Tioxolone niosomes exert antileishmanial effects on Leishmania tropica by promoting promastigote apoptosis and immunomodulation
Maryam Hakimi Parizi, Iraj Sharifi✉, Saeedeh Farajzadeh, Abbas Pardakhty, Mohammad Hossein Daie Parizi, Hamid Sharifi, Ali Reza Keyhani, Mahshid Mostafavi, Mehdi Bamorovat,Ahmad Khosravi, Daryoush Ghaffari
1Leishmaniasis Research Center, Kerman University of Medical Sciences, Kerman, Iran
2Department of Pediatric dermatology, Kerman University of Medical Sciences, Kerman, Iran
3Pharmaceutics Research Center, Neuropharmacology Institute, Kerman University of Medical Sciences, Kerman, Iran
4Department of Pediatrics, Kerman University of Medical Sciences, Kerman, Iran
5HIV/STI Surveillance Research Center, and WHO Collaborating Center for HIV Surveillance, Institute for Futures Studies in Health, Kerman University of Medical Sciences, Kerman, Iran
6Research Center of Tropical and Infectious Diseases, Kerman University of Medical Sciences, Kerman, Iran
Keywords:Niosome Tioxolone Leishmania tropica Apoptosis Objective: To explore the antileishmanial effect of tioxolone and its niosomal form against Leishmania tropica.Methods: Tioxolone niosomes were prepared by the hydration method and were evaluated for morphology, size, release study, and encapsulation efficiency. The cytotoxicity of tioxolone and its niosomal form was measured by MTT assay, leishmanicidal activity against promastigote and amastigote by MTT assay, apoptosis by flow cytometry, IL-12, IL-10 and metacaspase gene expression levels by q-PCR.Results: Span/Tween 40 and Span/Tween 60 niosomes had good physical stability as depicted in their size distribution curves and high encapsulation efficiency (>99%). The release profile of the entrapped compounds showed Fickian’s model of tioxolone delivery based on diffusion through lipid bilayers. With the IC50 value for amastigote as (24.5±2.1) µg/mL and selectivity index as 10.5, the Span/Tween 60 niosome (NT2) had a superior effect to other drugs. The CC50 value and IC50 of promastigote value for NT2 were (257.5±24.5) µg/mL and (164.8±20.6) µg/mL, respectively. The flow cytometric analysis showed that tioxolone and niosomal forms induced apoptosis of Leishmania tropica promastigotes in a dose-dependent manner. NT2 increased the expression level of IL-12 and metacaspase genes and decreased the expression level of the IL-10 gene.Conclusions: Niosomes of tioxolone play an immunomodulatory role in increasing Th1 cytokine profile and inhibiting the Th2 cytokine profile. It could be used for treatment of anthroponotic cutaneous leishmaniasis.
1. Introduction
Cutaneous leishmaniasis (CL) is a vector-borne, neglected tropical disease caused by over 20 obligatory intracellular protozoa of the Leishmania species. It is transmitted by the bite of phlebotomine sandflies[1]. The clinical manifestations of the disease vary considerably in CL and are quite complex, from self-limitingABSTRACTto even death if left untreated[1]. The most affected countries are Afghanistan, Algeria, Brazil, Iraq, Iran, and Syria. In the world,1-1.5 million new cases of the disease are reported annually; almost 431 million people are at risk of CL in CL high-burden countries[2].
In the Old World, including Iran, two clinical and epidemiological forms of CL are present. Zoonotic CL caused by Leishmania(L.) major infects small gerbils as the main reservoir host and Phlebotomus papatasi is the principal vector. While anthroponotic CL due to L. tropica infects humans as the major reservoir and Phlebotomus sergenti is the main vector. Anthroponotic cutaneous leishmaniasis (ACL) is transmitted by the bite of female phlebotomine sand flies through an urban life cycle anthropologically (man-to-man transmission)[2]. According to recent reports, the total number of CL cases in Iran is 21 148 annually and the population at risk of CL is 58%(18/31 provinces)[2]. But it is believed that the actual number of cases is 2-5 times higher than reported[3].
In the absence of a practical and efficacious vaccine, the firstline therapeutic options for CL usually rely on the injection of pentavalent antimonials such as Glucantime®[meglumine antimoniate (MA)][3]. However, these compounds have many side effects, variable efficacy, high cost, and drug-resistance. Moreover,treatment has some complications, such as painful injection,especially in children when the lesion is located on the face.Additionally, injection is not recommended when the parasite has progressed to the lymph nodes and cartilages. Systemic therapeutics with pentavalent antimonials or the second-line compound including pentamidine, amphotericin B, or oral miltefosine are valuable alternatives, but their uses are also limited due to their unwanted adverse effects and cost[4].
An alternative treatment for CL skin problems involves topical treatments. Advantages of using this therapy are superior compliance of patients, reduction of costs, minimized drug resistance, and avoidance of systemic toxicity. Topical treatment is currently limited to the least severe forms of CL without risk of dissemination. Topical treatments such as cryotherapy, thermotherapy, photodynamic therapy, imiquimod cream, ketoconazole and allopurinol have been reported, but these have only been effective in combination with other therapies[5].
