具备微电解特性的改性泡沫铜去除强力霉素
刘雨知,王 晨,邹东雷,董招君*
具备微电解特性的改性泡沫铜去除强力霉素
刘雨知1,王 晨2,邹东雷1,董招君1*
(1.吉林大学新能源与环境学院,吉林 长春 130000;2.南京大学环境学院,江苏 南京 210023)
通过改进的还原方法把纳米零价铁(nZVI)负载在多孔泡沫铜(CF)上,制备出具有微电解特性的泡沫铜材料(MCF),并利用SEM,SEMMAPPING和EDX对其表面的形态特征及其元素分布进行了表征.考察了不同去除方式,不同MCF投加量和不同强力霉素(DC)初始浓度对降解效果的影响.结果表明:负载等量nZVI的MCF处理效果明显优于nZVI;当DC初始浓度为50mg/L,MCF的投加量为4.0g,反应20min时,DC去除率可达到99%;动力学分析表明,MCF降解DC符合准一级反应动力学,且随着MCF的投加量增加,反应速率常数增大,投加量为5.0g时,值最大为0.0609min-1.
泡沫铜;微电解;纳米零价铁;强力霉素
环境中残留的抗生素已公认是新兴的污染物[1-4].强力霉素(DC)作为四环素类抗生素(TCs)的一种常被用于畜禽养殖业[5],其在使用中并不会完全被畜禽吸收,大部分会通过畜禽粪便进入环境中[6].虽然在地表水中浓度较低但危害持久,会引起病原体抗生素抗性基因的进化,危害人类环境[7-8].近年来,各种环境修复方法已研究用来去除和降解水环境中的抗生素,如吸附作用[9-10],脱氯作用[11],高级氧化法[12],光催化法[13]和超声催化法[14].常规的生物处理很难使其完全降解和矿化,这主要是因为抗生素对微生物的新陈代谢有抑制作用[15].由于DC的低浓度高危害以及难生物降解特性,开发有效的,快速的去除水环境中DC的方法势在必行[16].
传统微电解法(TME)是一种优良的预处理技术[17].TME类似于零价铁(Fe0)腐蚀[18],由铁屑和活性炭(AC)在电解质溶液形成无数的微小腐蚀原电池来去除和降解污染物[19-21].
近年来,基于TME的双金属体系同样受到了研究者的广泛关注.掺杂入TME的贵金属不仅具有催化作用而且可以和铁形成双金属体系,能加速铁的腐蚀作用,从而提高TME降解污染物的能力[22-23].铜,相对其他贵重金属更加经济,同时又具有良好的催化性能,负载铜的微电解材料已被用来去除许多污染物[22,24].有研究表明[25],铁/铜与铁/镍组合成双金属系统降解1,1,1-TCA效率远远高于零价铁单独降解作用. TME制备的填料容易板结,铁泥容易堵塞管道进而会导致处理污染物效率的急剧下降.在此基础上,开发处理效果好,不易板结,铁泥量少的微电解材料是本研究的重要内容.泡沫铜(CF)是一种在铜基体上均匀分布着大量连通或不连通孔洞的新型多功能材料.CF具有良好导电性,同时延展性也很好.CF可用于制备电池负极(载体)材料,催化剂载体等[26].此外,由于纳米材料具有较大的比表面积和较高的表面活性[27-28],nZVI应用于微电解系统中将优于铁屑或铁粉应用于微电解体系中[27,29].
本文通过改进的还原方法将nZVI负载在CF上,并以DC为目标污染物,探究了MCF去除水中DC的影响因素、反应动力学及降解机理,旨在揭示nZVI和CF形成的双金属体系的微电解特性,为MCF在微电解工业中的应用提供参考.
1 材料与方法
1.1 材料
本实验采用的CF采购于江苏苏州昆山某泡沫金属材料公司,体积密度1g/cm3.强力霉素(98%)采购于山东西亚化学工业有限公司,七水合硫酸亚铁(FeSO4×7H2O),硼氢化钾(NaBH4),氢氧化钠,无水乙醇,皆为分析纯,采购于国药集团化学试剂有限公司.
主要仪器包括UV2600紫外-可见分光光度计, pHS-25型pH计,SHA-B型恒温水浴振荡器,DHG- 9055A型鼓风干燥箱,DZF-6050型真空干燥箱, KQ5200V型超声清洗机,配置能量色散X射线光谱仪的XL-30ESEM型扫描电子显微镜,TOC 5000A总有机碳分析仪,FA2004型分析天平等.
1.2 MCF的制备方法
将CF裁剪为(1´1´0.5)cm3大小小块,用体积比为1:4的稀硫酸溶液浸泡2h去除表面杂质后用蒸馏水洗净至中性,放烘箱中60℃烘干备用.
步骤一:称取0.4902g硼氢化钠(NaBH4)放入50mL烧杯中,用10mL超纯水将其溶解,制得预制液A.步骤二:称取1.0000g的FeSO4×7H2O在250mL烧杯中溶解于1/1(/)乙醇/水混合溶液中,然后超声10min,再取5g CF加入上述混合溶液中,再超声10min,使得CF与混合液充分混合,制得预制液B.步骤三:把预制液A溶液逐滴缓慢加入预制液B中.随着预制液A的加入,预制液B中立即会有黑色固体颗粒出现,搅拌反应10min.把从液相中分离出来的CF用25mL乙醇洗涤3次,最后在真空干燥箱中烘干2h去除多余水分.
1.3 降解实验
取一定量MCF添加到250mL锥形瓶中,加入体积为100mL初始浓度为50mg/L的DC溶液,将锥形瓶放入恒温震荡箱中持续反应,反应温度为25℃,转速为160r/min.在不同的时刻取出上清液,过0.22μm的滤膜后用紫外-可见分光光度计法测量DC的浓度,检测波长=277nm,以DC剩余浓度以及TOC作为衡量指标,分别考察不同反应时间下MCF投加量以及DC初始浓度每个实验平行3组,取均值.
