Evaluation of in vitro and in vivo immunostimulatory activities of poly (lactic-co-glycolic acid) nanoparticles loaded with soluble and autoclaved Leishmania infantum antigens: A novel vaccine candidate against visceral leishmaniasis
Emrah Sefik Abamor, Adil Allahverdiyev✉, Ozlem Ayse Tosyali, Melahat Bagirova, Tayfun Acar, Zeynep Mustafaeva, Serap Derman
1Yildiz Technical University, Chemical and Metallurgical Engineering Faculty, Bioengineering Department, 34220, Esenler-Istanbul, Turkey
2Beykent University, Faculty of Engineering and Architecture, Biomedical Engineering Department, 34398, Sariyer-istanbul, Turkey
Keywords:Visceral leishmaniasis Vaccine Delivery Immunostimulant Poly lactic-co-glycolic acid (PLGA)Nanoparticle
ABSTRACT Objective: To prepare and characterize poly lactic-co-glycolic acid (PLGA) nanoparticles loaded with soluble leishmanial antigen or autoclaved leishmanial antigen and explore in vitro and in vivo immunogenicity of antigen encapsulated nanoparticles.Methods: Water/oil/water double emulsion technique was employed to synthesize PLGA nanoparticles, and scanning electron microscopy, Fourier transform infrared spectroscopy and Zeta-potential measurements were used to identify the characteristics of nanoparticles.Cytotoxicity of synthetized nanoparticles on J774 macrophage were investigated by MTT assays. To determine the in vitro immunostimulatory efficacies of nanoparticles, griess reaction and ELISA was used to measure the amounts of NO and cytokines. During the in vivo analysis, Balb/c mice were immunized with vaccine formulations, and protective properties of nanoparticles were measured by Leishman Donovan unit in the liver following the infection.Cytokine levels in spleens of mice were determined by ELISA.Results: MTT assay showed that neither soluble leishmanial antigen nor autoclaved leishmanial antigen encapsulated nanoparticles showed cytotoxicity against J774 macrophage cells. Contrary to free antigens, both autoclaved leishmanial antigen-nanoparticle and soluble leishmanial antigen-nanoparticle formulations led to a 10 and 16-fold increase in NO amounts by macrophages, respectively. Leishman Donovan unit calculations revealed that soluble leishmanial antigen-nanoparticles and autoclaved leishmanial antigen-nanoparticles yielded 52% and 64% protection against visceral leishmaniasis in mouse models. Besides, in vitro and in vivo tests demonstrated that by increasing IFN-γ and IL-12 levels and inhibiting IL-4 and IL-10 secretions, autoclaved leishmanial antigen-nanoparticles and soluble leishmanial antigennanoparticles triggered Th1 immune response.Conclusions: Both autoclaved leishmanial antigen-nanoparticles and soluble leishmanial antigen-nanoparticles formulations provide exceptional in vitro and in vivo immunostimulatory activities. Hence, PLGA-based antigen delivery systems are recommended as potential vaccine candidates against visceral leishmaniasis.
1. Introduction
Visceral leishmaniasis (VL), otherwise known as kala-azar, is thought to be one of the most severe and life-threatening vectorborne parasitic diseases. With the prevalence in more than 90 countries, VL new cases is estimated to be diagnosed in between 200 and 400 thousand, and about 60 000 deaths occur worldwide every year[1-3]. VL is regarded as the second most deadly tropical disease after malaria. Factors such as global warming, wars, and migration have been found to increase VL mortality rates[4-6]. Despite some antileishmanial drugs as first-line treatment options for the cure of VL, there are many disadvantages (i.e., toxicity, drug resistance, and costliness) limiting the administration in the treatment process[7,8].Therefore, there are increasing studies that aim to develop vaccines against VL recently. However, although the great effort in these studies, researchers have not yet been able to build a vaccine formulation. The global prevalence of VL is increasing rapidly and highlights the importance of the development of new approaches and vaccine formulations[9].
Until now, only first generation vaccine candidates (including parasite fractions, soluble antigens or whole killed parasite antigens)enter into phase Ⅲ clinical trials. Some studies showed that soluble leishmanial antigen (SLA) and autoclaved leishmanial antigen(ALA) were successful immunostimulants and could stimulate Th1 immune response against the disease[10-13]. Such antigens have also been proven to be reliable, simple, controllable, and affordable;however, the appropriate adjuvants are necessary to ensure full protection[10,14].
Limited long-term stability, enzymatic degradation, and poorly immunogenic features are disadvantages of the administration of antigens in a biological system. Hence, to boost the immunogenicity of the vaccine, particularly peptide or protein-based antigens,powerful vaccine delivery and adjuvant systems are necessary[15].Various synthetic polymers can be used as adjuvants in a vaccine delivery system, Poly(lactic-co-glycolic acid) (PLGA) is a copolymer which is approved by the Food and Drug Administration owing to its biodegradability, reliability, and biocompatibility and it has been examined broadly for the preparation of nanoparticular systems of vaccine delivery[16,17]. PLGA nanoparticles provide efficient platform for the delivery of protein-based antigens due to their potential to protect the antigens from enzymatic or ionic degradation until the uptake by antigen presenting cells (APCs)[18]. In addition to their prominent roles in the delivery of antigens into desired tissues or cells, PLGA nanoparticles also exhibit adjuvanticity to increase the immunogenic characteristics of used antigens. For these reasons,vaccine studies have gradually put more emphasis on the use of PLGA for the delivery of protein-based antigens.
A thorough search of the relevant literature showed that very few studies have been conducted to examine the protective properties of antigen encapsulated PLGA nanoparticles as a vaccine formulation against leishmaniasis. Tafagodhi et al. conducted a study to investigate the protective responses of PLGA nanospheres containing autoclaved Leishmania (L.) major (ALM) antigen and CpG oligodeoxynucleotides (ODN) adjuvant against cutaneous leishmaniasis. The researchers concluded that PLGA nanospheres with only ALM or ALM in conjunction with CpG ODN yielded adequate protection against cutaneous leishmaniasis[19]. Because the delivery of immunogens to activate the humoral and cellular immune system for VL is challenging, this system might also generate desired outcomes against VL.
Despite the studies mentioned above, however, to the best of our knowledge, no study developed a new vaccine candidate against VL and explored the immunogenic properties of PLGA nanoparticles including soluble or autoclaved L. infantum antigen (SLA or ALA).To fill this gap, this study aimed to prepare and describe SLA or ALA entrapped PLGA nanoparticles and develop an insight into their in vitro and in vivo immunogenicity on macrophage in mice models.
