Operative treatment of metastatic breast cancer in the spine with regard to molecular phenotypes
Daniel Adler, Wojciech Pepke, Michael Akbar
Spine Center, Department of Orthopaedic Surgery, Trauma Surgery and Division of Spinal Cord Injury, Ruprecht-Karls-University, Heidelberg 69118, Germany.
Abstract With more than one million new diseases per year breast cancer is the most common malignancy in women. Metastatic breast cancer remains an incurable disease and the spinal column is most likely affected by metastases. A significantly prolonged patient survival is the consequence of modern oncologic treatment options in the last decade. Surgical treatment of vertebral metastases has become an increasing focus for spine surgeons. With the turn of the millennium it was possible to classify breast cancer into four intrinsic phenotypes with various survival rates. Well known scoring systems help surgeons to evaluate the patient´s prognosis and to choose adequate treatment options. However, tumor entities are differentiated without regard to the molecular subtypes. In this article we describe surgical treatment options in metastatic lesions to the spine with regard to molecular phenotypes of breast cancer malignancy. It is crucial to correctly estimate the expected survival time to plan invasiveness of therapy regarding metastatic spine surgery.
Keywords: Spine, metastases, breast cancer, phenotypes, subtypes, molecular level, prognosis
INTRODUCTION
Breast cancer is one of the most common cancer diseases metastasizing to the spine and the second leading cause of deaths in woman related to cancer[1]. Modern treatment and diagnostic concepts including bisphosphonates go along with considerable longer survival times and a reduced rate of skeletal complications[2-4]. Until 2015 mean survival times of 27 months were described, actual studies report mean survival times of 55 months with a 85% incidence of spinal metastases[2-15]. An increased need for surgical treatment of complications related to spinal metastases is the consequence. Due to mortality and morbidity rates related to metastatic breast cancer every surgical treatment should be considered carefully. Well evaluated scoring systems (revised Tokuhashi-or Tomita score, revised Bauer score, Van-der-Lindenscore, Karnofsky index) help surgeons to individually chose optimal treatment methods and to predict life expectancies in cancer patients. But these scores only differentiate between tumor entities whereas respective tumor phenotypes are not taken into account[16-21]. With the turn of the millennium five different intrinsic subtypes of breast cancer were reported by means of gene expression profiles[14-22]. Four phenotypes are decisive in daily clinical routine: luminal A, luminal B, HER2-enriched and basal like/triple-negative. With an accordance of 70%-80%[23]these phenotypes are assessed with immunohistochemical pathologic markers (e.g., ER, PgR, HER2, KI-67/grading) as standardized gene expression profiling is available with partial coverage[24]. In this article we describe the molecular parameters of metastatic breast cancer to predict survival time more precisely and to adequately calculate surgical therapies.
ASSESSMENT OF PROGNOSIS
The development of oncologic treatment options in primary breast cancer (in particular medical therapy) led to considerable improved survival rates in the last decade[2-9,12-15,25-27]. Immunohistochemical diagnostic tools provide a sufficient molecular phenotyping in breast cancer [Table 1].
Visceral, skeletal or cervical spine metastases, surgical complications and advanced patient´s age are reported with different data in literature and therefore are not recommended as negative predictors[13]. In contrast, unimodal postoperative therapies, a disease-free interval less than 24 months, a high number of axillary lymph node metastases and progress after first-line therapy mark assured predictors of shorter survival[4,10,13]. Furthermore, patients’ wishes and preferences, symptoms, biological age, intrinsic breast cancer subtype, tumor burden (number of metastasized organs) and prior therapies have to be taken into account[4].
MOLECULAR PREDICTORS
Around the turn of the millennium, Sørlie und Perou[14,22]published fundamental studies to classify breast cancer at the molecular level with relevance to clinical course of disease. As tumor-biological classifications increasingly become more complex, modern oncologic diagnostic and therapy options interact with highly specialized regulatory mechanisms of cells and cell cycles[8,28]. The actual breast cancer classification [Table 1] is based on histopathological examinations of a biopsy from the breast. Classifying breast cancer[1-3,5-9,12-15]by estrogen receptor (ER), progesterone receptor (PgR), human epidermal growth factor 2 (HER-2) and a proliferation marker (KI-67) is global standard[23,24,28-33]. With these molecular genetic parameters breast cancer is differentiated into the four intrinsic phenotypes, which are clinically relevant [Table 1]. Hormone receptor status comprises the combination of ER and PgR status[3,8,12]. Furthermore, endocrine responsive subtypes (Luminal A and-B), triple negative and HER-2 over expressing immunophenotypes are differentiated[2,3,8,13,14,23]. Luminal-A and Luminal-B phenotypes are clinically distinguished by proliferation rate (e.g., KI-67), grading or a multigen signature[2,3,5-9,15,24,30].