Among several approaches, the use of vesicular formulations could increase penetration and sustained-release effect of drugs across the skin[6]. These formulations improve the drug release profile, reduce undesirable side effects, decrease effective amount of drug and increase their survival time in biological systems to enhance drug efficacy. The unique construction of these vesicles is niosome produced from nonionic surfactants in the aqueous phase[6].This vesicle is found to be efficient in topical drug delivery as it can enhance residence time of drugs in the skin, and promote the drug to penetrate into the skin in stratum corneum and to deliver in the deeper layer of the epidermis. Also, niosome enhances the penetration of different substances in cells such as Langerhans cells, thus, it can be effective to treat intracellular infection such as Leishmania infection[6-8]. Aflatoonian et al. showed that niosomal topical dapsone gel could be used as an efficient alternative treatment to cryotherapy in CL due to its fewer side effects[8]. In addition, Farajzadeh et al. reported that a combination of niosomal zinc sulphate with intralesional glucantime had equal efficacy versus a combination of cryotherapy plus intralesional glucantime in the treatment of acute CL. So, it can be used in cases that are resistant to first-line treatments[9]. Asadi et al. showed that the topical niosomal formulation of paromomycin with better penetration and efficacy could be used as a new topical drug delivery system for treatment of CL[10]. Also, in another study, it was mentioned that ketoconazole niosomal gel enhanced its penetration across the stratum corneum[11].
Daie Parizi et al. reported the therapeutic effect of topical application of tincture containing a mixture of two drugs, tioxolone and benzoxonium chloride. This formulation was named Thio-Ben.They evaluated the efficacy of Thio-Ben and found that the effect of this drug was not significantly different from that of MA. Also, due to the topical application of Thio-Ben, its side effects were much lower than MA[12].
Benzoxathiol derivatives, especially 6-hydroxy-1, 3-benzoxathiol-2-one (called also tioxolone) have been used for local treatment of acne and psoriasis vulgaris. They are also known as anti-fungal and anti-bacterial. The reports showed that benzoxathiol derivatives had anti-inflammatory and anti-tumorigenic effects through inhibition of NF-κB and STAT3 activation[13,14].
Since the niosomal formulation of this drug may potentially be more effective, this study was designed to evaluate the possible effect of the niosomal form of tioxolone on L. tropica in in vitro model.
2. Materials and methods
2.1. Chemicals and reagents
MA (Glucantime®) 1.5 g/5 mL solution for injection as a positive control drug was purchased from Sanofi-Aventis, France. Penicillinstreptomycin (10 000 U/mL) was obtained from Thermo Fisher Scientific, USA and was frozen at -20 ℃until testing. 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT)powder (Thiazolyl blue), product No. M 5655 was purchased from Sigma-Aldrich®, USA. Fetal bovine serum was purchased from Gibco, USA. DMEM medium with sodium pyruvate and RPMI-1640 medium with stable glutamine were purchased from Biosera,France. Sorbitan esters (Span) and their pegylated derivatives(Tween), cholesterol, and 6-hydroxy-1, 3-benzoxathiol-2-one(tioxolone) were purchased from Sigma-Aldrich®, USA. Ethanol,dimethyl sulfoxide, and chloroform were purchased from Merck(Germany). Phycoerythrin (PE) Annexin ⅤApoptosis Detection KitⅠ was purchased from BD PharmingenTM, USA. Phosphate buffered saline was purchased from Cassion Lab, USA.
2.2. Niosome preparation
Tioxolone niosomes were prepared by the hydration method described by Pardakhty et al[15]. Briefly, the appropriate amounts of
non-ionic surfactants (Span/Tween 20, 40, 60, and 80), cholesterol,and tioxolone (5 000 µg/mL) were dissolved in chloroform in 100 mL round-bottom flasks. The solvent was evaporated using a rotary evaporator (Buchi, Switzerland) at 180 rpm and 70 ℃ for 15 min.The thin layers of lipids formed on the internal wall of the flask were hydrated by adding 5 mL of deionized water at 70 ℃ for 30 min. These formulations were stored at room temperature for further study.
2.3. Characterization of niosomes
2.3.1. Morphological evaluation of lipid vesicles
The type and shape of vesicles and probable niosomal constituents,crystallization/separation or aggregation were observed using optical microscopy (Zeiss, Germany) with emergent micrographs captured.
2.3.2. Niosomes size analysis
Size of non-ionic surfactant vesicles was assessed by measuring their dynamic light scattering with Master Sizer 2000 E (Malvern,UK). Results were presented as average volume diameters of vesicles. All measurements were performed in triplicate. Also,physical stability of niosomes was determined by size variations over 3 days, 1, 3, and 6 months after production[16].
2.3.3. Release study
Release profiles of entrapped materials were determined by dialysis method[16] in selected formulations, after passing 1 mL of the formulations from the dialysis bag (cellulose acetate membrane,Sigma-Aldrich, Germany) at 37 ℃ at certain time intervals (0, 15,30, 60, 90, 120, 150, 180, 210 and 240 min). One mL of the receiver containing 96% ethanol-deionized water (50/50 volume %) was removed and replaced with the same amount of fresh receptor phase.The drug concentration in the samples was measured using the spectrophotometric method at 287.9 nm.