1.4 数据处理
具备微电解特性的MCF降解DC的动力学分析采用准一级动力学模型:
d/d= -
积分得:
ln/0=-
式中:(min)为反应时间;(mg/L)为时刻溶液中DC的浓度;0(mg/L)为初始时刻溶液中DC的浓度;(min-1)为速率常数.
2 结果与讨论
2.1 材料表征

(a)未改性的CF的SEM图;(b)反应前MCF 的SEM图;(c)反应后的MCF的SEM图
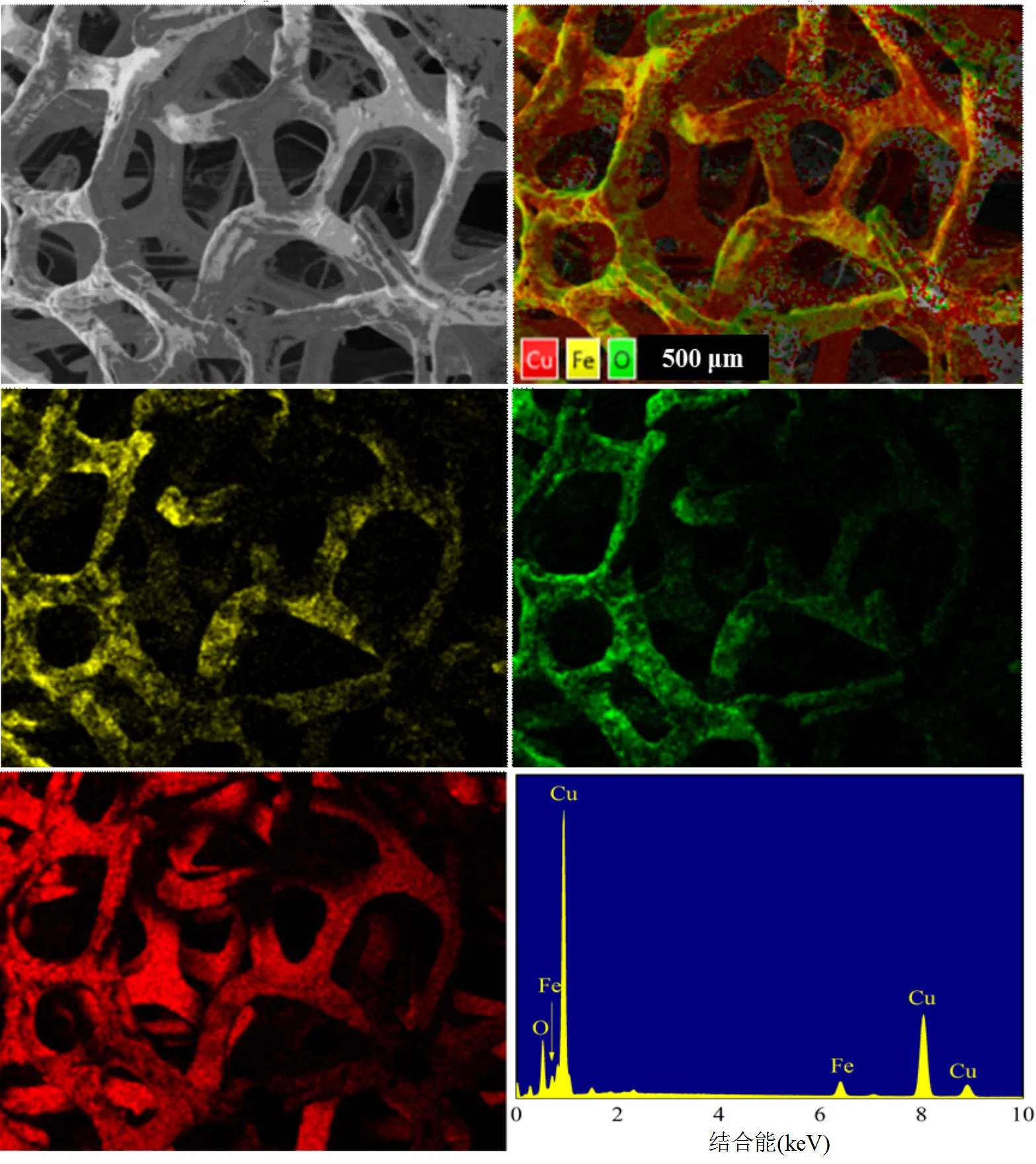
图2 MCF元素分布mapping图
从图1可以看到,CF负载nZVI前后的变化情况.由图1(a)可知,新购置的未负载nZVI的CF呈现金属光泽,而图1(b)表明,在MCF的表面看到明显的黑色nZVI覆在上面,CF也失去了其金属光泽.当放大到500μm时,未负载的CF骨架明显十分光滑,而负载了nZVI的CF骨架则显得十分粗糙.随着倍数的进一步放大,如图中放大到1μm的圆圈所示,图1(a)中表面没有任何物质,而图1(b)中则出现了很多链状物质,说明经过负载后,已将nZVI负载到了CF骨架和空隙中.MCF表面形成了40nm左右的nZVI小球,并呈现链状相连,最终形成网状结构.从1(c)可以看出反应后的MCF表面nZVI小球参与反应被氧化后,形成棉絮状结构依附于CF表面.
为了解具备微电解特性的MCF负载情况,对MCF材料做了元素分布,如图2所示. Fe元素均匀地分布在CF的骨架上,表明实验中MCF制备负载地较为均匀.材料表面含有氧元素,这是因为原本的CF在工业制备过程无法保证完全隔绝氧,导致材料本身含有5%的氧;其次,负载过程中为了简化制备过程,并不是全过程完全隔绝氧气,会导致MCF表面有部分nZVI被氧化.
