两组间比较采用配对样本t检验;GrahPad Prism 5.0 统计绘图软件处理、分析数据、绘制图形。以P < 0.05为差异有统计学意义。
2 结果
2.1 耐药株与亲本株形态学上的改变
在诱导细胞耐药的过程中可以观察到存活下来的耐药株,其表现出与亲代细胞系不同的形态,多不规则,核大,伪足增多。见图1。
PC-9亲本细胞未处理(左);2 μmol/L吉非替尼处理7 d(中);2 μmol/L吉非替尼处理20 d(右)
图1 Gefitinib诱导过程中对细胞的影响(40×)
2.2 CCK8法检测PC-9-GR耐药株的建立
CCK8法检测两组细胞株的吉非替尼药物IC50,结果显示PC-9-GR显著高于PC-9(P < 0.01)。见图2。
2.3 PC-9及PC-9-GR细胞中ALDHbright CSCs百分比比较
采用Aldeflour?誖试剂盒及流式细胞仪进行检测,结果PC-9-GR ALDHbright CSCs百分比显著高于PC-9(P < 0.01)。见图3。
2.4 两组细胞株中SIRT1蛋白的表达情况比较
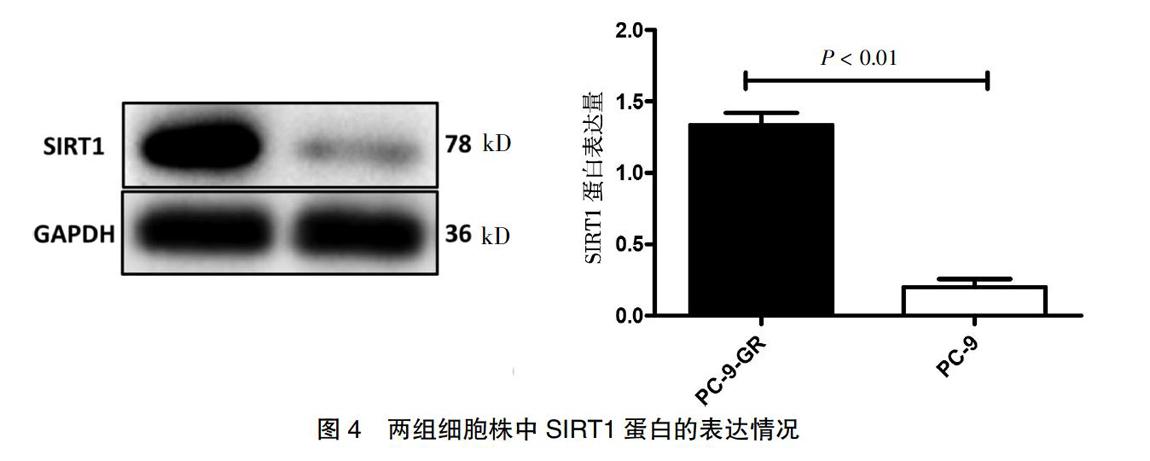
将两组细胞消化后提取蛋白,经Western blot检测SIRT1蛋白,并对蛋白灰度值进行定量分析,结果显示PC-9-GR中SIRT1蛋白显著高于PC-9(P < 0.01)。见图4。
2.5 两组细胞株中SIRT1 mRNA的表达情况
將两组细胞消化后提取RNA,进行qPCR检测,并进行定量分析,结果显示PC-9-GR中SIRT1 mRNA显著高于PC-9(P < 0.01)。见图5。
图5 两组细胞株中SIRT1 mRNA的表达情况
2.6 SIRT1小分子抑制剂TV6逆转PC-9-GR获得性耐药表型
分别采用TV6、吉非替尼、TV6联合吉非替尼处理两组细胞。PC-9-GR成球能力显著高于PC-9(P < 0.05);PC-9用药后,其成球能力均降低(P < 0.05);PC-9-GR应用吉非替尼后,其成球能力较对照组差异无统计学意义(P > 0.05);应用SIRT1特异性抑制剂TV6后,两组细胞株的成球能力均较对照组明显降低(P < 0.05);而PC-9-GR双药联合后其成球能力较其对照组显著降低(P < 0.05)。见图6。
3 讨论
对于肺癌,特别是EGFR优势突变的患者对EGFR-TKI的反应良好,但最终会产生耐药并屈服。多个研究团队[8,11-19]已阐明其耐药性的分子和生物学机制,如EGFR T790M突变、上皮细胞-间充质转化(EMT)等,干细胞样特性在肺癌的意义已经有所研究[7-9,15-21]。因此,本研究提出了科学假说,即TKI获得性耐药的产生与药物本身富集CSCs有关。
药物耐药与肿瘤干细胞间的关系可能是复杂的。Shien等[11]报道EGFR获得性耐药与肿瘤干细胞样特性相关。通过流式细胞仪检测,本研究也表明吉非替尼耐药细胞的ALDHbright比例显著高于亲本株。
SIRT1在肿瘤的发生、发展中起重要作用[10,20-21]。既往研究[22-23]发现急性髓细胞白血病干/祖细胞表现出SIRT1过表达和对抑制剂的敏感性。Zhang等[24]也观察到多数AML样本中SIRT1表达增加。但有研究[20,25]表明SIRT1具有抑制肿瘤生长的作用。Powell等[25]的研究表明,缺失SIRT1可诱导前列腺上皮内瘤变的发生。
本研究显示,SIRT1在非小细胞肺癌肿瘤干细胞表型及自我更新中发挥重要调控作用,对于靶向CSCs有助于逆转获得性耐药现象具有一定的意义。虽然TV6的药理学性质可能不是药物开发的合适候选者,但它可能是癌症潜在治疗的有用化合物。本研究结果也支持进一步调查Tenovin衍生物和其他抑制剂,以期延缓TKI治疗的耐药。