2. Materials and methods
2.1. Materials
PLGA (lactide: glycolide=50:50; inherent viscosity 0.45-0.60 dL/g, Mw~38-54 kDa P50/50), fluorescein isothiocyanate,polyvinyl alcohol (PVA), 3-(4,5-dimethyl triazole-2-il)-2,5-diphenyl tetrazolium bromide (MTT), dimethyl sulfoxide was obtained from Sigma Aldrich (St. Louis, USA), and dichloromethane was obtained from Ridel de Haen. Also, ultra-pure water was obtained from the Millipore Milli-Q Gradient system.
2.2. Preparation of polymeric nanoparticles (NPs)
For the encapsulation of SLA or ALA into PLGA nanoparticles, the water/oil/water (w/o/w) double emulsion solvent evaporation method was employed. Antigen (SLA or ALA) solution (50 mg/mL) in ultra-pure water was initially added to the organic phase as an inner aqueous phase (40 mg/mL PLGA in dichloromethane). A probe sonicator (Bandelin Sonopuls, Germany) was used to emulsify the aqueous phase in the organic phase for 90 s at 100 W electric power input and an amplitude of 80% over an ice bath. The first emulsion was mixed into 10 mL aqueous solution of PVA (3% w/v). To achieve the double emulsion (w1/o/w2), this solution was emulsified by the same settings in an ice-water bath and diluted in 35 mL PVA solution(0.1% w/v). The organic phase (dichloromethane) was vaporized on a magnetic stirrer plate at 350 rpm and room temperature for overnight. The resulting particles were centrifuged at 10 000×g for 40 min (Sartorius-Biofuge), and then ultra-pure water was used to wash them. An equivalent volume of ultra-pure water was similarly encapsulated in P50/50 for the free NPs (without any antigen) to serve as the control group. The nanoparticles that were obtained(Free-NPs, ALA-NPs, and SLA-NPs) were lyophilized and stored at-80 ℃.
2.3. Physicochemical characterization
2.3.1. Determination of antigen encapsulation efficiency(EE) and antigen-loading capacity (LC)
The bicinchoninic acid (BCA) assay was employed to determine the EE and LC[20]. To this end, the supernatant obtained from the ultracentrifugation of each NPs formulation by the BCA techniques was analyzed. EE and LC were calculated according to the following equations (1) and (2).

2.3.2. Zetasizer measurements
Dynamic light scattering (Malvern Zetasizer, ZS, Malvern, UK)technique was employed to determine the particle size, zeta potential,and polydispersity index (PDI) of NPs[21]. Measurements were performed in triplicate at 25 ℃, using 0.8872 cP viscosity and 1.330 refractive indexes for the solutions, dielectric constant 79; f(ka) 1.50(Smoluchowski)[22]. During the measurements, all samples were diluted to 1:30 dilution factors with ultra-pure water (Millipore).
2.3.3. Fourier transform infrared (FTIR) spectrometry and scanning electron microscopy (SEM) measurements
IR-Prestige 21 FTIR spectrophotometer (Shimadzu, Japan) was used in the range of 4 000 cm-1-600 cm-1and a resolution of 4 cm-1.Also, each sample (Free-NPs, ALA-NPs, SLA-NPs) was scanned 16 times as described[23]. To describe the structural changes between free antigen and antigen-loaded NPs after the encapsulation procedure, FTIR spectroscopy was used.
An SEM (A JSM-7001FA, Jeol, Japan) was used to assess the particle morphology of NPs after coating with gold (3 min) under vacuum conditions[24]. The surface morphology of the samples was analyzed at 7-kV pulse at different resolutions.
2.3.4. In vitro antigen release
The in vitro release of SLA or ALA antigen from nanoparticle formulations was conducted in pH 7.4 phosphate buffer solutions(PBS) in accordance with the dissolution method presented by Arasoglu et al.[25] with minor modifications. A total of 5 mg of lyophilized antigen-loaded nanoparticles were dispersed in 5 mL PBS 0.01% sodium azide. Following this, the suspension was incubated at 37 ℃ at pH 7.4 in a shaking incubator (60 rpm).The medium was entirely removed, and fresh medium (PBS) was replaced each time at time intervals that were predetermined (1, 2,4 h, 1, 2, 3, 4, 5, 10, 15, 20, 25, 30, 35 days). The BCA assay was employed to measure the amount of released antigen.
2.4. In vitro assay
2.4.1. Parasite cultivation
L. infantum promastigotes (EP126) were cultured in RPMI-1640 medium (GIBCO BRL., Grand Island, NY, USA) complemented with 10% fetal bovine serum (FBS), 80 µg gentamicin/mL at 27 ℃incubator.
2.4.2. Preparation of SLA
The freezing/thawing method was applied to prepare the SLA. L.infantum promastigotes, which were cultured at the log phase, were centrifuged at 3 500 rpm for 20 min to obtain the parasite pellet.In order to remove medium content and cell debris, the pellet was washed three times with sterile PBS. Following this procedure, the remaining pellet was reconstituted with 1 mL of PBS. Afterward, the pellets were frozen in liquid nitrogen for 5 min. The next step was dissolution in a 37 ℃ water bath for 5 min. The process was repeated for five times. The resulting lysate was centrifuged at 10 000 rpm for 15 min to eliminate insoluble fractions. UV-spectrophotometer was used to measure the amount of antigenic protein in mL, which was kept at -40 ℃ until being used. The following formula was tested to calculate the protein concentration:
Protein concentration (mg/mL)=(A280×1.55)-(A260×0.76) (3)
2.4.3. Preparation of ALA
L. infantum promastigote cultures were centrifuged three times at 3 500 rpm for 20 min. The resulting pellet was reconstituted with 1 mL PBS. Then, it was autoclaved at 121 ℃ under 1.1 atm pressure for 15 min. UV-spectrophotometer was again used to measure the amount of antigenic protein in ml. It was kept at -40 ℃ until being used. The same formula with SLA was employed to assess the protein concentration.
2.4.4. Culture of J774 macrophages
J774 cells were cultured in RPMI 1640 medium containing 10% FBS,80 µg gentamicin/mL per mL (8% FBS-RPMI) in flask at 37 ℃ in 5%CO2-air.