2.2 不同材料对DC去除效果的影响
本实验设置了3组对照实验来考察不同材料对DC去除效果的影响.对照组分别为Control组:不添加任何材料; nZVI组:仅加入0.015g nZVI; MCF组:约4.0g的MCF.实验结果如图3所示.
由图3可知,Control组在90min内DC的浓度几乎不变;仅加入nZVI的组,DC的浓度缓慢下降,在90min时,去除率只有39%,由于nZVI的还原作用, nZVI对DC的去除具有一定效果,但效果并不显著;而加入了具有微电解特性的MCF组,在20min时,DC的去除率迅速达到97%,90min时,DC仅剩不足0.4%,表明本文构建的微电解体系对于DC的降解有显著的效果.其他学者在研究nZVI和纳米Fe/Cu颗粒对硝酸盐去除结果表明,纳米Fe/Cu颗粒的双金属体系对模拟地下水中硝酸盐的去除效果亦优于nZVI[30-31].
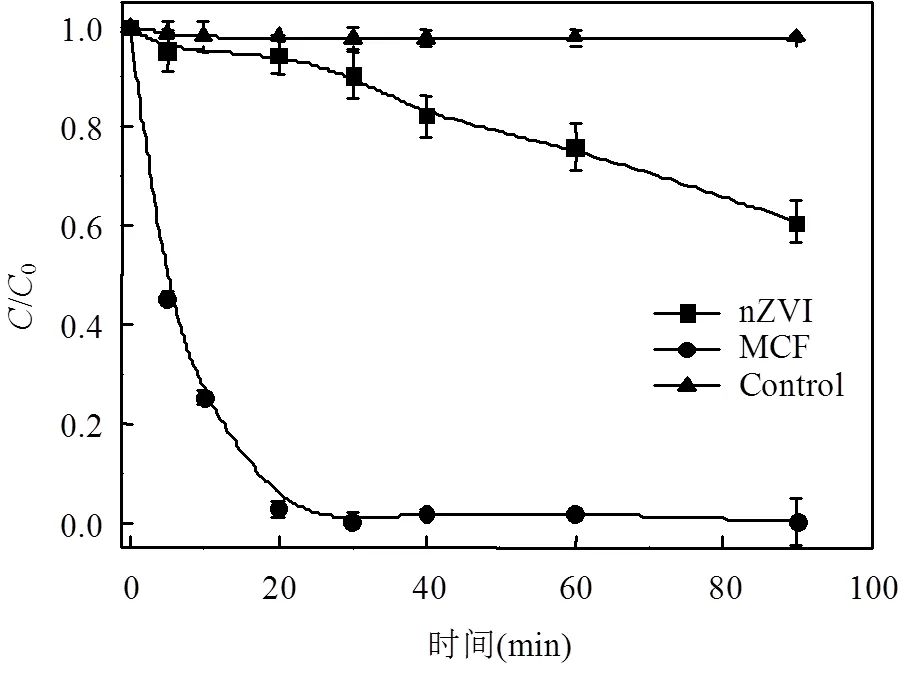
图3 不同材料对DC的去除效果对比
2.3 MCF的投加量对于DC降解的影响
本文构建的微电解体系对于DC的去除本质上是nZVI和CF在DC溶液中形成了无数微小的腐蚀原电池.当投入DC溶液中的MCF材料数量不同时,形成的微小腐蚀原电池数量也不同,因此对于DC的去除效果也有差异.分别加入1.0,2.0,3.0,4,5g MCF来考察MCF的投加量对于DC降解的影响.
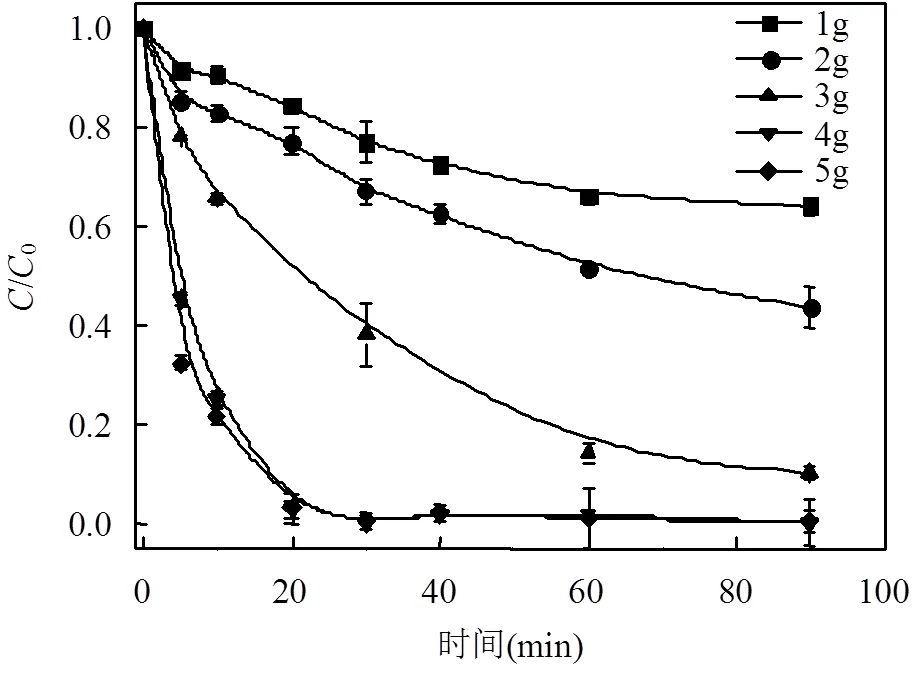
图4 MCF的投加量对于DC降解的影响
由图4可知,具备微电解特性的MCF的投加量对于DC的降解过程有重要的影响.对于100mL, 50mg/LDC,当投加量为1.0g时,90min DC的去除率仅为36%;而当投加量逐渐增加为2.0,3.0,4.0和5.0g时,90minDC的去除率依次为:57%,90%,99%,和99%.由此可见,随着投加量的增加,DC的去除率也随之增加,整体上呈现正相关.此外,当投加量增加至4.0g时,20min时去除率就达到了99%以上,由此可以推测,投加量的增加不仅可以提高DC的去除效果,还可以加快微电解处理DC的速度,更高效地去除溶液中的DC.当投加量进一步增加到5.0g时,去除率提高并不明显,说明即使再加入更多的MCF材料,对DC去除率影响较小,而更多的MCF材料添加明显不符合经济效益.综上所述,本实验最终确定的最优投加量为4.0g.