综上所述,本研究发现,在PC-9-GR中,应用TV6抑制SIRT1特异性表达后,干细胞比例减少,成球能力下降。提示SIRT1在非小细胞肺癌CSCs表型、干细胞样特性及自我更新中发挥重要调控作用。因此,抑制SIRT1对CSCs的靶向可能有助于延缓TKIs获得性耐药。
[参考文献]
[1] Mok TS,Wu YL,Thongprasert S,et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma [J]. N Engl J Med,2009,361(10):947.
[2] Singh A,Settleman J. EMT,cancer stem cells and drug resistance:an emerging axis of evil in the war on cancer [J]. Oncogene,2010,29(34):4741-4751.
[3] Jiang F. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer [J]. Mol Cancer Res,2009,7(3):330-338.
[4] Marcato P,Dean CA,Pan D,et al. Aldehyde Dehydrogenase Activity of Breast Cancer Stem Cells Is Primarily Due To Isoform ALDH1A3 and Its Expression Is Predictive of Metastasis [J]. Stem Cells,2011,29(1):32-45.
[5] Luo Y,Dallaglio K,Chen Y,et al. ALDH1A Isozymes are Markers of Human Melanoma Stem Cells and Potential Therapeutic Targets [J]. Stem Cells (Miamisburg),2012, 30(10):2100-2113.
[6] Sullivan JP,Spinola M,Dodge M,et al. Aldehyde Dehydrogenase Activity Selects for Lung Adenocarcinoma Stem Cells Dependent on Notch Signaling [J]. Cancer Res,2010, 70(23):9937-9948.
[7] Kim IG,Kim SY,Choi SI,et al. Fibulin-3-mediated inhibition of epithelial-to-mesenchymal transition and self-renewal of ALDH+ lung cancer stem cells through IGF1R signaling [J]. Oncogene,2014,33(30):3908-3917.
[8] Arasada RR,Amann JM,Rahman MA,et al. EGFR Blockade Enriches for Lung Cancer Stem-like Cells through Notch3-Dependent Signaling [J]. Cancer Res,2014, 74(19):5572-5584.