The common classification is based on the ubiquitous availability of these diagnostic methods, which enable comparable studies and therapy concepts. With regard to metastatic breast cancer mean survival times of 60 months are reported in the current literature[4,10,34]in case of endocrine responsive phenotypes (Luminal-A and-B) compared to 26 months until the year 2015[2,3,35]. Compared to PGR positive (PgR+) status, patients with PgR negative (PgR-) status have a 59% higher mortality risk[3]. In patients with hormone receptor negative (HR-) status the mean survival time is reduced by 11 months and mortality risk is 52% higher compared to hormone receptor positive (HR+) status[6]. Biggest progress regarding survival times and therapy options was achieved in HER-2 enriched phenotypes in the last decade. Until some years a HER-2 positive status was associated as a negative predictor with a mean survival time of 5-9 months. After the introduction of new Anti-Her-2 therapeutics (Trastuzumab, Pertuzumab, T-DMI) the mean survival time is reported with 56.5 months[2-4,10,34,36].
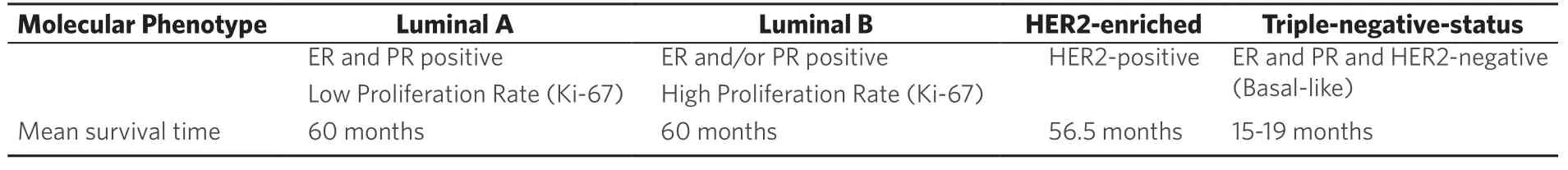
Table 1. Molecular phenotypes of metastatic breast cancer with related survival times[26]

Table 2. Karnowsky Performance Scale from 1949[34]
Whereas triple negative phenotypes (ER-, PgR-, HER-2-) were reported with a mean survival time of 6-9 months until the year 2015, actual data show a prolonged survival time of 15-19 months[2-6,9,10,13-15,26-28,34,35,37,38].
SCORE SYSTEMS
Life expectancy in metastasized tumor diseases is estimated with well known and evaluated score systems. The Karnofsky-Index [Table 2] dating from 1948 describes patients´ general condition and physical resilience[17,21,39]. Furthermore, the Karnofsky-Index is part of different score systems (Tokuhashi and revised Tokuhashi score, Oswestry Disability Index, Van-der-Linden-Score)[18,20,21,39,40]. Patients describe pain with the numeric or visual analogue scale (NAS/VAS)[41]. Vertebral stability is estimated by the Spinal Instability Neoplastic Score (SINS) [Tables 3 and 4][21,42], Harrington Score[21,43]or Taneichi score[44]. The revised Tokuhashi score [Tables 5 and 6][18,34]and Tomita score [Tables 7 and 8][19]are worldwide accepted to determine individual life expectancy in malignancy and to plan optimal treatment options. The modified Bauer score[16], Van-der-Linden score[20]and Oswestry-risk index[39]are named for the sake of completeness.
The revised Tokuhashi[18]and Tomita score[19]merely discern tumor entities (breast, prostate, lung, thyroid) and in both scores breast cancer is assessed with a favorable prognosis. The four different phenotypes of breast cancer are not included. A life expectancy limited to 5 to 9 months was published until 2015 concerning HER-2 enriched and triple negative phenotypes[2-6,9,10,13-15,26-28,34,35,37,38,45]. Therefore, modifications in the revised Tokuhashi[18]and Tomita score[19]were suggested[2,3]and highly invasive anterior-posterior spine surgery should be evaluated critically. Based on present-day knowledge we can no longer emphasize these recommendations. Due to better oncologic treatment options actual studies estimate mean survival times of 15-19 months with regard to the triple negative and 56.5 months concerning HER-2 enriched phenotypes[4,6,7,10,15,26]. A mean survival time of 15-19 months implies that several patients live longer and these patients have to be filtered out. According to Tomitaet al[19]and Tokuhashiet al[18]anterior spine surgery is indicated with a life expectancy of 12 months or more. If the patient is in good general condition and asks for surgery, we therefore recommend anterior-posterior techniques after critical evaluation. Furthermore, in 2012 Majeed[45]evaluated 55 patients according to the revised Tokuhashi[18]and Tomita score[19]and reported continuously longer survival times than initially estimated. Majeed[45]concluded, that prognosis of survival times is not reliable with the current scoring systems.
