High-risk HPVs, microbiota and epithelial carcinogenesis: state of the art and research contribution of in vitro 3D models
Diletta Francesca Squarzanti, Rita Sorrentino, Barbara Azzimonti
1Laboratory of applied Microbiology, Center for Translational Research on Allergic and Autoimmune Diseases, Department of Health Sciences, Medical School, Università del Piemonte Orientale, Novara 28100, Italy.
2Department of Health Sciences, Medical School, Università del Piemonte Orientale, Novara 28100, Italy.
3Consorzio Interuniversitario Nazionale per la Scienza e Tecnologia dei Materiali, Firenze 50121, Italy (Local Unit of Università del Piemonte Orientale, Novara 28100, Italy).
#Authors contributed equally.
Abstract Persistent high-risk human papillomavirus (HR-HPV) infection is associated with anogenital and head & neck squamous epithelial (HNSCC) tumors, which altogether cause about 550,000 new cases every year.Several evidences suggest that the microbiota could have a role on the inflammatory, epithelial mesenchymal transition and tumorigenesis processes promoted by HR-HPV infection, yet the mechanisms involved remain to be clarified.In this review we report the state of the art on this topic and on the most promising in vitro developed models for studying the host-pathogen interactions.Using MEDLINE, several terms were searched and combined to select the most pertinent papers.The investigation was limited to the international indexed articles published in PubMed in the last 10 years.This review reports the latest knowledge in the field of the microbial-associated anogenital tumors and HNSCC.In addition, we also discuss the in vitro epithelial culture systems that reproduce the pathophysiological features of the tumoral microenvironment and the in vivo response to microbial agents,thus representing a useful tool for analyzing at cellular and molecular levels the role played by infective agents in tumorigenesis.
Keywords: High risk human papillomavirus, microbiota, Fusobacterium nucleatum, epithelial-mesenchymal transition, anogenital cancer, head & neck squamous cell carcinoma, colorectal cancer, transepithelial electrical resistance
INTRODUCTION
As referred in the last human papillomavirus (HPV) cancer report of the HPV Information Centre[1],unremitting HPV infection, mainly promoted by high-risk HPV (HR-HPV) 16 and 18 E6 and E7 oncoproteins, is the main microbial etiological factor till now recognized that is necessary, yet not sufficient, for the development of anogenital and of head & neck cancers (namely of the tonsil, tongue and oropharynx).
Despite the progresses in vaccination and screening prevention led to the reduction of cases number[2,3],these tumors are still diffuse among the general population, with the highest mortality rate in countries with a low socio-economic level[3].
Cervical cancer is the 3rd most frequent female tumor in the world (2nd in the age range 15-44) with about 600,000 new cases/year and a 50% mortality rate[4,5].
In the last three decades oropharyngeal tumors, which include some 450,000 new cases/year (mostly affecting older tobacco and alcohol users worldwide), have increased also in young people, due to sexual habits changes predisposing to HR-HPV infection[6].
It has been reported that oncogenic HPV-related oropharyngeal tumors have a better prognosis than those HPV negative[7,8].On the other hand, it is known that HR-HPV-mediated cervical oncogenesis generally develops after a long latency (about 10-30 years from infection).Thus, it remains a conundrum the fact that tumor latency and prognosis differ in the two populations of patients, regardless of the treatment and of the fact that both types of cancer share a common infective cause[9].Seemingly, HR-HPV infection is notper seenough to cause these cancers, though viruses are classified as Class 1 carcinogens by the International Agency for Research on Cancer[4].Recent evidences suggest that the microbiota of the cervical and oropharyngeal niches (that includes bacteria, protozoa, viruses and fungi, although sometimes the term specifically refers only to bacterial communities) can have both a harmful and a protective role.Further, the microbiota can influence persistent HPV-mediated inflammation, epithelial mesenchymal transition (EMT) and tumorigenesis, although the mechanisms involved are still largely unknown and controversial[10].In fact, just as a balanced bacteriota may be constituted by both pathogens and commensals, the human virota can include, among the others, both low risk and HR-HPVs, without inducing morbidity for its host if the immune surveillance and the whole microbiota itself are able to control its load and persistence[11].
Therefore, an open question is if commensal HPV types, belonging to the human virota, play a protective role against the oncogenic ones or not[12-15].What is sure is that cancer often arises from a metabolic imbalance.Given this assumption, the metabolic profile of the microenvironment, in which host cells directly cooperate with viruses and bacteria, could reveal a snapshot of their functional and complex interactions that are at the basis of HPV infection, persistence susceptibility, inflammation, EMT,uncontrolled cell proliferation and cancer progression.
To learn more about it, researchers have developed severalin vitroco-culture systems that could mimic the physiological appearance of the tumoral microenvironment[16].
On this basis, the purpose of this study was to show the state of the art on epithelial carcinogenesis and on the most suitablein vitro3D models till now developed for the study of host-pathogens interactions.