2.3.4 Encapsulation efficiency
The un-entrapped drug was separated by dialysis method, and then niosome was digested by isopropanol. The resultant solution was finally analyzed by the spectrophotometry method to calculate the entrapped drug[16] as per the following equation:
% Encapsulation efficiency=(Total drug-free non entrapped drug)/Total drug×100
2.4. Parasite strain and culture
The standard strain of L. tropica (MHOM/IR/2002/Mash2) was prepared from the Leishmaniasis Research Center (Kerman,Iran). The parasite was cultured in RPMI-1640, 1 µL penicillinstreptomycin (10 000 U/mL) and 15% heat-inactivated fetal bovine serum, and then incubated at (25±1) ℃.
2.5. Cytotoxicity assay
In this study, the cytotoxic effects of tioxolone and its niosomal forms against murine macrophage cells (J774 A.1 ATCC®TIB-67TMpurchased from the Pasteur Institute of Iran) were determined.Macrophages (5×104cells/mL) with various concentrations of drugs (0-500 µg/mL) were cultivated in 96-well tissue culture plates at 37 ℃ and 5% CO2for 48 h. MTT assays were used to investigate the cytotoxicity of the formulations of tioxolone in comparison with the standard drug. MTT at 5 mg/mL was dissolved in RPMI-1640 without phenol red in our experiment. The solution was filtered through a 0.2 µm filter and stored at 2-8 ℃ for frequent use, or frozen for extended periods. MTT stock solution (5 mg/mL) was added routinely to each culture to equal one-tenth of the original culture volume and then incubated for 3 to 4 h. After that, the medium was removed and acidic isopropanol (0.04-0.10 mol/L HCL in absolute isopropanol)was added to stop the reactions. When working with cell suspension,the dye was added directly and dissolution was accomplished by titration. Absorbance of converted dye was measured by an ELISA reader (BioTek-ELX800 Winooski, Vermont, USA) at a wavelength of 490 nm. The percentage of living cells for each repetition (cell viability) was obtained by the following formula:

Where A is average optical density (OD) of treat (media+macrophage cells+drug), B is average OD of negative control (media) and C is average OD of positive control (media+macrophage cells). From the percentage of cell viability, dose-response curves and 50%cytotoxicity concentration values (CC50) were calculated using probit analysis in SPSS software.
A transformed version of proportions was used instead of regressing the actual proportions in probit regression. In probit transformation,each proportion was replaced with the value of the standard normal curve below which is the observed proportion of the area. The regression equation derived could be used to estimate CC50, where the value of probit is set to zero. The output of the model in the graphs and tables is used to calculate CC50.
2.6. Anti-leishmanial activity against promastigote
Leishmanial activity of tioxolone and its niosomal forms at the promastigote stage of L. tropica was evaluated by MTT assay. Initially,promastigotes in logarithmic growth phase (105cells/mL) were added into a 96-well tissue culture plate. Then, varying concentrations of tioxolone, its niosomal forms, or MA alone (positive control)were added to each well and incubated at (25±1) ℃ for 48 h(concentrations based on cytotoxicity results). After incubation, 10µL of MTT solution (5 mg/mL) was added into each well and they were allowed to incubate at 25 ℃ for 4 h. Promastigotes without drug and complete medium with no promastigote and drug were used as untreated control and blank, respectively. All experiments were repeated in triplicate. Finally, absorbance was measured at 490 nm.Dose-response curves and 50% inhibitory concentration values (IC50)were calculated using probit analysis in SPSS software as mentioned for calculation of CC50in the previous section.
2.7. Anti-leishmanial activity against intramacrophage amastigote
A total of 100 µL of macrophage culture (1×105per mL) was added to each slide (75 mm×25 mm) and placed in a sterile Petri dish, which was incubated for 4 h at 37 ℃ and 5% CO2. Host cells were then infected at a 1:10 ratio ( macrophage: promastigote )in stationary phase parasites (L. tropica) and further incubated for 24 h at 37 ℃, 5% CO2, and 85% relative humidity. The next day,drug concentrations similar to promastigote assay were added in triplicates. After confirming macrophage infection levels above 80% (with light microscopy), 100 µL of drugs diluted in DMEM medium were added to the infected macrophages. In addition, the macrophages containing amastigotes without drug and complete medium without parasite and drug were considered untreated control and blank, respectively. After incubation for 48 h at 37 ℃, the medium was removed and the slides fixed with 100% methanol for 2 min. They were then stained with Wright Giemsa for 15 min. The number of amastigotes in 100 macrophages was evaluated for each treatment by direct observation under light microscope.
Percentage inhibition was calculated by using the following formula:
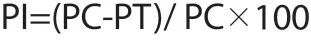
Where PI is the percentage of inhibition, PC the number of amastigotes/100 infected macrophages in the control slide and PT the number of amastigotes/100 infected macrophages in the drugtreated slide. The IC50was obtained using probit test in SPSS software as mentioned for calculation of CC50in the 2.5 section. In addition, the selectivity index (SI) was calculated by the following formula: SI=50% cytotoxicity concentration on macrophages/50%inhibition concentration on amastigotes.