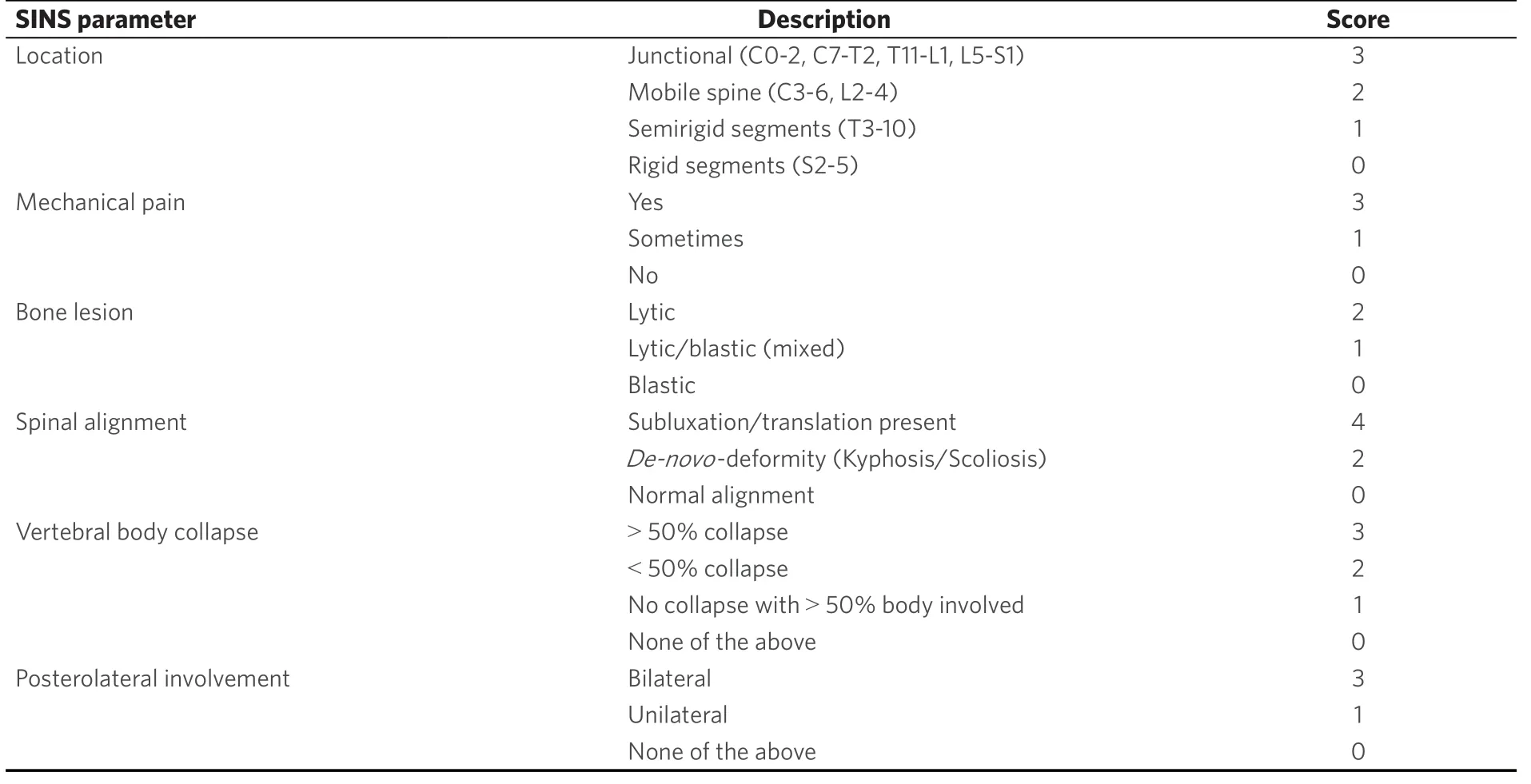
Table 3. Spinal Instability Neoplastic Score for the determination of vertebral body stability from 2010[23]
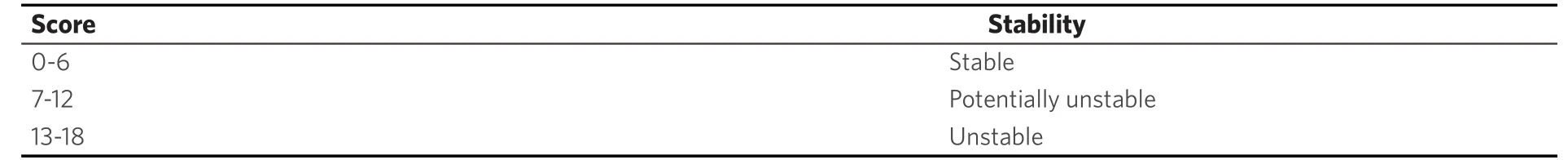
Table 4. Interpretation of Spinal Instability Neoplastic Score[23]
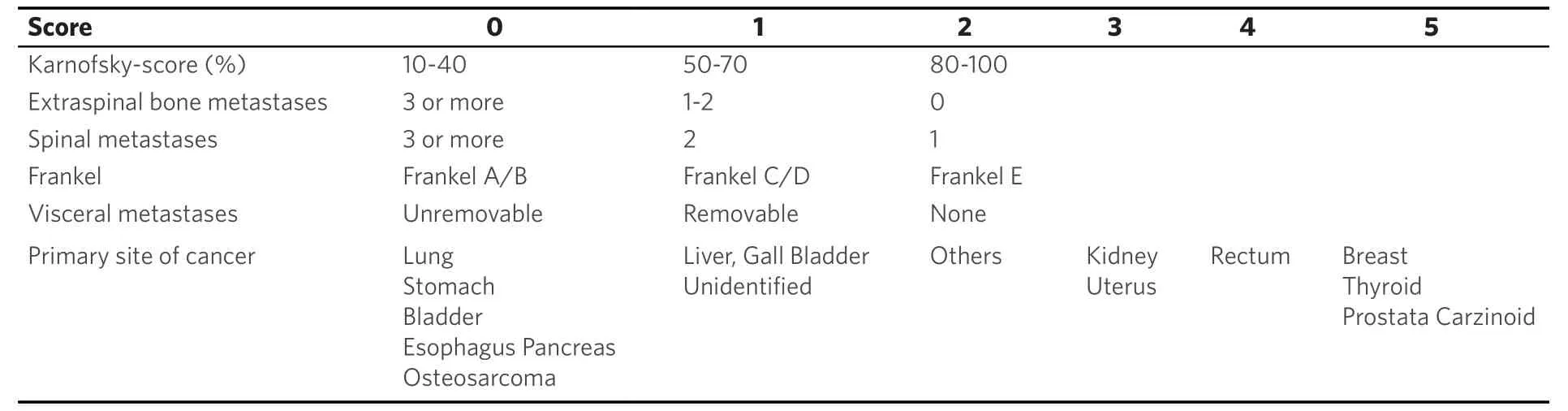
Table 5. Revised Tokuhashi-Score parameter[48]

Table 6. Iterpretation of the revised Tokuhashi-Score[48]
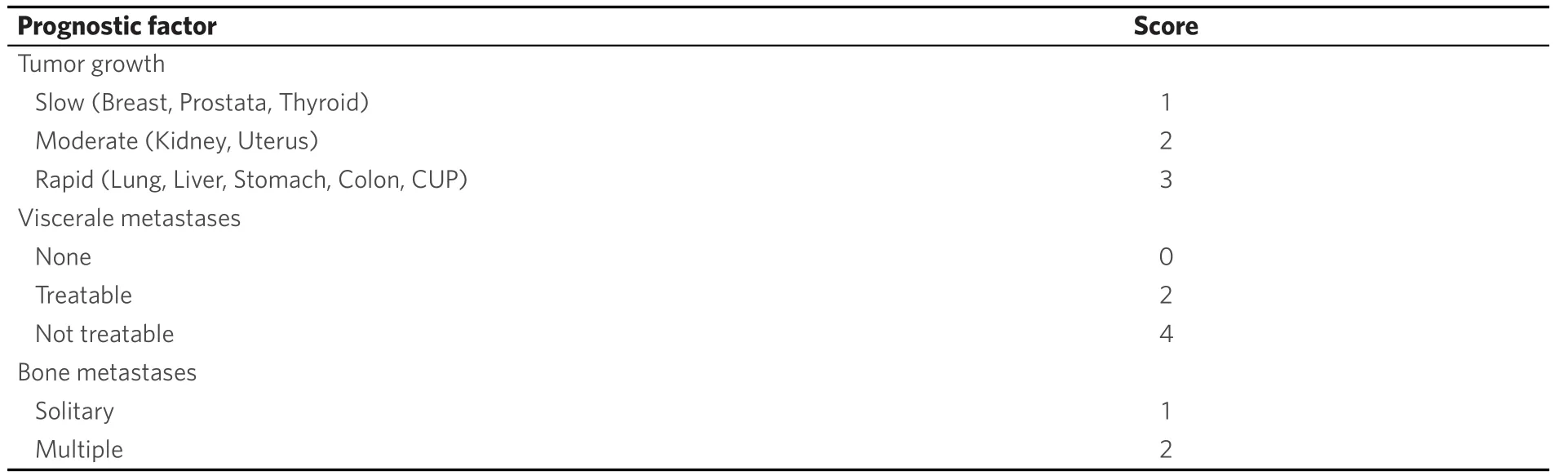
Table 7. Tomita-Score[50]
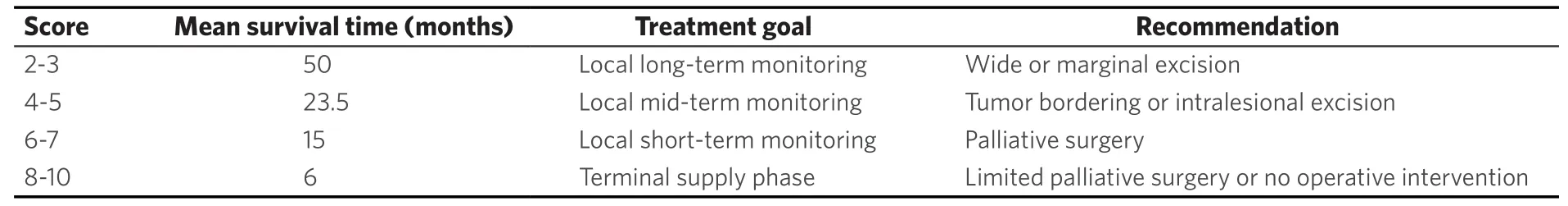
Table 8. Interpretation of the Tomita-Score[50]
THERAPEUTIC OPTIONS REGARDING SPINAL METASTASES
With an incidence of 85% breast cancer metastases to the spine may lead to instability with pain, pathologic fractures and neurologic deficits in up to 10%[21,29,46]. Furthermore, there are severe socioeconomic aspects with 50% of woman have to change the working environment and 37% of woman involved can temporarily or permanently work no longer[4]. The primary therapy of painful metastases to the spine without relevant loss of stability is radiation and spine surgeons are not necessarily