By using MEDLINE, terms such as “microbiota”, “virota”, “virus”, “epitheliotropic virus”, “HPV”,“bacteriota”,“bacteria”, “Fusobacterium nucleatum”, “Porphyromonas gingivalis”, “EMT”, “eukaryotic cells”,“keratinocytes”, “epithelial cells” “epithelial infection”, “epithelial cancer”, “anogenital cancer”, “head and neck squamous cell carcinoma (HNSCC)”, “epithelial models”, “2D”, “3D”, “3D epithelial cultures”, “rafts”,“spheroids”, “reconstructed tissues”, both in single and mutually combined, were searched in titles and abstracts in order to find PubMed indexed most pertinent articles; only original research articles published in peer review journal were considered.Papers including EBV or other epitheliotropic viruses other than HPV were excluded.
The literature search was limited to the most recent scientific publications (10 years); six thousand and five hundred nine appropriate abstracts and full papers were carefully read and reviewed; those relevant (216)were reconsidered based on the above described inclusion and exclusion criteria and then 70 were reported in detail and included in the text.
Overall, our findings highlight the most recent advances in the field of host-pathogens interactions.We focused on thein vitro3D modeling for the study of the microbial induced epithelial carcinogenesis and emphasized its ability in mimicking at best the role of bacteria and viruses within anin vivomicroenvironment.
mICRObIOTa, ImmUNe RespONse aND epITHeLIaL CaNCeR
Several studies, among which that conducted by Kyrgiouet al.[10], underlined that vaginal microbiota may drastically influence host innate immune response, infection susceptibility and cervical cancer development, but without proving causality and thus making necessary further longitudinal studies.In this direction, Ilhanet al.[17], through a triple approach based on liquid chromatography mass spectrometry,16S rRNA gene sequencing and immunoassay, evaluated and integrated the metabolic signature of the vaginal microbiota and the inflammatory status changes of 78 women, including HPV-negative/positive controls, low-/high-grade cervical-dysplasia or -cancer affected females.These authors found that unique cervicovaginal metabolites and microbes allow to discriminate clearly the affected patients from healthy subjects, thus providing novel cancer hallmarks.For example, the lipidic three-hydroxy-butyrate,eicosenoate, and oleate/vaccenate are distinctive of cancer patients, while sphingolipids, plasmalogens and linoleate positively correlate with cervicovaginal inflammation.
Regarding this niche, the absence of dysbiosis and a dominance of peculiar gram-positive bacteria belonging to the Lactobacillus genus may inhibit several pathogens, preserve aminoacidic and nucleotide metabolisms and avoid the inflammatory state.Conversely, Fusobacterium spp.,F.nucleatumin primis, promotes cell survival, proliferation, dysregulated carcinogenesis and cervical cancer through the induction of the WNT/Beta catenin signaling pathway, as emerged by research in colorectal cancer (CRC)[12,18,19].
Since it has been demonstrated a significant correlation between bacteria belonging to the Fusobacteria genus, mainlySneathia sanguinegens, and high-grade squamous intraepithelial lesions, they could be hired as microbiological markers of clinically significant cervical diseases[10].
This evidence has also been observed in another study by Mitraet al.[12], who found that a relative abundance of Fusobacterium spp.associates with higher levels of cytokines such as IL-4 and TGF-1β, which have been shown to generate local immunosuppression, HPV immune evasion and cancer development.
MoreoverF.nucleatuminversely correlates with CD3+-T cell counts, whose aberrant signaling and function is typical in cervical carcinogenesis.
Al-Hebshiet al.[15]have reported an abundance of the periodontal pathogenF.nucleatum, selected by the tumor environment, followed to a lesser extent by Campylobacter, Parvimonas and Prevotella spp.in oral squamous cell carcinoma, while the Streptococci are frequently associated with a healthy status.
Although the results obtained from clinical studies vary in the oral cancer associated microbiota composition, partially due to differences in the methodological approaches, the functional data consistently show an enrichment of a virulent, chronic inflammatory bacteriota that, as a passenger within the tumor environment, contributes to the worsening of the tumoral disease.
HIGH-RIsK HpVs, mICRObIOTa, epITHeLIaL meseNCHYmaL TRaNsITION aND CaNCeR pROGessION
As known, EMT associates with cell migration, invasion, resistance to chemotherapy and anoikis.During this process, several signaling pathways, such as those involving TGF-β, Wnt, Hedgehog, β-catenin, Notch,Nanog, STAT3 and ERK, are activated in the cell leading to a pro-metastatic behavior[20,21].In this context,EMT can be also regulated by HR-HPVs[22,23].
Despite anogenital and oropharyngeal squamous cell carcinomas (OPSCC) promoted by these viruses have a more favorable prognosis respect to HPV negative ones, EMT usually associates with an aggressive tumoral behavior, making HPV role an open issue.In a study on a cohort of 296 OPSCC-patients, EMT was confirmed as an adverse marker of evolution of HPV related carcinomas[24].