[9] de Aberasturi AL,Redrado M,Villalba M,et al. TMPRSS4 induces cancer stem cell-like properties in lung cancer cells and correlates with ALDH expression in NSCLC patients [J]. Cancer Letters,2016,370(2):165-176.
[10] Wang Z,Chen W. Emerging Roles of SIRT1 in Cancer Drug Resistance [J]. Genes Cancer,2013,4(3/4):82-90.
[11] Shien K,Toyooka S,Yamamoto H,et al. Acquired Resistance to EGFR Inhibitors Is Associated with a Manifestation of Stem Cell-like Properties in Cancer Cells [J]. Cancer Res,2013,73(10):3051-3061.
[12] Jnne PA. Challenges of detecting EGFR T790M in gefitinib/erlotinib-resistant tumours [J]. Lung Cancer,2008, 60 Suppl 2:S3-S9.
[13] Bai XY,Zhang XC,Yang SQ,et al. Blockade of Hedgehog Signaling Synergistically Increases Sensitivity to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Non-Small-Cell Lung Cancer Cell Lines [J]. PLos One,2016,11(3):e0149370.
[14] Morgillo F,Amendola G,Della Corte CM,et al. Dual MET and SMO negative modulators overcome resistance to EGFR inhibitors in human non-small cell lung cancer [J]. J Med Chem,2017,60(17):7447-7458.
[15] Bora-Singhal N,Perumal D,Nguyen J,et al. Gli1-Mediated Regulation of Sox2 Facilitates Self-Renewal of Stem-Like Cells and Confers Resistance to EGFR Inhibitors in Non-Small Cell Lung Cancer [J]. Neoplasia,2015,17(7):538-551.
[16] Cheng J,Yang K,Zhang Q,et al. The role of mesenchymal stem cells in promoting the transformation of androgen-dependent human prostate cancer cells into androgen-independent manner [J]. Sci Rep,2016,6(1):16993.
[17] Ichihara E,Westover D,Meador CB,et al. SFK/FAK signaling attenuates osimertinib efficacy in both drug-sensitive and drug-resistant models of EGFR-mutant lung cancer [J]. Cancer Res,2017,77(11):2990-3000.
[18] Li H. Efficacy of EGFR Tyrosine Kinase Inhibitors in Non-Small-Cell Lung Cancer Patients with/without EGFR-Mutation:Evidence Based on Recent Phase Ⅲ Randomized Trials [J]. Med Sci Monit,2014,20:2666-2676.
[19] Cappuzzo F,Ciuleanu T,Stelmakh L,et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer:A multicentre,randomised,placebo-controlled phase 3 study [J]. Lancet Oncol,2010,11(6):521-529.
[20] Yang H,Bi Y,Xue L,et al. Multifaceted Modulation of SIRT1 in Cancer and Inflammation [J]. Crit Rev Oncog,2015,20(1/2):49-64.
[21] Raffaele P,Mauro C,David DM,et al. Sirtuins and Cancer:Role in the Epithelial-Mesenchymal Transition [J]. Oxid Med Cell Longev,2016,2016:1-9.
[22] Li L,Osdal T,Ho Y,et al. SIRT1 Activation by a c-MYC Oncogenic Network Promotes the Maintenance and Drug Resistance of Human FLT3-ITD Acute Myeloid Leukemia Stem Cells [J]. Cell Stem Cell,2014,15(4):431-446.
[23] Zeisig B,So C. A Knockout Combo:Eradicating AML Stem Cells with TKI plus SIRT1 Inhibition [J]. Cell Stem Cell,2014,15(4):395-397.
[24] Zhang W,Gao C,Konopleva M,et al. Reversal of Acquired Drug Resistance in FLT3-Mutated Acute Myeloid Leukemia Cells via Distinct Drug Combination Strategies [J]. Clin Cancer Res,2014,20(9):2363-2374.
[25] Powell MJ,Casimiro MC,Carlos CC,et al. Disruption of a Sirt1-dependent autophagy checkpoint in the prostate results in prostatic intraepithelial neoplasia lesion formation [J]. Cancer Res,2011,71(3):964-975.