2.4 DC初始浓度对于DC去除的影响
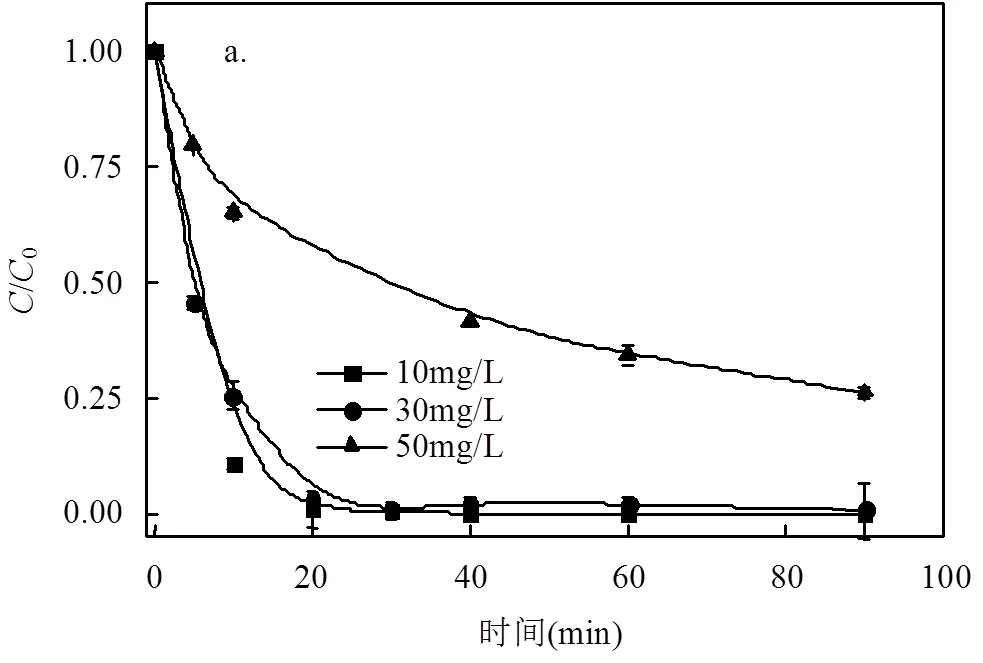
为研究DC初始浓度对于DC去除的影响,本研究设置3个DC溶液浓度梯度,分别为10,30,50mg/L.当投加量为4.0g时,微电解反应去除DC的过程更高效,为了更明显地反映出MCF材料对于不同初始浓度DC的去除效果,本实验选取的投加量为3.0g,其余实验参数与前文一致.
通过图5可知,当DC初始浓度较低时,如10, 30mg/L, MCF可在20min内快速去除溶液中的DC,DC的去除率达到了99%和97%,而50mg/L的DC去除率为73%,这主要是因为微电解对于DC的去除过程是发生在MCF材料表面,在DC溶液中可以形成以nZVI小球为阳极,泡沫铜为阴极的微电解体系,nZVI小球和泡沫铜表面接触的间隙发生着微观的腐蚀电化学反应,二者接触的间隙也就是活性位点存在的地方.而表面反应是DC去除速率的控制步骤,表面活性位点决定了其降解DC的效能,只要保证足够的表面反应活性位点就能使得MCF降解DC快速有效.同时本文也考察了DC反应90min后的矿化度,3个浓度梯度的TOC去除率分别为58%,52%和35%,说明MCF矿化DC的能力较强.
2.5 MCF降解DC的反应动力学
本实验研究了DC初始浓度为50mg/L时,不同MCF投加量下,其去除溶液中DC的反应动力学.如图6所示,5种投加量下的ln(/0)与时间均成良好的线性关系,图7中拟合的直线斜率表示MCF投加量和反应速率常数的关系.5组MCF处理DC的反应速率常数和拟合的相关系数2值如表1.5组反应拟合的相关系数2均大于0.9,表明具备微电解特性的MCF处理DC模拟废水的动力学过程符合准一级动力学模型.