involved. Potentially unstable and painful lesions with a SIN Score > 7[42](+/-neurological deficits) are demonstrated to the consulting spine surgeons. Mandatory in every patient is a critical individual evaluation of prognosis to choose correct therapy options[4,8,11,14,21,29,35,38,45-48]. Improving or maintaining the quality of life is the decisive therapeutic target in an incurable palliative situation. Wishes and priorities of these patients beside the status of metastases, previous therapy lines and general condition mainly influence the appropriate treatment[4].
Beside local radiation orthotic devices are the main conservative treatment tools to stabilize the spinal column and reduce pain. Concomitantly as systemic osteoprotective therapy a bisphosphonate in combination with calcium and Vitamin D or Denosumab (monoclonal antibody) mark the standard additive medication in advanced breast cancer with bone metastases. With the best response in diverse tumor entities up to 62% of recalcification post radiatio is described in breast cancer spinal metastases[4,8,21,47,49,50].
Various surgical treatment options can reduce pain and stabilize the spine. Bilateral, percutaneous balloon kyphoplasty as a minimal invasive treatment tool[13,36,46,47,49,51-54]may not restore vertebral height but correlates with pain reduction. Thermal ablation of vertebral metastases with radio frequency ablation (RFA)[50,53,55-57]may be combined with kyphoplasty to reduce the likelihood of tumor recurrence. Posterior instrumentation with a screw and rod system is the gold standard in spine surgery to stabilize unstable tumor lesions. In case of spinal stenosis due to tumor the decompression of neural structures is reached via laminectomy and tumor debulking[13,29,43,46,47,50,52-55]. According to Tomitaet al[19]palliative anterior surgery with vertebral body replacement (VBR) [Figures 1-3] can be recommended in patients with a life expectancy > 12 months[46,47,58,59]. Highly invasive surgical options likeen-blocspondylectomy in Tomita technique or vertebral column resection with a mandatory 360 reconstruction [Figure 4] mark curative treatment options in case of solitary spinal metastases[46,47,58,59].