2.4.5. Cell viability measurement
A tetrazolium dye assay (MTT) (Sigma Chemical Co. (St. Louis,MO, USA) was used to measure the in vitro viabilities of J774 macrophage cells to which different formulations were administered.1×104cells which were seeded in 96 well plates were incubated at 37 ℃overnight. Each well was refreshed with RPMI 1640 complemented with 5% FBS and different concentrations of nanoparticles (from 10 to 500 µg/mL) were used. Only, the medium was added into the control wells. Following 48 h of incubation, 10 µL of MTT (10 mg/mL) was added into each well and incubated for 4 h at 37 ℃, 5% CO2. The viable cells formed purple-colored formazan crystals, which were made soluble by adding to each well 100 µL dimethyl sulfoxide.A multiwell scanning spectrophotometer (Thermo Scientific Microplate Reader) was used at a wavelength of 570 nm to evaluate the optical density.
2.4.6. Nitric oxide assay
For the determination of immunostimulatory effects of free antigens,free nanoparticles, and antigen-loaded nanoparticles, the nitric oxide assay was performed on J774 macrophage cell line. J774 macrophages(1×104/100 µL medium for each well) which were seeded into 96-well plates (CORNING) were cultivated for 24 h at 37 ℃ in 5%CO2. The cells were then co-incubated at 37 ℃ for 48 h with suspensions comprising different concentrations of SLA, ALA,Free-NPs, SLA-NPs, and ALA-NPs (ranging between 10 and 500µg/mL). On the other hand, to determine stimulatory effects of nonencapsulated antigens on nitric oxide production of macrophages,free SLA and ALA were used as the positive control, whereas PBStreated macrophages were designated as the negative control. Only the medium was added into the control wells. Following exposure of 48 h, supernatants were gathered from each well to measure the levels of NO production. The Griess method was employed to assess the nitrite levels. In a 96-well, a standard nitrate solution (NaNO2)was serially diluted in duplicate. The diluted medium was used as the standard blank. After adding supernatants (50 µL) on the plate, equal volumes of Griess reagents (1% sulfanilamide, 0.1%naphthyl ethylenediamine, and 2.5% H3PO4) were quickly added and incubated at room temperature for 10 min. The absorbance levels were evaluated with an ELISA reader at 540 nm (Thermo Scientific)after incubation. The findings were revealed as µM of NO based on a standard curve which was formed by known concentrations of NaNO2dissolved in the culture medium.
2.4.7. Measurement of cytokine levels
1×104cells/100 µL were seeded into 96-well plates to find out the effects of SLA, ALA, Free-NPs, SLA-NPs, and ALA-NPs on cytokine levels secreted from J774 cells. Subsequent to an overnight incubation at 37 ℃, the cells were treated with 500 µg/mL concentration of each sample for 72 h. Cell-free supernatants were then collected and stored at -20 ℃ until use. An ELISA kit was used by the manufacturer’s instructions (EBIOSCIENCE, Inc.) to determine the concentrations of IFN-γ, IL-12, IL-10, and IL-4 in the supernatants of J774 cell cultures.
2.5. In vivo assay
2.5.1. Immunization of Balb/c mice
In vivo experiments were performed in Bezmialem University Experimental Application and Research Center. The ethical clearance and approval of the animal protocol for conducting experiments on animals was granted from Experimental Animal Ethics Committee (ethics approval protocol number: 2013/128 date:26.07.2013). Inbred, male, six to eight week old Balb/c mice were used in experiments. To investigate the protective effect of vaccine candidates against leishmaniasis, twenty five experimental animals were separated into five groups and immunized with formulations including SLA, ALA, SLA+PLGA, and ALA+PLGA. Nonvaccinated group was accepted as control. All test formulations with either free antigens or antigen entrapped PLGA nanoparticles were prepared in 200 µL PBS supplemented with 240 µg SLA and ALA. The mice were then intraperitoneally vaccinated with the vaccine formulations at two-week intervals. The control mice were only exposed to PBS. Prior to each vaccination, blood samples were collected from submandibular regions of mice and centrifuged at 3 000 rpm for 15 min. Later, the ELISA method was employed to measure the antibody levels in blood samples.
2.5.2. Infection of animals
The mice were intraperitoneally vaccinated with 1×107metacyclic L. infantum promastigotes at stationary phase. At the 4th week after infection, blood samples were collected, and parasites were detected by microculture method. A total of 70 µL blood sample in hematocrit capillary tubes were centrifuged at 3 000 rpm for 5 min. Buffy-coat layer was then removed by micropipettes and mixed with RPMI 1640 media, including 20% FBS. Afterward, the mixture transferred into capillary tubes again was incubated at 37 ℃ for 72 h. Finally,an inverted microscope (20×dimension) was used to detect the parasite existence in the capillary tubes. It was scored as “6+” when the parasite number in investigated zone was more than 50, as “5+”between 40 and 50, as “4+” between 30 and 40, as “3+” between 20 and 30, as “2+” between 10 and 20, and as “+” less than 10.
2.5.3. Sacrification of experimental animals
Thirty days after the vaccination procedure, all experimental control group mice were sacrificed, and in each animal the liver and spleen were taken out for weight measurement. Subsequently,Leishman Donovan unit (LDU) measurements were used to evaluate the amounts of L. infantum parasites. In addition, both the spleens of both mice groups were examined in terms of cytokine levels.
2.5.4. Determination of parasite load
The removed livers and spleens were rinsed with PBS. Then, small pieces were cut from the organs and gently and homogenously touched onto the surface of clean slides to prepare impression smears. All smears were then fixed with methanol for 10 min,and Giemsa dye (1:10) was used to stain the smears for 30 min.Subsequent to the incubation period, distilled water was used to wash the smears which were then viewed using an inverted microscope with immersion oil at 100×. Throughout the analysis,200 liver or spleen cells were examined for each slide. Besides,infective L. infantum amastigotes were counted. LDU measurements(LDU=number of amastigotes/number of host cell nucleus×organ weight as mg) were used to calculate the parasite burdens in each group. Additionally, to specify the protective efficacies of studied formulations, the reduction in the prevalence of parasite burdens in control and experimental groups were defined using LDU values. To this end, the following formula was used:

2.5.5. Measurement of spleen cytokine production Splenocytes isolated from the spleens of vaccinated animals were cultivated in 6-well plates containing RPMI 1640 medium enriched by 10% FBS. To stimulate splenocytes for cytokine secretion, they were exposed to 10 µg ALA or SLA when they reached enough confluency in each well. Following this procedure, treated cells were incubated at 37 ℃ for 72 h. Finally, to measure cytokine responses,supernatants were collected and an ELISA kit was used by the manufacturer’s instructions (EBIOSCIENCE, Inc.) to determine the concentrations of IFN-γ, IL-12, IL-10, and IL-4 in the supernatants of splenocyte cell culture.