2.8. Apoptotic cell determination
The apoptosis kit used for flow cytometry was the two-channel PE Annexin Ⅴand 7-amino actinomycin (7-AAD) with PE AnnexinⅤApoptosis Detection Kit Ⅰ (BD PharmingenTM). 1×106L.tropica promastigotes were seeded into the microtube and the highest concentrations, as well as concentrations of 50 µg/mL and 12.5µg/mL of each drug were added following incubation at 25 ℃ for 48 h. Promastigotes were washed twice with cold PBS and were suspended in 1× binding buffer. Then, 100 µL of the solution (1×105parasites) was transferred to a 5 mL culture tube and 5 µL of PE Annexin Ⅴand 5 µL 7-AAD were added. Promastigotes were incubated for at least 15 min at room temperature (25 ℃) in the dark. Finally, 400 µL of 1× binding buffer was added to each tube,which was then analyzed by flow cytometry (BD FACSCalibur™,USA) within 1 h.
PE Annexin Ⅴstaining enters cells losting their entire membrane,which accompanies either apoptosis or necrosis processes. Cells that are PE Annexin Ⅴand 7-AAD negative are considered viable; cells that are PE Annexin Ⅴpositive and 7-AAD negative are in early apoptosis; and cells that are both PE Annexin Ⅴand 7-AAD positive are in late apoptosis or already dead.
2.9. mRNA transcripts
Levels of relative expression of interleukin-12 (IL-12) and interleukin-10 (IL-10) in murine macrophage cells (J774 A.1) and metacaspase genes in promastigotes were detected by quantitative real-time PCR(q-PCR) assay. In brief, RNA was extracted from various concentration(200 µg/mL, 100 µg/mL, 50 µg/mL and 12.5 µg/mL) of superior drug(NT2), MA and untreated control group using the RNeasy mini kit(Qiagen, Chatsworth, CA, USA) based on the producer’s protocol.The cDNA was synthesized by a first-strand cDNA synthesis kit(Takara Bio, Inc., Shiga, Japan).

Figure 1. Particle size distribution graphs by frequency at 3 days, 1, 3, and 6 months and light microscopy pictures (×100 magnification). A, B: Span/Tween 40 (6:4 molar ratio) and C, D: Span/Tween 60 (6:4 molar ratio).
The qPCR reaction was carried out in duplicate with the Rotorgene Cycler System (Rotorgene 3000 Cycler System, Corbett Research,Sydney, Australia) and a SYBR Green experiment (SYBR Premix Ex Taq™ Ⅱ, Takara Bio, Inc., Shiga, Japan).
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as reference genes. The gene expressions of IL-12 and IL-10 in murine macrophage cells (J774 A.1) and metacaspase in promastigote were detected by q-PCR assay[17]. The primers are as shown in Table 1. At first, the test was performed at 95 ℃ for 1 min and then the cDNA was amplified by 40 three-step cycles (10 s at 95 ℃ for denaturation of DNA, 15 s at 58 ℃ for primer annealing, and 20 s at 72 ℃ for extension). The final temperature was 65 ℃ for 1 min. The ΔCT was measured by means of the following formula:

Gene expression level was specified by the 2-ΔCtmethod. Moreover,the fold increase (FI) was measured through the comparative threshold method (2-ΔΔCT).
2.10. Statistical analysis
Data were entered into a computer using the SPSS software version 20 (Chicago, IL, USA). ANOVA and independent t-test were used to analyze the difference among the treatment groups. The 50%inhibitory concentration (IC50) and 50% cytotoxicity concentration(CC50) values were analyzed by probit with SPSS software. P<0.05 was considered a significant level.
3. Results
3.1. Niosome characterization
Different formulations of tioxolone were prepared. Based on the morphology of the niosomes (round multilamellar vesicles) and the particle size distribution, the best formulations were chosen as Span/Tween 40 (ST40, 6:4 molar ratio) and Span/Tween 60(ST60, 6:4 molar ratio) (Figure 1). Niosomes were formed in the presence of different amounts of cholesterol as spherical bilayer vesicles. Both formulations demonstrated lognormal particle size distribution curves (Figure 1A & C). The selected formulations had good physical stability as depicted in their size distribution curves during 3 days, 1, 3, and 6 months at room temperature. Tioxolone in ST40 (NT1) and ST60 (NT2) niosomes displayed high encapsulation efficiency (more than 99%).
The release profile of entrapped compounds in the selected formulations demonstrated Fickian’s model of tioxolone delivery based on diffusion through lipid bilayers (Figure 2).
3.2. Optimize concentration without toxicity
Based on the CC50, dosages lower than the toxic doses (CC50)were selected for anti-leishmanial assays. The CC50values for tioxolone and its niosomal forms (NT1and NT2) were (169.4±15.3)µg/mL, (134.4±10.5) µg/mL, and (257.5±24.5) µg/mL against J774,respectively.