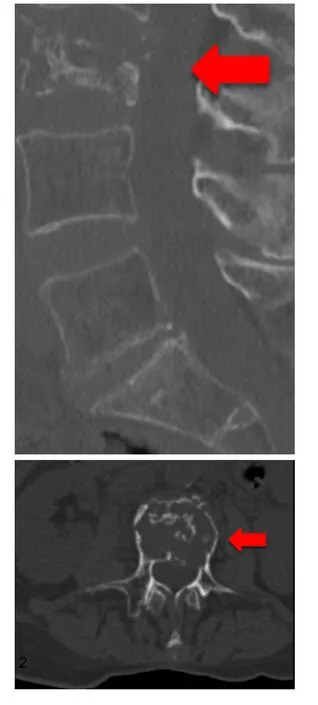
Figure 1. 73-year-old female patient with multiple metastatic breast cancer ER: 95%; PgR: 0%; HER2-Score 1+; Ki-67: 20%. Sagittal and axial section of a total spine CT scan: unstable metastatic destruction of L3
The various techniques mentioned above can be combined as required. Additional measures like (partial-) resection of soft tissue, thoracic wall or pelvis with potential correction or reconstruction of deformity complete the port folio[47]. Modern adjuvant oncologic concepts comprise radio-, chemo- or hormone therapy[8,47,50].
Due to the wide range of therapy options mentioned above it is impossible for a sole attending physician to determine the individual therapeutical regimes. Such complex decision making demands an interdisciplinary setting like a tumor conference or board[4]. The spine surgeon determines the individual therapy of spinal metastases in breast cancer patients. An improved quality of life results of preserved mobility and autonomy with less pain. If possible, pathologic fractures and neurologic deficits up to paraplegia should be avoided[2,3,8,18,19,29,42,43,47,50].
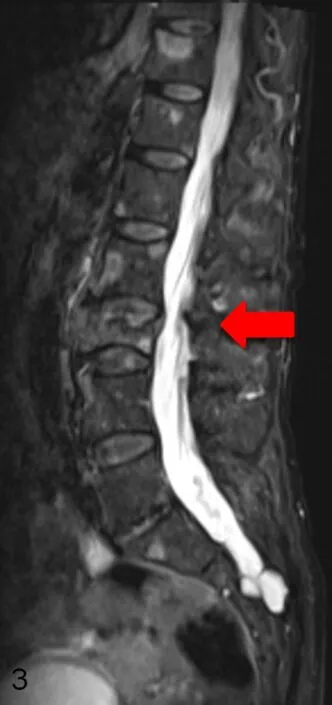
Figure 2. Sagittal section (T2-weighted) of a total spine MRI with contrast agent: metastatic destruction of L3 and multiple metastases
SPECIAL CASE: SOLITARY METASTASIS
Prognostic statements are especially important in case of a solitary, locally curable metastasis (principle of limited metastasis[4]) and a treatable primary tumor in a curative way. For us the question was, if solitary metastasis with any phenotype is always to be treated the same way [Figure 5].
Either a Ct-controlled or an open biopsy finally ensures the histopathological phenotype. Until the year 2015 the curative therapeutic approach withen-blocspondylectomy according to Tokuhashiet al[18]and Tomitaet al[19]was only recommended in hormone positive receptor status (luminal A and B) with a median survival time of 26 months[2,13]. In triple-negative or Her-2 enriched phenotypes with an estimated survival time of 5-9 months, a limited posterior instrumentation (screw and rod system) and decompression of neural structures was indicated[2,13]. Modern oncologic treatment concepts provide clearly longer survival times and therefore, according to Tokuhashiet al[18], curative treatment options (mean survival time > 12 months) can principally be applied to all phenotypes in breast cancer with solitary metastasis. In the worst case (triple-negative phenotype) the mean survival time is actually reported with 15-19 months[2-4,10,34,35].
Indicatingen-blocspondylectomy will never be an automatism and controversial discussions in tumor boards can be expected. Critically evaluation of the individual wish, priorities, general condition, comorbidities, previous lines of treatment and the biological age must influence the decision[4]. In tumor conferences a (neo-)adjuvant systemic therapy (endocrine therapy, Anti-HER-2 therapy, chemotherapy, osteoprotective therapy) is determined according to tumor load (TNM system) and biology. An adjuvant loco regional radiation therapy is mandatory in case of short edges of the resected areas in preparation slides[4].