2.6. Statistical analysis
The data were analyzed for statistical significance using analysis of variance (ANOVA), and the difference among means was compared by the high-range statistical domain using Tukey’s test. A level of probability was accepted significantly when the P value was below 0.05. Additionally, results associated with cytokine levels are also presented as mean±SD of the triplicate measurement.
3. Results
3.1. Physicochemical characterization
3.1.1. Antigen EE and LC
The results indicate that SLA showed a higher EE than ALA, as 31.4% and 19.1%, respectively. Further, the DL was 16.8% for SLA and 14.1% for ALA.
3.1.2. Particle size, PDI and zeta potential
The mean particle size was (154.4±2.2), (316.3±26.4), (199.1±8.4) nm with PDI of (0.18±0.11), (0.46±0.16), (0.37±0.13), and zeta potential as (-27.6±0.9), (-24.7±2.2), (-25.9±1.4), respectively. According to the results, the particles were in a nano-range, having a narrow size distribution. The PDI values demonstrated that homogeneous nanosuspensions were obtained. Besides, it was found that nanoparticles had high stability according to zeta potential showing a surface charge on the particles.
3.1.3. FTIR and SEM analysis
As demonstrated in Figure 1A and 1B, the FTIR spectra of the frozen-thawed and autoclaved antigen-loaded nanoparticles were provided comparatively with free antigen and empty nanoparticles spectrum. Absorption peaks are delivered to a specific functional group of molecules in the FTIR spectra. Information about the involvement of agents to the surface of the nanoparticles could be obtained from the FTIR analysis of nanoparticles. FTIR analysis showed that no adsorbed active substance was presented on the surface of the particles and antigens were encapsulated successfully to PLGA nanoparticles. Figure 1C and Figure 1D present the SEM results of SLA-NPs and ALA-NPs, respectively. It confirmed that the particles were discreetly spherical with mono-dispersed size distribution for all types of NPs. Furthermore, the results of the SEM analysis were consistent with the results of particle size measurement by the Zeta-Sizer.
3.1.4. Release study
Figure 2 presents the biphasic release pattern of SLA-NPs and ALANPs. An initial burst release occurred during the first 24 h (14.6% for SLA-NPs and 12.3% for ALA-NPs), followed by a slower sustained release up to 75.7% and 65.9% as the main characteristics of the antigen release pattern. The difference between the two nanoparticles in terms of cumulative release was resulted from the differences in particle size with a low swelling of polymer and a long diffusion way in the release media, resulting in a slow diffusion of ALA antigens from the polymeric matrices.
3.2. In vitro assay
3.2.1. Cytotoxicity analysis
Cytotoxicity values of antigen-loaded nanoparticles at concentrations varying between 10 and 500 µg/mL are shown in Figure 3. Neither SLA nor in ALA encapsulated nanoparticles showed cytotoxicity against J774 macrophage cells. Hence, all investigated concentrations of antigen-loaded nanoparticles could be used to determine immunogenic properties such as nitric oxide production and cytokine secretion.

Figure 1. FTIR spectra and scanning electron microscopy images of SLA-NPs and ALA-NPs. SLA-NPs: Soluble leishmanial antigen nanoparticles, ALA-NPs:autoclaved leishmanial antigen nanoparticles; A: FTIR spectra of SLA-NPs, B: FTIR spectra of ALA-NPs, C: Scanning electron microscopy images of SLANPs, D: Scanning electron microscopy images of ALA-NPs.
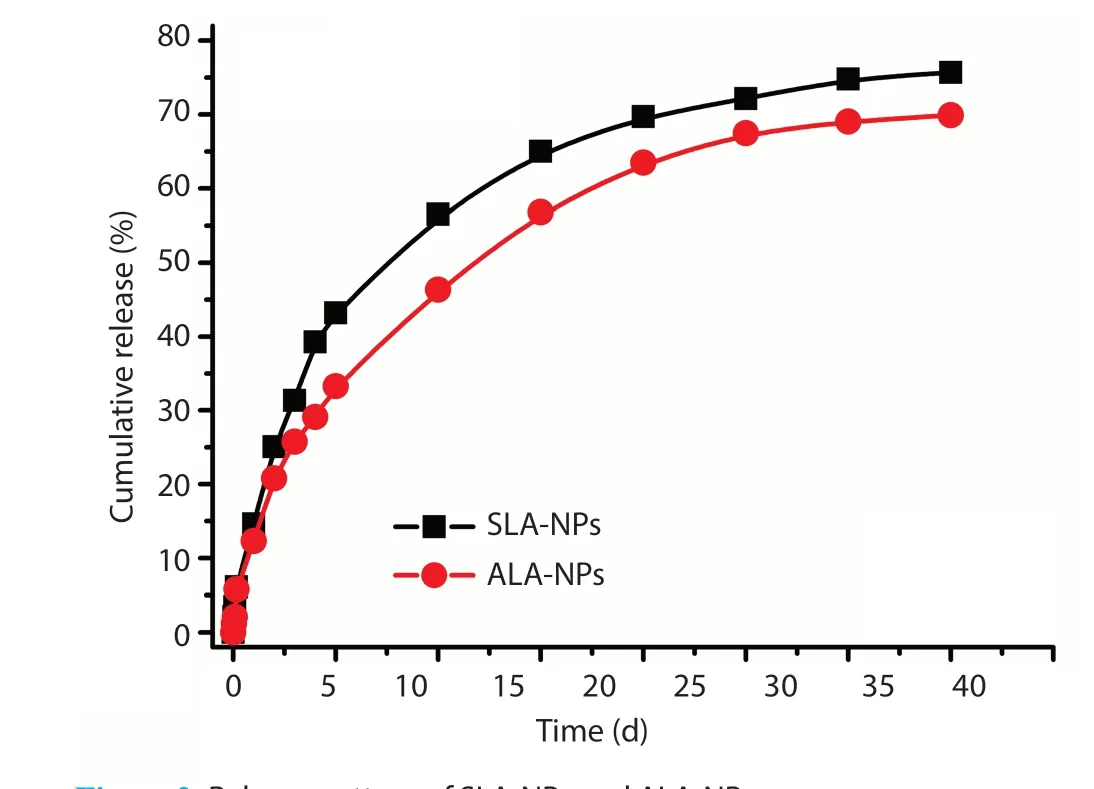
Figure 2. Release pattern of SLA-NPs and ALA-NPs.