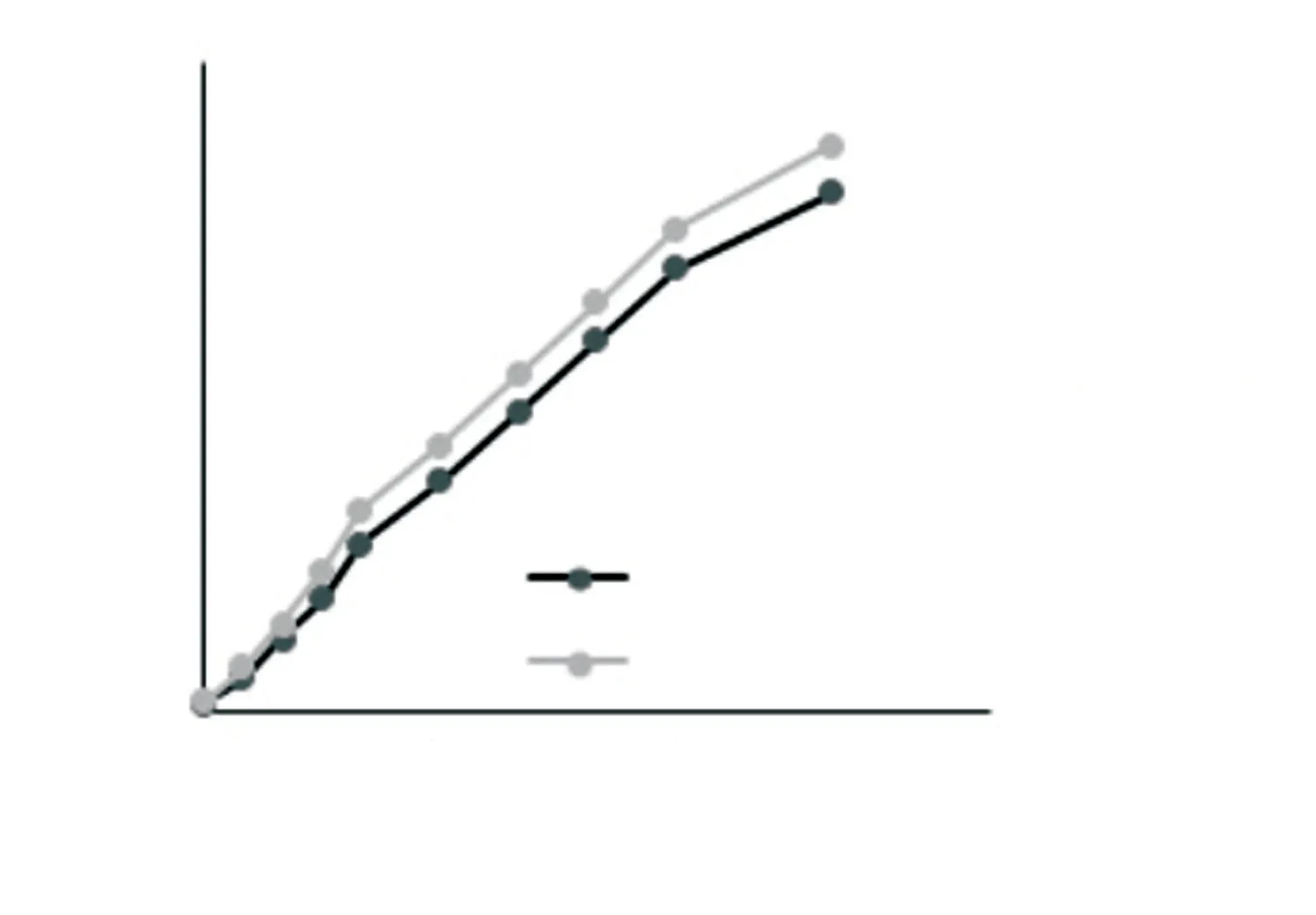
Figure 2. Released amount of tioxolone (%) from the selected formulations at different time intervals.
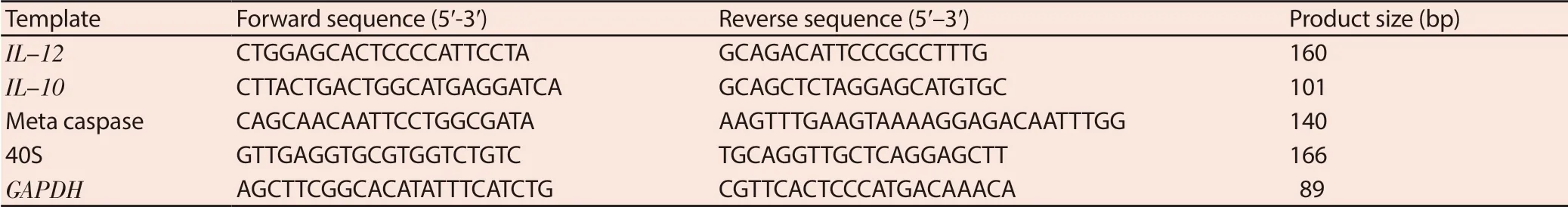
Table 1. Sequences of forward and reverse primers and reference genes for quantitative real time PCR.

Table 2. Effect of tioxolone, niosomal forms of tioxolone and meglumine antimoniate on intramacrophage amastigote of Leishmania tropica.
3.3. Anti-leishmanial activity against promastigote
Various concentrations of tioxolone, niosomal forms of tioxolone(NT1and NT2) and MA showed percentage of inhibition of L. tropica promastigotes in a dose-dependent manner, as presented in Figure 3A. The IC50values of tioxolone, NT1and NT2were (56.1±5.2)µg/mL, (94.3±13.3) µg/mL, and (164.8±20.6) µg/mL against promastigotes of L. tropica, respectively. However, the IC50value of MA was much higher [(536.5±40.0) µg/mL] as the positive control drug. The IC50values of tioxolone, NT1, and NT2were significantly lower than the MA (P<0.001). These results also revealed that tioxolone had a more pronounced leishmanicidal effect on the promastigote of L. tropica in comparison with NT1and NT2, although the difference was not significant with NT1.