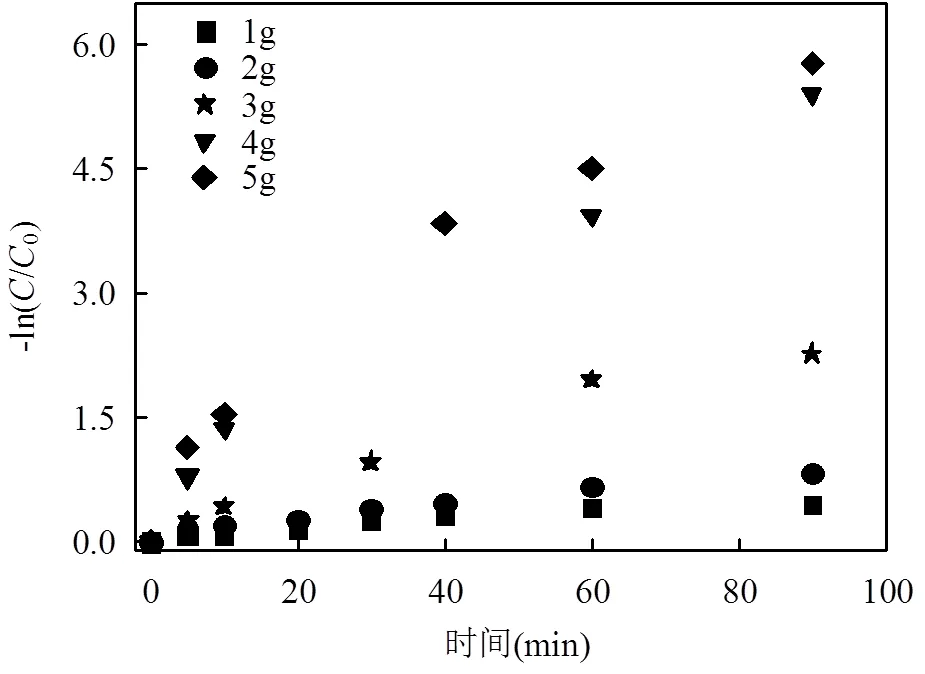
图6 不同投加量下MCF降解DC反应动力学关系
由表1及图6,图7可知,当处理100mL,50mg/L的DC模拟废水,MCF的投加量为5.0g时,值为0.0609min-1,随着投加量的减少,依次减小,直至减少到投加量为1.0g时,仅为0.0050min-1.这是由于随着MCF投加量的减少,形成的微小腐蚀原电池数量减少,同时表面积减少,不有利于MCF材料吸附和降解溶液中的DC,使得反应速率常数显著下降.
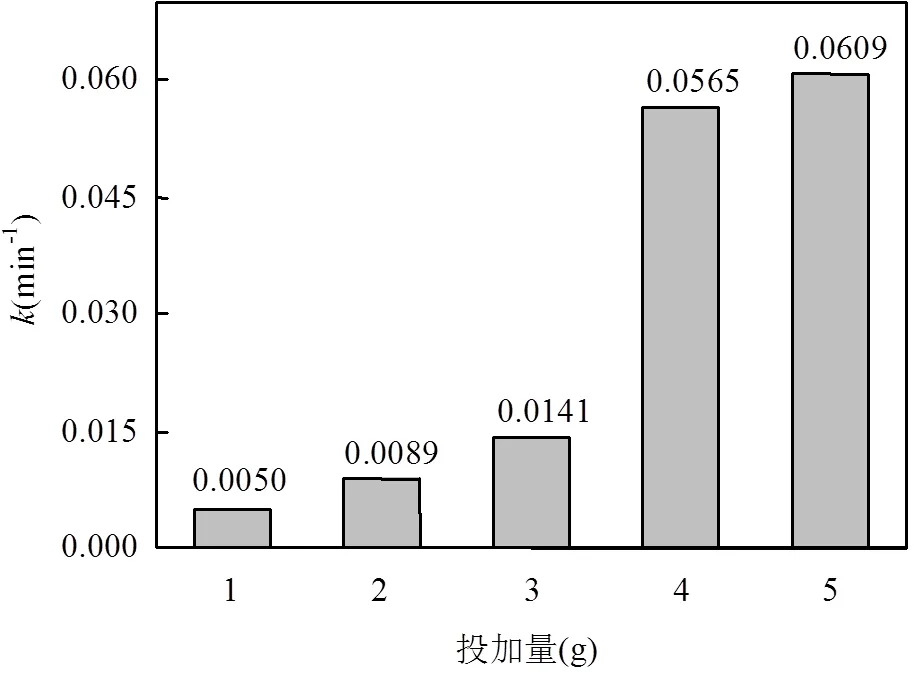
图7 不同投加量下MCF处理DC反应速率常数
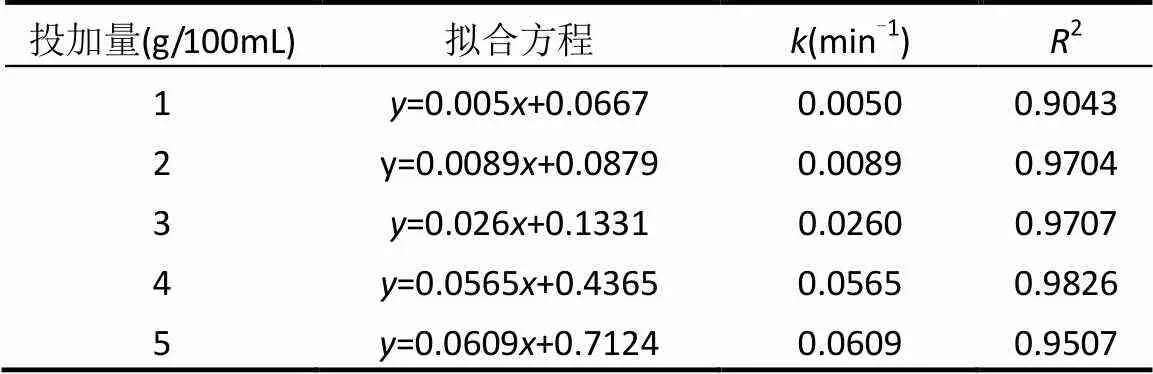
表1 不同投加量下MCF降解DC准一级动力学拟合
2.6 MCF降解DC反应机理
由图8可见,当DC初始浓度过低时,如10mg/L, MCF可在5min内快速去除溶液中的DC,反应过程中的DC在200~400nm范围内的吸光度均很低.这表明MCF不仅能快速去除溶液中的DC,最终也不会引起DC转换为其他带苯环,C=O和C=N(吸收峰在280nm左右)的中间产物滞留在溶液中,故而不会造成二次污染.