3.2.2. Determination of nitric oxide amounts
Figure 4A & B show that treatment with free antigen at concentrations between 10 and 50 µg/mL didn’t increase nitric oxide compared to the control group. However, at concentrations higher than 100 µg/mL(200, 300, and 500 µg/mL), the levels of nitric oxide were significantly increased by SLA and ALA (P<0.05). Figure 4C & D showed that compared to the control, the exposure to 500 µg/mL of SLA and ALA encapsulated PLGA nanoparticles significantly increased the production of nitric oxide (P<0.05). While compared with free antigens, SLA- and ALD-loaded nanoparticles showed significantly higher levels of nitric oxide (P<0.05).
3.2.3. Determination of secreted cytokine levels from macrophages
Figure 5A & B indicate that both SLA and ALA encapsulated PLGA nanoparticle formulations increased the levels of IFN-γ and IL-12 levels compared to PBS-treated controls. It was found that cytokine levels in macrophages increased by approximately 2.5-3.0 folds when they were exposed to SLA-NPs and ALA-NPs formulations compared to control macrophages (P<0.05). On the other hand, we also found that exposure to free antigens yielded higher levels of IFN-γ and IL-12 cytokines than exposure to SLANPs and ALA-NPs formulations (P<0.05). It may be related to sustained release of antigens from PLGA nanoparticles.
Figure 5 C & D demonstrates the IL-10 and IL-4 levels produced by macrophages exposed to SLA-NPs and ALA-NPs formulations;SLA and ALA antigens. SLA-NPs and ALA-NPs formulations yielded nearly 1.5 folds more IL-10 amounts in contrast to the control. It was also found that since ALA and SLA produced 2 and 2.5 folds IL-10 quantities in contrast to the control, produced IL-10 levels were superior for the application of free antigens(P<0.05). Similarly, it was determined that SLA-NPs and ALANPs formulations increased the production of IL-4 amounts when compared to the control. However, macrophages exposed to free antigens secreted higher IL-4 levels than those exposed to SLA-NPs and ALA-NPs formulations (P<0.05).
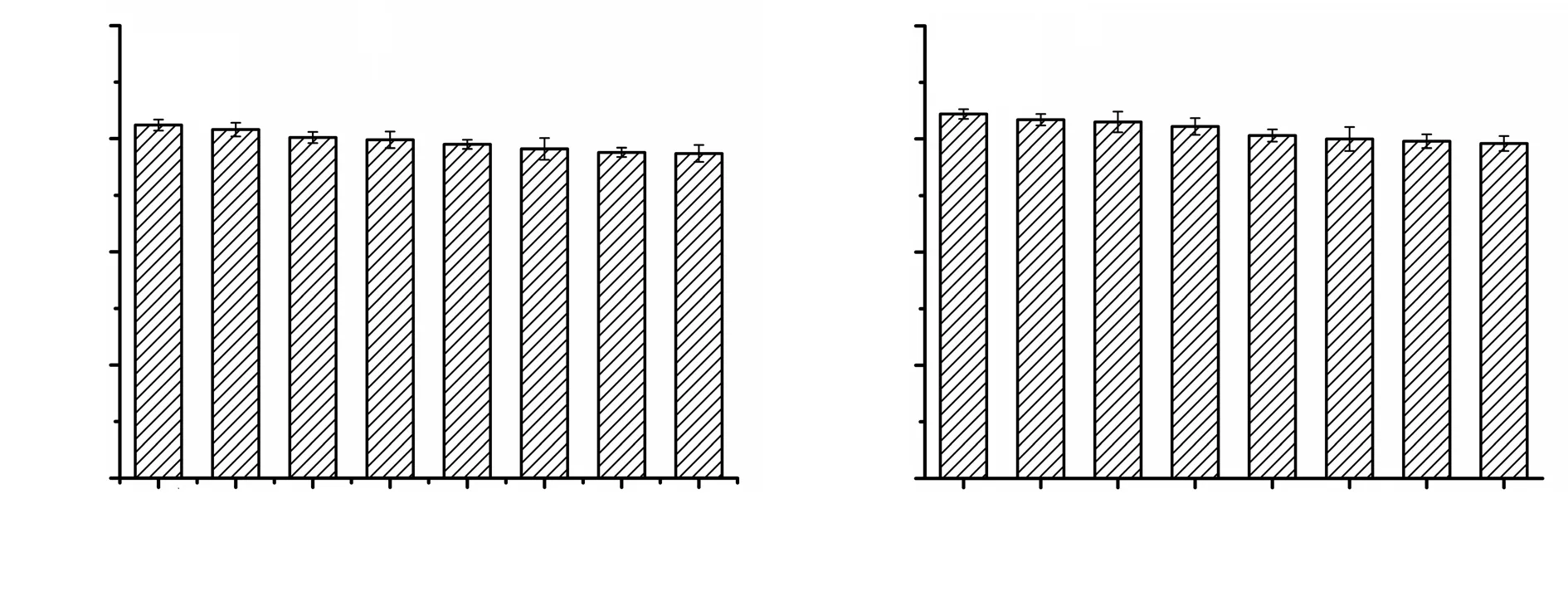
Figure 3. Cytotoxicity of SLA-NPs (A) and ALA-NPs (B) on J774 murine macrophages.