3.4. Anti-leishmanial activity against intramacrophage amastigote
Anti-leishmanial activity of drugs in the macrophage model was evaluated by counting the number of amastigotes in 100 macrophages in triplicate (Table 2). Various concentrations of tioxolone, NT1, NT2and MA were all able to inhibit the multiplication rate of amastigotes significantly in each macrophage as compared with the untreated control (P<0.05). The IC50values of tioxolone, NT1, NT2and MA were (49.8±3.4) µg/mL, (23.3±2.8) µg/mL, (24.5±2.1) µg/mL,(101.8±4.2) µg/mL against amastigotes of L. tropica, respectively. In addition, the amastigote inhibition rate of NT2was higher than the other drugs and the standard drug (Figure 3B). Besides, NT2also displayed the highest SI (10.5).
3.5. Apoptotic cell determination
The levels of apoptotic cells, necrotic cells, and viable cells in three different concentrations of each drug were determined and compared with the untreated control and positive control (MA). The highest(58.9%) rate of apoptosis in promastigotes occurred at 150 µg/mL concentration in tioxolone, while it was 0.34% in the untreated control (Figure 4A). Also, NT2(200 µg/mL), NT1(100 µg/mL), and MA (100 µg/mL) showed the high apoptosis (37.35%, 29.11%, and 27.4%, respectively). The levels of necrosis in tioxolone (150 µg/mL),NT2(200 µg/mL), NT1(100 µg/mL), and MA (100 µg/mL) were 11.2%, 6.38%, 6.13%, and 8.93%, respectively (Figure 4).
3.6. qPCR results
The genes expression of IL-12 and metacaspase were increased,while expressions of IL-10 were decreased in NT2and MA group comparing with untreated group (Figure 5). IL-12 and metacaspase expression showed significant difference between MA and NT2in all the concentrations (P<0.05), but the significant difference in IL-10 expression was observed only at 12.5 µg/mL concentration (P<0.05)(Figure 5).
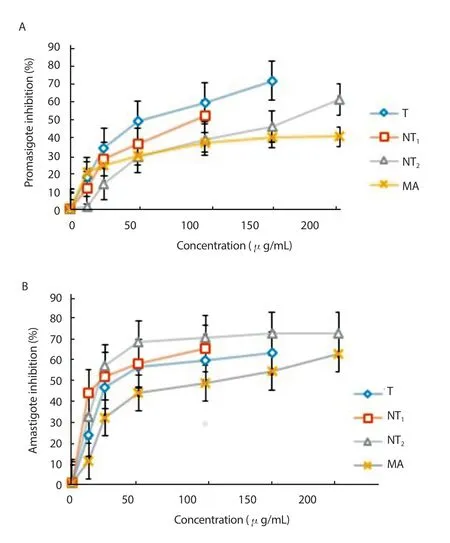
Figure 3. Inhibition of Leishmania tropica promastigotes (A) and amastigote(B) treated with various concentrations of tioxolone (T), niosomal forms of tioxolone (NT1 and NT2) and meglumine antimoniate (MA) after 48 h incubation. Bars represent the mean±standard deviation of inhibition rates.
4. Discussion
Pentavalent antimonials have been the standard, first-line drugs against leishmaniasis for several decades. The emergence of resistance to antimonials, especially in anthroponotic CL foci, has required newer treatment modalities like combination therapies and vesicular forms of drugs[18,19]. Also, the use of topical drugs instead of oral, intravenous and/or intramuscular treatments can reduce systemic side effects[20-22]. A problem with topical use is their low penetration rate into the stratum corneum barrier. Recently, to overcome this problem as well as to facilitate the gradual release of the drug, vesicle structures such as niosomes have been used[6,7].