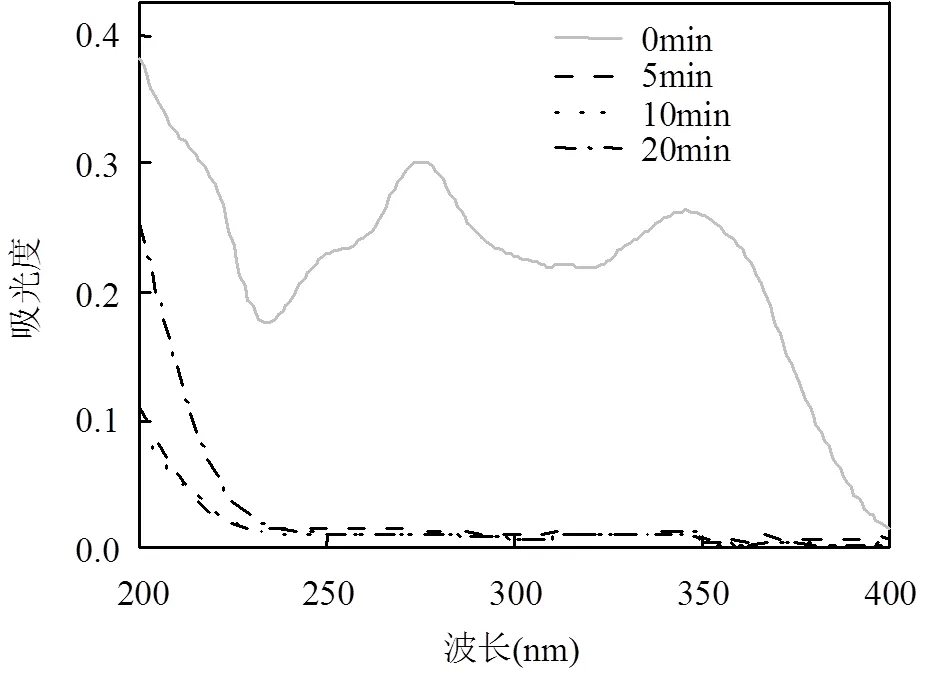
图8 10mg/L DC在反应0~20min时紫外全扫描图
MCF投加量3g/100mL
根据实验结果和相关研究,图9提出了在水环境中MCF降解DC反应机理.在阳极(nZVI)处,电子由nZVI提供,然后通过腐蚀原电池[32]转移到DC和阴极(CF).水电离产生的H+在阴极上得到电子,形成活性氢[H],进一步转变为H2,在这过程中[H]部分降解DC.CF不光作为阴极,也在微电解体系中起到催化作用,加速了阳极(Fe)的腐蚀,从而提高了降解DC的能力[33].
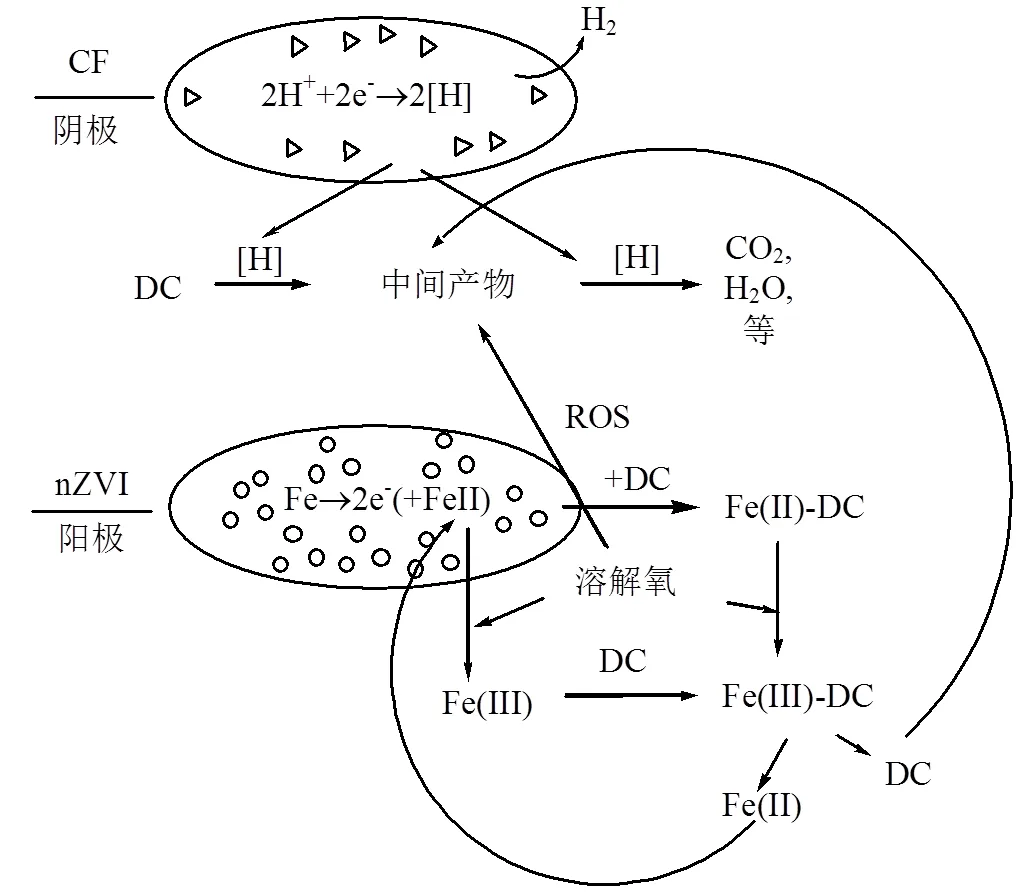
图9 MCF降解DC反应机理
利用nZVI在阳极上通过微电解反应提供的电子,Fe被氧化成Fe(II),形成Fe(III)-DC络合物,Fe(II)通过溶解氧与DC形成 Fe(III)TC络合物和活性氧(ROS).同时,Fe(II)氧化过程中形成的ROS引起DC降解[34].Fe(II)在中性pH值下易被溶解氧氧化,形成稳定的Fe(III).Fe(III)可以与DC络合,氧化DC形成Fe(II)和DC×自由基[35].[H]可通过腐蚀原电池进一步降解DC×自由基.通过微电解反应,络合反应以及产生的ROS一起促使DC降解完全为碳酸和水等.