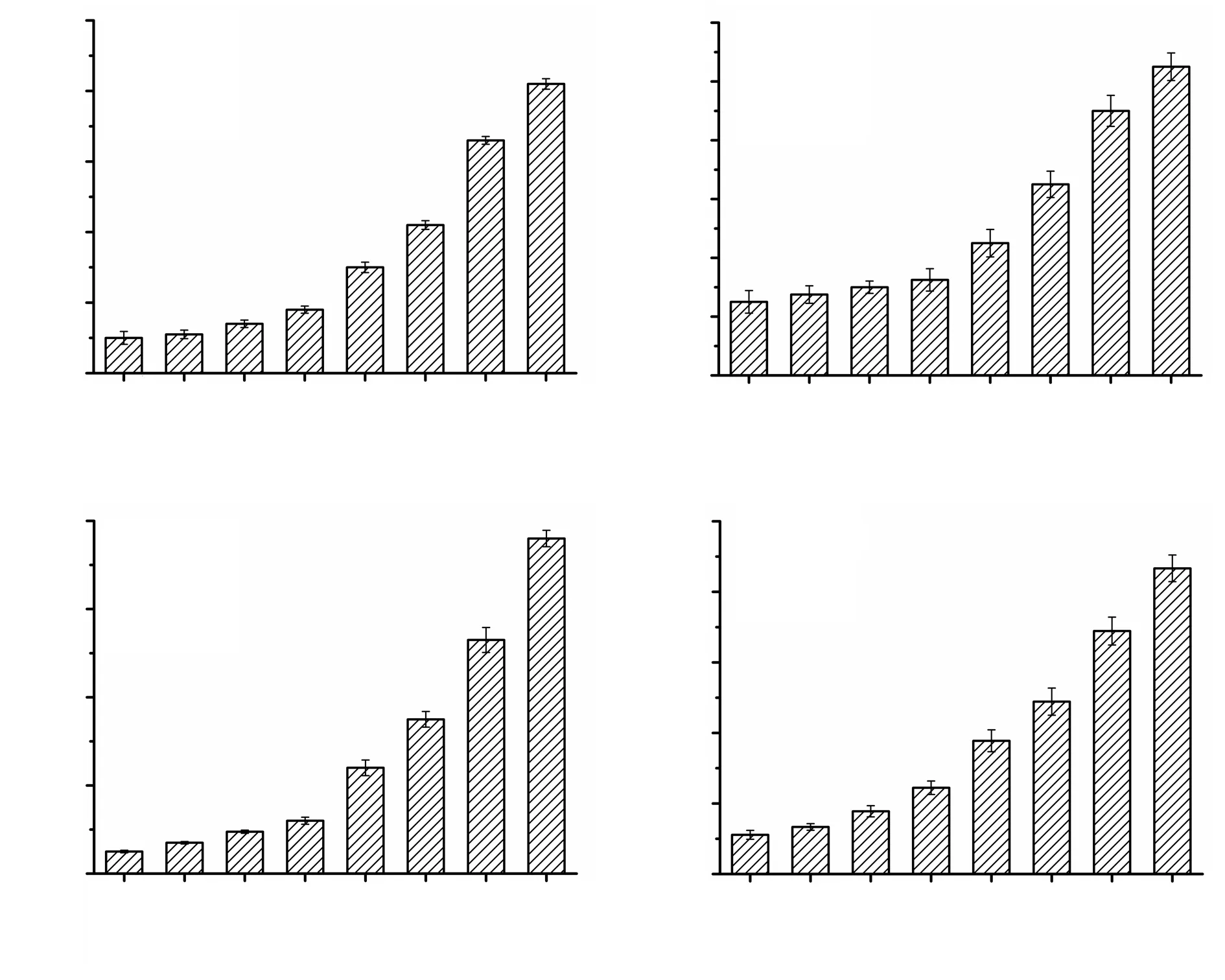
Figure 4. NO production of J774 cells exposed to SLA (A), ALA (B), SLA-NPs (C) and ALA-NPs (D). *P<0.05 compared with the PBS control group.
3.3. In vivo assay
3.3.1. Immunization with vaccine formulations
Figure 6 shows the antibody levels in blood serum of mice immunized with these formulations. The antibody levels were significantly higher in all the test groups when compared to the control group (P<0.05 or P<0.01). The mice immunized with ALANPs showed the highest levels of antibody levels. Besides, SLA-NPs nanoparticles led to an significant increase of nearly 2-folds in the antibody levels compared to SLA formulations (P<0.05).
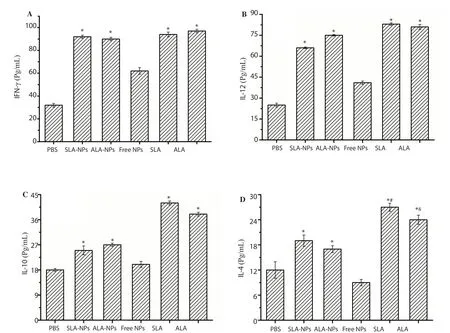
Figure 5. IFN-γ (A), IL-12 (B), IL-10 (C) and IL-4 (D) production by J774 cells. *P<0.05 compared with the control group; #compared with SLA-NPs group;&compared with ALA-NPs group.
3.3.2. Challenge with parasite infection
The groups immunized with antigen-encapsulated polymeric nanoparticles showed less parasite density. The rate of parasite densities was calculated as “6+”, “5+” and “5+” for the control group, the group immunized with ALA, and the group immunized with SLA, respectively. However, both ALA-NPs and SLA-NPs nanoformulations yielded the rate of parasite densities as “3+”,indicating that the parasite density in the peripheral blood of immunized mice was considerably reduced by antigen-encapsulated PLGA nanoparticles.
3.3.3. LDU measurement
Figure 7 shows no significant difference between the control group and the groups immunized with ALA and SLA samples alone in terms of parasite density in the liver. On the other hand, SLA-NPs and ALA-NPs nano-formulations significantly reduced LDU values in livers compared to the control group (P<0.05).
3.3.4. Determination of cytokine levels in spleen of mice
Table 1 demonstrates that neither antigens nor antigen-encapsulated PLGA nanoparticles increased IL4 and IL-10 secretion in the spleens of sacrificed mice significantly, compared with the control group.While all test formulations resulted in significant increase of IL-12 and IFN-γ secretion in the spleens (P<0.05). Levels of IL-12 and IFN-γ in ALA-NPs nano-formulations were the highest (P<0.05).