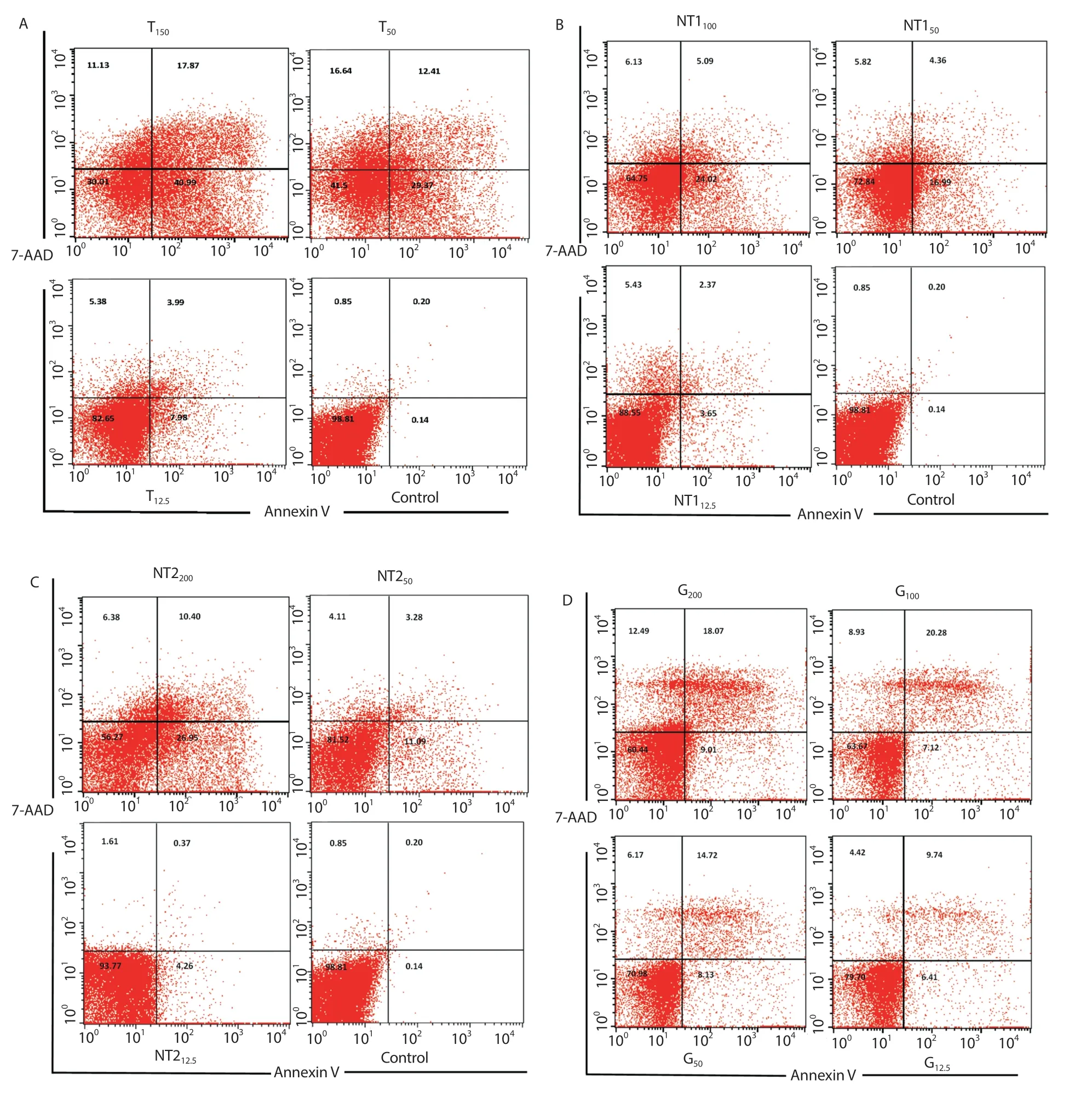
Figure 4. Flow cytometry results showing early and late apoptosis as well as necrotic cells after treatment with A: various concentrations of tioxolone (T), B &C: niosomal forms of tioxolone (NT1 & NT2) and D: meglumine antimoniate (MA) for 48 h.
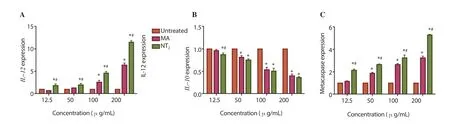
Figure 5. Effect of different concentrations of NT2 on genes expression of IL-12 (A), IL-10 (B), and metacaspase (C) of Leishmania tropica. One-way ANOVA followed by Bonferroni post hoc test. *P<0.05 compared to untreated parasites (control).#P<0.05 compared between MA and NT2.
The previous study has shown that tioxolone and benzoxonium chloride demonstrate a high index of antileishmanial effect on humans[12]. Tioxolone is a biologically active compound and possesses cytostatic, antipsoriatic, antibacterial, and antimycotic properties. Therefore, it has been widely used for various skin and scalp disorders for many years[14,23]. Because it contains ester and thioester groups, tioxolone is also applied in the synthesis of heterocycle-phosphor esters with potential antimicrobial activity[24].Additionally, the study of Tripp’s group showed that tioxolone is a potential carbonic anhydrase inhibitor and possesses several medical applications, such as treating glaucoma, Alzheimer’s disease, osteoporosis, and managing epilepsy and diuretics[24,25].Tioxolone inhibits the enzyme carbonic anhydrase, which catalyzes the conversion of carbon dioxide and water to bicarbonate ions and protons[26]. This reversible reaction helps maintain the acidbase balance within blood and tissues. Tioxolone could even inhibit this overexpression of carbonic anhydrase, leading to loss of the advantage over cancer cells[26]. In a study on some viruses, IC50of tioxolone achieved 16.24 µg/mL[27].
Determination of niosome size is necessary because this factor is very effective on the biodistribution and plasma pharmacokinetics of niosomes. During this study, NT2formulations had more sustainability from three days to sixth months. Due to long and saturated hydrocarbon chain of Span 40 (C16) and Span 60 (C18),gel state lipid bilayers will be formed[28] in comparison to short hydrocarbon chain of Span 20 (C12) or unsaturated chain of Span 80(C9) which both resulted in liquid state of lipid bilayers[29]. Liquid state lipid vesicles usually have less stability and encapsulation efficiencies[30]. This effect was previously reported for niosomes containing autoclaved L. major prepared by Span60[15]. On the other hand, incorporation of polyxylated derivatives of sorbitan esters,Tweens, will increase the steric stability of prepared niosomes[31].Considering that the hydration method of the fatty layer was used in manufacture of niosomes, the diameters of the niosomes were larger than 5 µm. The level of entrapment efficacy in both formulations was very high and the rate of release in the formulation showed that both of them were slow-release. Vesicular systems including niosomes are novel means to deliver drug in a controlled manner to enhance bioavailability and therapeutic effect over a longer period[32]. Tioxolone in free solution form dialyzed completely and rapidly during the first one hour (data not shown). On the other hand, the release profile of niosomal-entrapped material showed an incomplete release profile, less than 65% after 240 min. Two phases release profiles of encapsulated material which shown in our previous studies[15,30], have not been obtained in present research.This difference may be due to the high volume of the receptor compartment in dialysis method (200 mL) used in this study, in comparison to Franz diffusion cell (usually less than 40 mL).