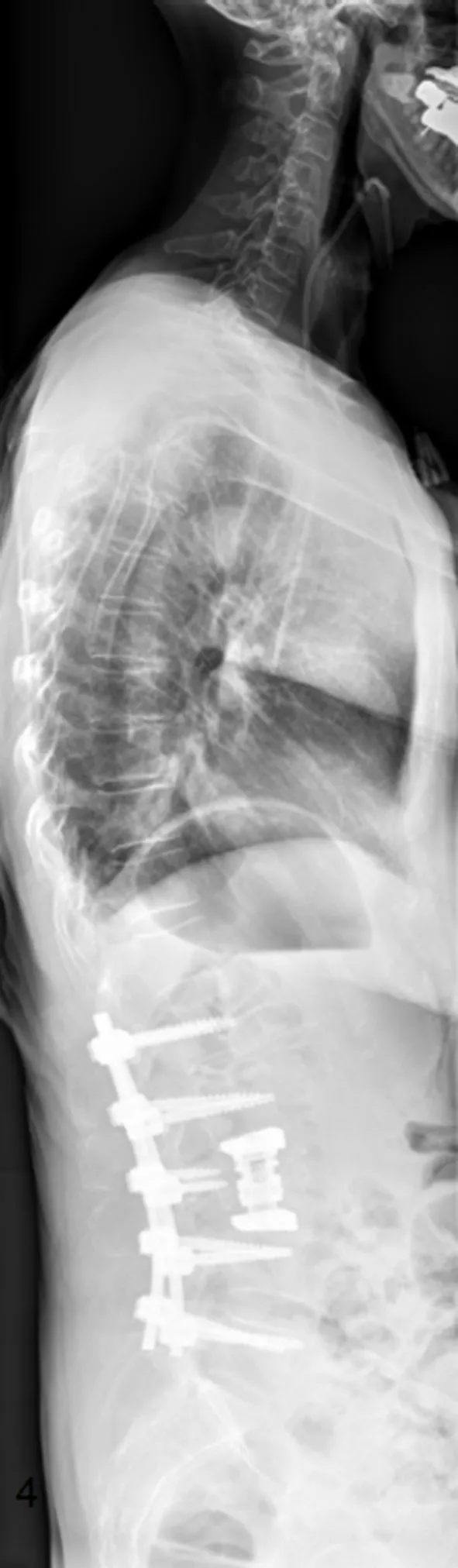
Figure 3. Postoperative sagittal total spine radiograph: posterior instrumentation L1-L5 with a screw and rod system, decompression via laminectomy, anterior stabilization with an insitu distractable vertebral body replacement of L3
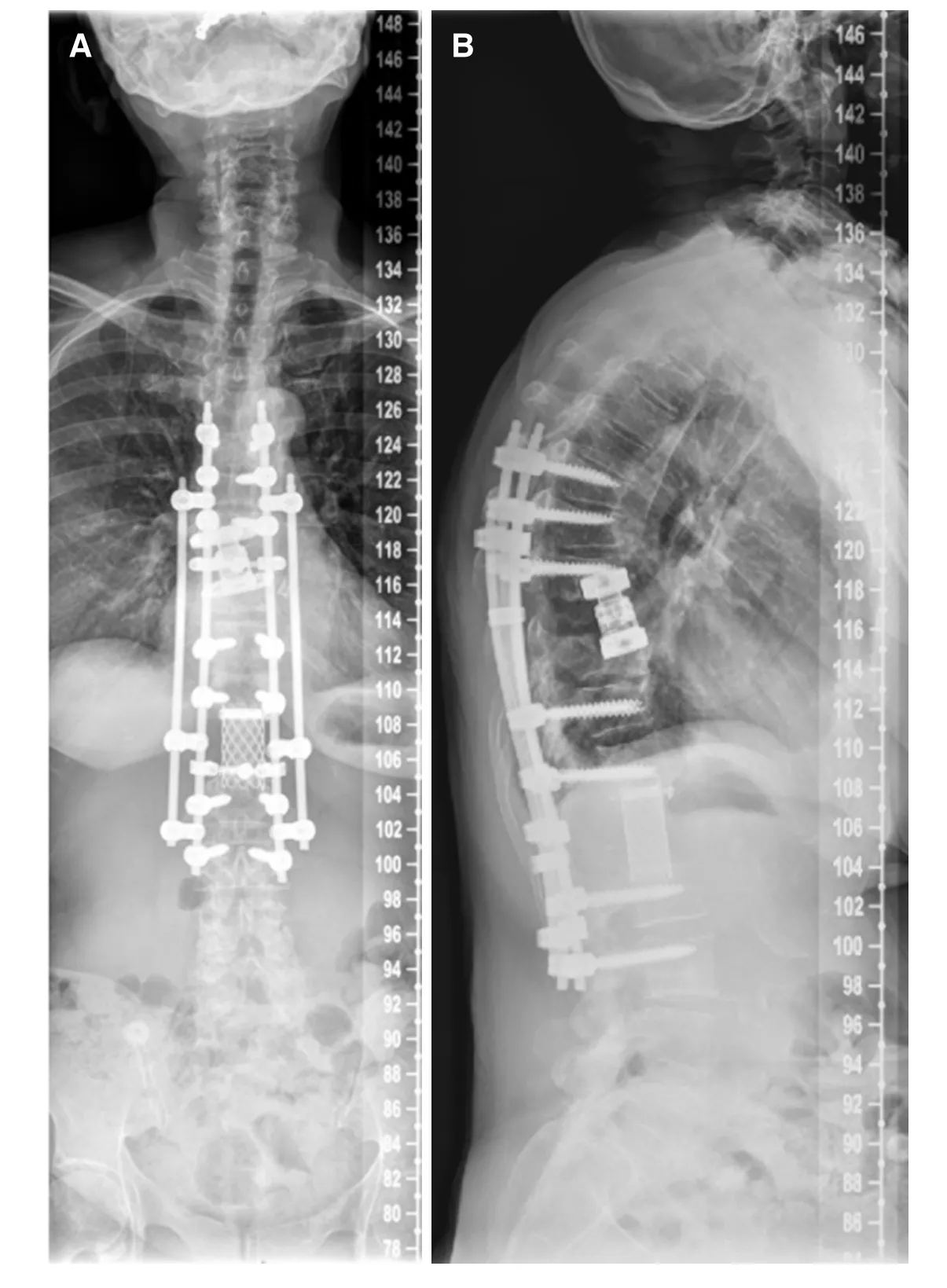
Figure 4. Two plane full spine radiographs of a 65 year old female patient with metastatic breast cancer(A, B). En-bloc spondylectomy in Tomita technique at the Th9 and L1 level with a time interval of 10 years. Anterior in situ distractible cage at the Th9 level and Harms cage at the L1 level, posterior 4-rod instrumentation Th6-L3
CONCLUSION
Evaluating prognosis by means of molecular tumor typing, previous course of disease and actual status of metastases is mandatory prior to elective surgery of breast cancer spine metastases.
Mean survival times of 60 months in endocrine responsive (Luminal A and B) phenotypes, 50 months in HER-2 enriched phenotypes and 15-19 months in triple negative phenotypes are described in actual literature.
The patients’s general condition, biological age, intrinsic breast cancer subtype, comorbidities, tumor burden (number of metastases to organs) and previous therapies influence decision making.
Regardless of molecular phenotype solitary spinal metastases may be treated with a curative therapeutic approach.