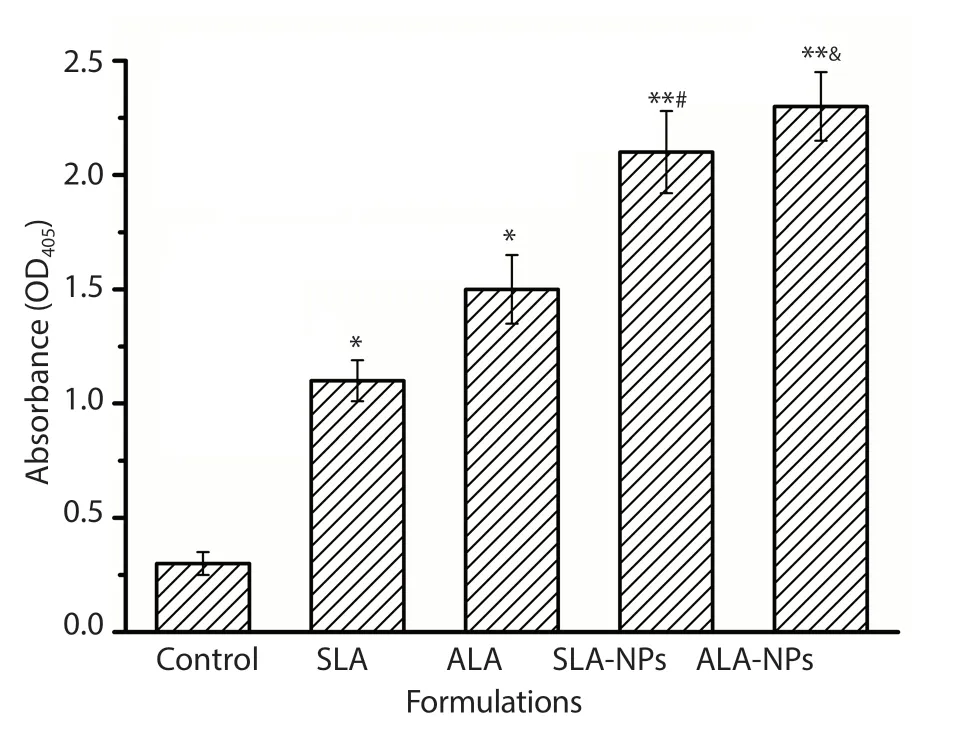
Figure 6. Antibody levels in mice vaccinated with different formulations following six immunization. *P<0.05 compared with the control group;**P<0.01 compared with the control group; #compared with the SLA group;&compared with the ALA group.

Figure 7. Mean Leishman Donovan unit values in livers of mice immunized with different formulations prior to parasite infection challenge. *P<0.05 compared with the control group.
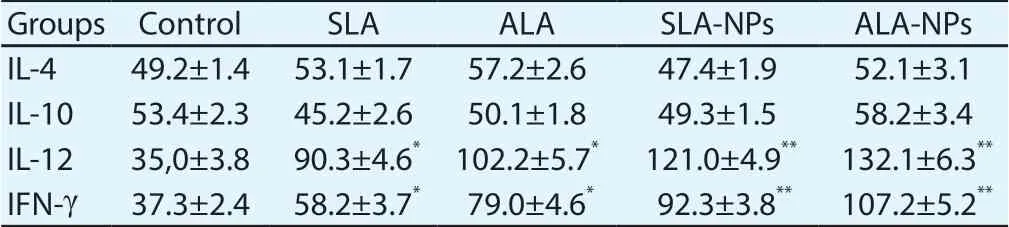
Table 1. Cytokine levels in spleens of mice immunized with different formulations (pg/mL).
4. Discussion
The present study examined in vitro and in vivo immunogenic properties of SLA and ALA encapsulated PLGA nanoparticles to develop a new vaccine candidate against VL. The findings in the study have demonstrated the strong stimulatory effects of SLA and ALA encapsulated PLGA nanoparticles on macrophages to produce significant quantities of nitric oxide. When compared to the control group, SLA and ALA encapsulated PLGA nanoparticles were found to lead to 16 and 10 folds increase in the production of nitric oxide,respectively. Besides, SLA and ALA entrapped PLGA nanoparticles significantly increased cytokine secretion of macrophages. It was found that SLA encapsulated PLGA nanoparticles resulted in an increase in the levels of IFN-γ and IL-12 by 3 and 2.5 folds, respectively. On the other hand, ALA encapsulated PLGA nanoparticles increased IFN-γ and IL-12 levels by 2.5 and three folds, respectively. Also, in vivo analysis revealed that SLA-NPs and ALA-NPs yielded 64% and 52% protection against VL in Balb/c mice models. Furthermore, SLA-NPs and ALA-NPs stimulated splenocytes to secrete IFN-γ and IL-12 cytokines while inhibited the increase in IL-4 and IL-10 levels in Balb/c mice models.
Scientists across the world have so far failed to develop an effective and reliable vaccine against leishmaniasis. One reason is the shortage of adequate adjuvant and insufficient delivery of antigenic molecules to the related cells in the innate and adaptive immune system[26,27]. The most important property of an ideal vaccine against Leishmania is that it should primarily target APCs, mainly belonging to the mononuclear phagocytic system and also dendritic cells. Besides, a perfect vaccine must effectively support higher proportions of engulfment of antigenic molecules to this type of cells[28]. Nanoparticles can be easily absorbed into dendritic cells and other kinds of APCs as they possess comparable dimensions with microorganisms[29-31]. Hence, nanoparticles are thought to play essential roles in the transfer of desired antigens into APCs and stimulating strong immune responses against infections[32].
Due to its biocompatibility, bioavailability, non-toxicity, and biodegradability, PLGA is one of the most commonly administered polymeric vaccine delivery instruments. Furthermore, high adjuvanticity property of PLGA nanoparticles contributes to the immunogenicity of antigens. Therefore, due to sustained antigen release profiles of nanoparticles, the use of PLGA nanoparticles as vaccine carriers leads to long-term interactions of APCs with antigens and durable protection linked with increased immunogenicity of antigens together with adjuvant features of PLGA[33-35].
Although such beneficial properties of PLGA nanoparticles have been proven, there are very limited studies to explore the immunogenic properties of Leishmania-specific antigen containing PLGA nanoparticulate delivery systems. Only a few studies were conducted to examine the immunostimulatory efficacies of PLGA nanoparticles, including ALA isolated from L. major promastigotes against cutaneous leishmaniasis[19]. However, no study has been undertaken to develop an insight into in vitro and in vivo immunogenic properties of SLA and ALA encapsulated PLGA nanoparticles isolated from L. infantum promastigotes and to define their protective efficacies as vaccine candidates against VL.
This study used the double emulsion solvent evaporation method to encapsulate soluble or autoclaved VL antigens to PLGA nanoparticular system. Properties of synthesized nanoparticles were defined in comparison to Free-NPs. The particle size of SLANPs and ALA-NPs were (316.3±26.4) and (199.1±8.4) nm, and encapsulation efficiency were 31.4% and 19.1%, respectively.Besides, the significantly negative zeta potential of all nanoparticles,which is a valuable property that affects the stability of particles,shows that produced nanoparticles have high stability due to the electrostatic repulsion.