Cytotoxicity assay showed that NT2had less toxicity than tioxolone and NT1against J774 A.1. In addition, the highest level of SI was obtained at NT2(10.5). It is generally considered that biological efficacy is not due to in vitro cytotoxicity when SI≥10, therefore,this formulation showed antileishmanial activity with no toxicity as represented by good SI[33]. The IC50value of tioxolone for promastigote was lower than NT1and NT2; on the other hand, its inhibitory effect was higher as exhibited by flow cytometry analysis.However, according to the IC50for amastigote and SI, the NT2formulation appears to have a better effect than the other drugs. The IC50values of tioxolone and its niosomal forms on promastigotes were higher than that on the amastigote. Despite being inside the macrophages within the vertebrate hosts including humans, the intrinsic susceptibility of amastigote at clinical stage is attributed to physiological and biochemical differences. As previously indicated by many authors, amastigotes are more susceptible to pentavalent antimonials. It is generally accepted that pentavalent antimonials act as prodrug and undergo biological reduction to trivalent antimony within the intra-macrophage amastigotes, which is much more active antileishmanial form. While promastigotes as non-clinical and extracellular form, are not able to reduce pentavalent antimonials[34].
Programmed cell death in the flagellated protozoan parasite Leishmania has been accepted by most authors. It has been described as apoptosis[35]. In Leishmania promastigotes, cells lack any detectable levels of phosphatidylserine, a phospholipid that is exposed at the surface of metazoan cells in response to apoptotic stimuli, and it is not an apoptosis marker in this parasite[36].Instead, several other phospholipid classes, such phosphatidic acid, phosphatidylethanolamine, phosphatidylglycerol, and phosphatidylinositol, have been identified as candidate lipids by annexin Ⅴ staining[37]. Our flow cytometry results showed tioxolone-induced apoptosis in a dose-dependent manner in L.tropica promastigotes.
IL-12 (one of the Th1 cytokines) is an important regulatory cytokine that initiates and regulates cellular immune responses and plays a crucial role in both innate and adaptive immunity of host against predominantly intracellular pathogens[38]. In our study, the level of IL-12 gene expression increased with increasing concentrations(NT2), which was significantly higher than the positive control (MA).It indicates that NT2plays an immunomodulatory role in induction of IL-12 and inhibition of Th2 cytokine gene profiling. This leads to increase in the Th1 cytokine profile, which could make it potentially applicable for treatment of ACL. Experimental studies indicate that IL-10 has potent immunosuppressive activity in leishmaniasis,including suppression of macrophage activation. Moreover, IL-10 production has a strong correlation with disease progression[39]. In our study, by increasing the concentration of the drug, the expression of IL-10 was declined.
Metacaspases are caspase family cysteine peptidases that have been implicated in cell death processes in plants, fungi, and protozoa[40].The single metacaspase of L. major has a major role in the cell cycle and programming cellular death[40]. The metacaspase of L. donovani has a function in cell death pathways[41]. This study also showed that NT2increased metacaspase in promastigotes of L. tropica, and could lead to immune responses towards host-protective Th1 response.Increasing the expression of the metacaspase gene contributes to apoptosis, which was consistent with the flow cytometry result[42].In ACL, since human is the main reservoir and transmission is anthroponotic, chemotherapy is the most effective means to control the disease[2]. Moreover, due to emerging resistance to antimonials[18], the impending challenges necessitate more effective treatment modality and the development of new types of drugs.
The niosomal formulation improves anti-leishmanial activities of tioxolone and promotes a protective immune response to L. tropica.Further investigations using in vivo and human model are needed.
Conflict of interest statement
The authors declare that they have no conflict of intereest.
Foundation project
The present study was part of a Ph.D. thesis and financially supported by the Iran National Science Foundation under Grant ID 95839151 to Saeedeh Farajzadeh.
 Asian Pacific Journal of Tropical Medicine2019年8期
Asian Pacific Journal of Tropical Medicine2019年8期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Pharmacological and analytical aspects of artemisinin for malaria:Advances and challenges
- The cholera epidemic of 2004 in Douala, Cameroon: A lesson learned
- Evaluation of in vitro and in vivo immunostimulatory activities of poly (lactic-co-glycolic acid) nanoparticles loaded with soluble and autoclaved Leishmania infantum antigens: A novel vaccine candidate against visceral leishmaniasis
- Molecular identification of hemoplasmas in free ranging non-human primates in Thailand
- Chlamydoconidium-producing Trichophyton tonsurans: Atypical morphological features of strains causing tinea capitis in Ceará, Brazil