3 结论
3.1 本研究通过改进的还原方法把nZVI负载在CF骨架上和空隙中,制备了具有微电解特性的MCF.
3.2 随着MCF投加量的增加,DC的去除率也随之增加,整体上呈现正相关.处理不同浓度DC时,保证足够的表面反应活性位点能使得MCF降解DC快速有效,最高去除率为99%.与此同时,MCF处理DC模拟废水的动力学过程符合准一级动力学模型.当MCF的投加量为5.0g时,值为0.0609min-1.
3.3 在水环境里MCF降解DC反应机理中,CF不光作为阴极, nZVI微球和CF表面接触的间隙发生着微观的腐蚀电化学反应;也在微电解体系中起到催化作用,加速阳极(nZVI)的腐蚀.最后通过微电解反应,络合反应以及产生的ROS一起促使DC降解完全为碳酸和水等.
[1] Xu J, Xu Y, Wang H, et al. Occurrence of antibiotics and antibiotic resistance genes in a sewage treatment plant and its effluent-receiving river [J]. Chemosphere, 2014,119:1379-1385.
[2] Cheng W, Li J, Wu Y, et al. Behavior of antibiotics and antibiotic resistance genes in eco-agricultural system: A case study [J]. Journal of Hazardous Materials, 2016,304:18-25.
[3] Kulshrestha P, Giese R F. Aga D S, Investigating the molecular interactions of oxytetracycline in clay and organic matter: insights on factors affecting its mobility in soil [J]. Environmental Science & Technology, 2004,38(15):4097-4105.
[4] Martnez J L. Antibiotics and antibiotic resistance genes in natural environments [J]. Science, 2008,321(5887):365-367.
[5] Bolobajev J, Trapido M, Goi A. Effect of iron ion on doxycycline photocatalytic and Fenton-based autocatatalytic decomposition [J]. Chemosphere, 2016,153:220-226.
[6] Daghrir R, Drogui P, Delegan N, et al. Electrochemical degradation of chlortetracycline using N-doped Ti/TiO2photoanode under sunlight irradiations [J]. Water Research, 2013,47(17):6801-6810.
[7] Khan M H, Jung J Y. Ozonation of chlortetracycline in the aqueous phase: Degradation intermediates and pathway confirmed by NMR [J]. Chemosphere, 2016,152:31-38.
[8] 周 贺,王双玲,徐梦瑶,等.管网多相界面下抗生素抗性菌的分布特征研究 [J]. 中国环境科学, 2017,37(6):2347-2351. Zhou H, Wang S L, Xu M Y, et al. Study on the distribution characteristics of antibiotic resistant bacteria (ARB) in the multi-phase interfaces of water pipe network [J]. China Environment Science, 2017,37(6):2347-2351.
[9] Chen G, Zhan L, Dong Y H. Oxidative degradation kinetics and products of chlortetracycline by manganese dioxide [J]. Journal of Hazardous Materials, 2011,193(20):128-138.
[10] 鲍艳宇,周启星,万 莹,等.3种四环素类抗生素在褐土上的吸附和解吸 [J]. 中国环境科学, 2010,30(10):1383-1388. Bao Y Y, Zhou Q X, Wan Y, et al. Adsorption and desorption of three tetracycline antibiotics in cinnamon soils of China [J]. China Environment Science, 2010,30(10):1383-1388.
[11] Li B, Zhang T. Different removal behaviours of multiple trace antibiotics in municipal wastewater chlorination [J]. Water Research, 2013,47(9):2970-2982.
[12] Kim T H, Kim S D, Kim H Y, et al. Degradation and toxicity assessment of sulfamethoxazole and chlortetracycline using electron beam, ozone and UV [J]. Journal of Hazardous Materials, 2012, 227-228:237-242.
[13] Salazar R, Bago J J, et al. Organic xerogels doped with Tris (2,2¢- bipyridine) ruthenium(II) as hydroxyl radical promoters: Synthesis, characterization, and photoactivity [J]. Chemical Engineering Journal, 2016,306:289-297.
[14] Honseini M, Safari G H, Kamani H, et al. Sonocatalytic degradation of tetracycline antibiotic in aqueous solution by sonocatalysis [J]. Toxicological & Environmental Chemistry, 2013,95(10):1680-1689.
[15] Yang S F, Lin C F, Wu C J, et al. Fate of sulfonamide antibiotics in contact with activated sludge – Sorption and biodegradation [J]. Water Research, 2012,46(4):1301-1308.
[16] Aydn E, M Ş, Taskan E, et al. Chlortetracycline removal by using hydrogen based membrane biofilm reactor [J]. Journal of Hazardous Materials, 2016,320:88-95.
[17] Yang X, Yu X, Wang W. Mechanism, kinetics and application studies on enhanced activated sludge by interior microelectrolysis [J]. Bioresource Technology, 2009,100(2):649-653.
[18] Fan L, Ni J, Wu Y, et al. Treatment of bromoamine acid wastewater using combined process of micro-electrolysis and biological aerobic filter [J]. Journal of Hazardous Materials, 2008,162(2/3):1204-1210.
[19] Gong D. Pretreatment of petroleum refinery wastewater by microwave-enhanced Fe/GAC micro-electrolysis [J]. Desalination & Water Treatment, 2014,52(13-15):2512-2518.