Close cooperation of all experts participating interdisciplinary tumor boards are essential to determine adequate therapy strategies.
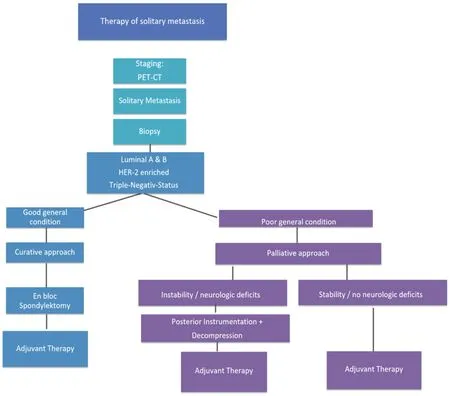
Figure 5. Heidelberg Spine Research Group Therapeutic Algorithm of Solitary Spine Metastasis in Breast Cancer
DECLARATIONS
Authors’ contributions
Made substantial contributions to conception and design of the study and performed data analysis and interpretation: Adler D, Akbar M
Performed data acquisition, as well as provided administrative, technical, and material support: Pepke W
Availability of data and materials
Data is available for readers.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2019.
 Journal of Cancer Metastasis and Treatment2019年5期
Journal of Cancer Metastasis and Treatment2019年5期
- Journal of Cancer Metastasis and Treatment的其它文章
- The moIecuIar interaction of ADAMTS-1 and fibuIin-1 and its potentiaI contribution to breast cancer bioIogy
- ESR1 alterations and metastasis in estrogen receptor positive breast cancer
- Training and evaluation of a knowledge-based model for automated treatment planning of multiple brain metastases
- Stem cells, immortality, and the evolution of metastatic properties in breast cancer: telomere maintenance mechanisms and metastatic evolution
- The lncRNA BORG: a novel inducer of TNBC metastasis, chemoresistance, and disease recurrence
- Autophagy in breast cancer metastatic dormancy: tumor suppressing or tumor promoting functions?