Subsequent to the synthesis and characterization of PLGA nanoparticles, the levels of nitric oxide produced were measured to define their impacts on macrophages. Because nitric oxide is a potent lethal factor that obstructs the proliferation of microorganisms, nitric oxide production is one of the most critical mechanisms mediated by macrophages to prevent infections of intracellular pathogens such as Leishmania parasites. IFN-γ, one of the most common Th1 cytokine,stimulates nitric oxide production. IFN-γ has the potential to activate inducible nitric oxide synthase enzyme in macrophages, which then transforms L-arginine to L-citrulline to generate nitric oxide by the help of nicotinamide adenine dinucleotide phosphate cofactor[36,37].On the other hand, in the case of an infection, Leishmania parasites suppress the formation of IFN-γ in macrophages, which leads to the suppression of JAK tyrosine kinase phosphorylation and finally cessation of the production of NO because of inactivation of inducible nitric oxide synthase enzyme[38]. Therefore, a potent vaccine candidate needs to trigger macrophages to produce nitric oxide in large amounts to support the immune response before Leishmania invasion. In the present study, both SLA-NPs and ALANPs led to an exceptional increase in the in vitro production of macrophages when compared to the application of free antigens.
Previous studies have reported the immunostimulating capacities of SLA and ALA for the production of nitric oxide[39,40]. The results of the present study supported these studies. Treatment of macrophages with SLA-NPs and ALA-NPs resulted in increased levels of nitric oxide, which may be attributed to the adjuvant properties of PLGA, and increased the immunogenicity of used antigens. We have previously conducted a study on the immune response of macrophages exposed to canine parvovirus synthetic peptide-loaded PLGA nanoparticles, and found the adjuvant capacity of PLGA nanoparticles to boost the immunogenicity of antigens[41]. We found that antigen-loaded PLGA nanoparticles increased the leves of nitric oxide by approximately two folds when compared to the application of free antigens. It was also observed that PLGA as an adjuvant boosted the immunogenic efficacies of antigens and provided sustained and targeted release. In another study conducted by Santos et al., it was found that compared with empty nanoparticles and free antigens, KMP-11 encapsulated PLGA nanoparticles stimulated macrophages to produce higher amounts of nitric oxide[42]. The results of these studies are consistent with this study, and all show the stimulatory effects of PLGA nanoparticles on macrophages in the production of nitric oxide.
The present study has also focused on the levels of cytokines produced by macrophages and produced in the spleen cells. Th1/Th2 paradigm is known to be an important factor during the process of leishmanial infection. Cytokines linked with Th1 immune response such as IFN-γ, IL-12, and IL-2 directly leads to the extermination of Leishmania parasites, while IL-4 and IL-10 cytokines, which stimulate Th2 immune response, result in the aggravation of the infection. Therefore, an ideal Leishmania vaccine candidate must alter this paradigm towards Th1 response to fight against the infection[43-45]. The results in this study showed that both SLANPs and ALA-NPs significantly increased IFN-γ and IL-12 levels compared to empty nanoparticles and control group. However,among the macrophages and splenocytes exposed to SLA-NPs,ALA-NPs and free nanoparticles, no significant difference was observed in IL-4 and IL-10 levels. In addition, when macrophages were treated with free SLA and ALA, the levels of IL-4 and IL-10 cytokines were not much higher than the levels of IFN-γ and IL-12.Thus, it can be inferred from these findings that both free antigens and antigen-loaded nanoparticles trigger the production of Th1 cytokines more than Th2 cytokines. These types of formulations can be selectively targeted to reduce Leishmania infection by the activation of the mechanisms linked with Th1 immune response.
Also, using LDU measurements, we tried to determine the protective effects of SLA-NPs and ALA-NPs. The LDU method is the measurement of parasite burden in livers and spleens of vaccinated animals to identify the protection rates of vaccine candidates against leishmaniasis. The results showed that SLANPs and ALA-NPs yielded 52% and 65% protection against VL,respectively. These rates were approximately 2-folds higher than free antigens. Certain properties of PLGA nanoparticles (i.e., sustainable release of antigens, directly targeting of APCs, and adjuvanticity)might account for high protection rates of ALA-NPs and SLA-NPs.In our previous study, we investigated the protective efficiencies of vaccine formulations containing conjugates of lipophosphoglycan(LPG) molecule of Leishmania parasite and polyacrylic acid polymer in in vivo mice models. LPG covers the whole surfaces of Leishmania parasites including flagellates and is accepted as one of the most immunogenic molecules of Leishmania species. We demonstrated that LPG-polyacrylic acid conjugates provided much more protection against parasite infection in contrast to free antigens indicating immunostimulatory activity of the polymer as an adjuvant[46]. In the present study, we obtained similar results and confirmed that use of leishmanial antigens together with polymeric constituents that are employed as adjuvants beside of delivery vehicles can increase vaccine potential of native Leishmania-specific molecules. In further studies, polymeric nanoparticles containing antigen cocktails may be applied in order to elevate protection rates against parasite infection.
In conclusion, the present study demonstrate that SLA-NPs and ALA-NPs significantly increased the levels of nitric oxide produced by macrophages in vitro and thus provided high protection against Leishmania infection in mice models. This suggests that the formulations in this study have potent immunogenic properties.Also, future studies may further develop nanotechnology-based new vaccine candidates against VL based on the results in this study. We propose that vaccine delivery systems, especially based on PLGA nanoparticles, might be used to eliminate the disadvantages of present antileishmanial vaccine formulations.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Acknowledgments
The authors express special thank to Scientific and Technological Research Council of Turkey (TUBITAK, Grant Number: 213S148)for financial support of this work.
 Asian Pacific Journal of Tropical Medicine2019年8期
Asian Pacific Journal of Tropical Medicine2019年8期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Chlamydoconidium-producing Trichophyton tonsurans: Atypical morphological features of strains causing tinea capitis in Ceará, Brazil
- Molecular identification of hemoplasmas in free ranging non-human primates in Thailand
- Tioxolone niosomes exert antileishmanial effects on Leishmania tropica by promoting promastigote apoptosis and immunomodulation
- The cholera epidemic of 2004 in Douala, Cameroon: A lesson learned
- Pharmacological and analytical aspects of artemisinin for malaria:Advances and challenges