[20] Liu W W, Tu X Y, Wang X P, et al. Pretreatment of coking wastewater by acid out, micro-electrolysis process with in situ electrochemical peroxidation reaction [J]. Chemical Engineering Journal, 2012, s200(16):720–728.
[21] Zhou H, Shen Y, Lu P, et al. Degradation of 1-butyl-3- methylimidazolium chloride ionic liquid by ultrasound and zero- valent iron/activated carbon [J]. Separation & Purification Technology, 2013,104(2):208-213.
[22] Hosseini S M, Ataie-ashtiani B, Kholghi M. Nitrate reduction by nano-Fe/Cu particles in packed column [J]. Desalination, 2011, 276(1–3):214-221.
[23] Zhou H, Lu P, Shen Y, et al. Identification of degradation products of ionic liquids in an ultrasound assisted zero-valent iron activated carbon micro-electrolysis system and their degradation mechanism [J]. Water Research, 2013,47(10):3514-3522.
[24] Trujilloreyes J, S Nchezmendieta V, Col Ncruz A, et al. Removal of indigo blue in aqueous solution using Fe/Cu nanoparticles and C/Fe- Cu nanoalloy composites [J]. Water, Air, & Soil Pollution, 2010, 207(1):307-317.
[25] And J P F, Roberts A L. Reaction of 1,1,1-Trichloroethane with Zero-Valent Metals and Bimetallic Reductants [J]. Environmental Science & Technology, 1998,32(13):1980-1988.
[26] Li H, Cheng F, Zhu Z, et al. Preparation and electrochemical performance of copper foam-supported amorphous silicon thin films for rechargeable lithium-ion batteries [J]. Journal of Alloys & Compounds, 2011,509(6):2919-2923.
[27] Sun Y, Li J, Huang T, et al. The influences of iron characteristics, operating conditions and solution chemistry on contaminants removal by zero-valent iron: A review [J]. Water Research, 2016,100:277-295.
[28] 李 卉,赵勇胜,韩占涛,等.蔗糖改性纳米铁原位反应带对硝基苯污染的模拟修复研究 [J]. 中国环境科学, 2015,35(11):3352-3358. LI H, Zhao Y S, Han Z T, et al. Simulated remediation for nitrobenzene pollution with in-situ reactive zone of sucrose-modified nanoscale zero valent iron [J]. China Environment Science, 2015,(11): 3352-3358.
[29] Zhang W X. Nanoscale Iron Particles for Environmental Remediation: An Overview [J]. Journal of Nanoparticle Research, 2003,5(3):323- 332.
[30] Shubair T, Eljamal O, Khalil A M E, et al. Multilayer system of nanoscale zero valent iron and Nano-Fe/Cu particles for nitrate removal in porous media [J]. Separation & Purification Technology, 2018,193:242-254.
[31] Khalil A, Eljamal O, Saha B B, et al. Performance of nanoscale zero- valent iron in nitrate reduction from water using a laboratory-scale continuous-flow system [J]. Chemosphere, 2018,197:502-512.
[32] Fp V D Z, Bisschops I A, Lettinga G, et al. Activated carbon as an electron acceptor and redox mediator during the anaerobic biotransformation of azo dyes [J]. Environmental Science & Technology, 2003,37(2):402-408.
[33] Liu Y Z, Wang C, Sui Z Y, et al. Degradation of Chlortetracycline Using Nano Micro-Electrolysis Materials with Loading Copper [J]. Separation & Purification Technology, 2018,203:29-35.
[34] Wang H, Yao H, Sun P, et al. Transformation of Tetracycline Antibiotics and Fe(II) and Fe(III) Species Induced by Their Complexation [J]. Environmental Science & Technology, 2016,50(1): 145-153.
[35] Wang H, Yao H, Sun P, et al. Oxidation of tetracycline antibiotics induced by Fe(III) ions without light irradiation [J]. Chemosphere, 2015,119(2):1255-1261.
Removal of doxycycline by modified copper foam with micro electrolysis characteristics.
LIU Yu-zhi1, WANG Chen2, ZOU Dong-lei1, DONG Zhao-jun1*
(1.College of New Energy and Environment, Jilin University, Changchun 130000, China;2.School of the Environment, Nanjing University, Nanjing 210023, China)., 2019,39(7):2864~2870
Modified foam copper (MCF) with micro-electrolytic properties was prepared in this study through an improved reduction method by loading nano-zero-valent iron (nZVI) on porous copper foam (CF). The surface morphology and element distribution of CF before and after nZVI loading were analyzed using SEM, SEMMAPPING and EDX. Besides, The study investigated the effects of removal methods, MCF dosages and initial DC concentrations on the degradation of DC with nZVI loading on MCF. The removal efficiency of DC using nZVI with MCF was much better than that without MCF. When DC concentration was 50mg/L, MCF dosage was 4.0g, and the reaction time was 20min, the removal efficiency of DC could reach as high as 99%. In addition, it was found that DC degradation with MCF fitted the pseudo-first-order reaction kinetics, and the reaction rate constantbecame larger with the increase of MCF dosage. When the MCF dosage was 5.0g, the maximumvalue was 0.0609min-1. Finally, the mechanism of DC degradation with MCF was explored based on all the results and it provided a certain theoretical basis for MCF practical application.
copper foam;micro-electrolysis;nanoscale-zero-valent iron;doxycycline
X703.5
A
1000-6923(2019)07-2864-07
刘雨知(1992-),男,安徽宣城人,吉林大学博士研究生,主要从事水处理新材料研究.发表论文7篇.
2018-12-24
吉林大学无机合成与制备化学国家重点实验室开放课题(2016-25)
* 责任作者, 工程师, dongzhaojun@jlu.edu.cn