In another research, the expression of five EMT markers was assessed in normal and tumoral cervical tissues, revealing that E-cadherin and β-catenin expression decreases gradually, while that of N-cadherin,vimentin and fibronectin increases during malignant progression[25].Moreover, Suet al.[26]studied the role of DNA methylation of the ten-eleven translocation methyl-cytosine dioxygenase 1 (TET1), which oxidizes 5-methylcytosine to 5-hydroxymethylcytosine within cervical lesions.At a precancerous level,TET1 inhibits EMT, interacting with chromatin modifiers and downregulating mesenchymal genes such asZEB1andVIM[26].In vitroculture of cervical cancer cells revealed that p68, an ATP-dependent RNA helicase, induces EMT through the transcriptional activation of the TGF-β1 pathway, inducing cancer progression[27].
Finally, Panget al.[28]reported that HPV18 E6 regulates YB-1 mRNA expression by promoting EMT and cervical lesion progression through the enhanced Snail expression.
Moreover, both HPV and EMT upregulate programmed cell death ligand-1 (PD-L1) expression, an immune checkpoint and therapeutic target in OPSCC, whose efficacy is currently under investigation[29,30].Precisely, PD-L1 is overexpressed in about half of OPSCC that seem to have a better response to anti-PD-1 therapy[31].
Regarding the involvement of bacterial microbiota in EMT,F.nucleatumhas been frequently found in stools and bioptic specimens of CRC affected patients, and more recently also of OPSCC patients.It has been suggested that this bacterium triggers EMT to malignant transformation by interacting with E-cadherin, as suggested byin vitroexperiments on NCM460 cells[32,33].In a cohort of stage III/IV CRC affected patients, Yanet al.[34]found a significant correlation between the presence ofF.nucleatumand tumor progression also in relation to chemotherapy efficacy, suggesting its role as a biomarker for atherapeutic approach improvement.Panebiancoet al.[35]also confirmed this link.Moreover, the same authors underlined that not only gut bacteria, but also the intratumor ones, may influence patients’response toward chemo- and immuno-therapeutic drugs andvice versa[36].
More recently, it has emerged that EMT can also be promoted byPorphyromonas gingivalis, an anaerobe frequently found in chronic periodontitis.In fact, it has been observed that this bacterium causes an early EMT of chronically infected human oral epithelial cells (OECs) that, through PI3K/Akt activation,ultimately lead to the loss of E-cadherin and to the accumulation of β-catenin, together with an increased expression of Zeb1, vimentin, MMP-2, -7, and -9.Moreover, upon stimulation with this bacterium, OECs migratory capacity increases proportionally[37-39].
BothF.nucleatumandP.gingivalisdetermine an enhanced transcription of mesenchymal markers, an increase of TGF-β1, TNF-α and EGF and a downregulation of those associated to epithelial layer integrity,as evidenced by transepithelial electrical resistance measures[40].
Recently, a new tumor metastasis model has been proposed.Till now it was supposed that epitheliallike cancer cells acquire mesenchymal features in order to produce metastasis by exploiting the vascular system, and then switch back to an epithelial phenotype to establish the new tumor.The existence of a stable population of hybrid epithelial/mesenchymal cells, possessing epithelial and mesenchymal characteristics with both tumorigenic and metastatic properties, has been hypothesized[41,42].Moreover,in predisposed individuals, with defects in the innate immunity response or with a specific epigenetic background, bacterial or viral infections could lead to EMT, causing a chronic inflammatory state, through the activation NF-kB and MAPK[43].Considering this and the emerging role of the microbiota on EMT and cancer progression, more rational and shared laboratory models should be used to better investigate the microbiota composition[44]and its relationship with the human host.
IN VITRO epITHeLIaL mODeLs FOR sTUDYING THe HOsT-paTHOGeNs INTeRaCTIONs
The first studies focused on host-pathogens connections started in the 1970s with Todaro[45]and Taylor-Robinson[46], who described the interactions between oncogenic viruses and human cells, and mycoplasma pneumonia and ciliated tracheal epithelium, respectively.Since then,in vitromodels acquired popularity in the microbiology field, due to their reproducibility, higher versatility and high-throughput data acquisition respect toin vivomodels[47][Table 1][5,16,38,45-62].Undeniably, due to the improvement in imaging and screening techniques and to thein vitromodels features, nowadays it is possible to directly analyse characteristics such as: specific surface-adhesion processes (i.e., biofilm formation), spreading capabilities,immune system escapes aptitudes, migration in 3D matrices, and host’s cell-type-specific interactions in general.
The development of functional co-culture systems mostly depends on their ability to allow the physiological growth of cellular components in optimized culture media inside trans-well devices or microfluidic chambers.In this regard, Di Giulioet al.[50]developed a model in whichStreptococcus mitiswas grown with human gingival fibroblasts in saliva sterilized stocks enriched with 1% sucrose as culture medium.With this system, the authors demonstrated thatS.mitiscan penetrate within fibroblasts, via a FAK-integrin β1-vinculin-actin mediated process, which is modulated by the saliva and the microenvironment themselves.
Sterilized artificial saliva has also been used in more complex models such as the multi-culture trans-well system developed by Millhouseet al.[51], in which a complex multispecies biofilm, composed byS.mitis,F.nucleatum,P.gingivalisandAggregatibacter actinomycetemcomitans, was grown on a glass slide and cocultivated in a trans-well together with immortalized oral keratinocytes (OKF6-TERT2).With this system,these authors validated the efficacy of commercially available oral hygiene products.
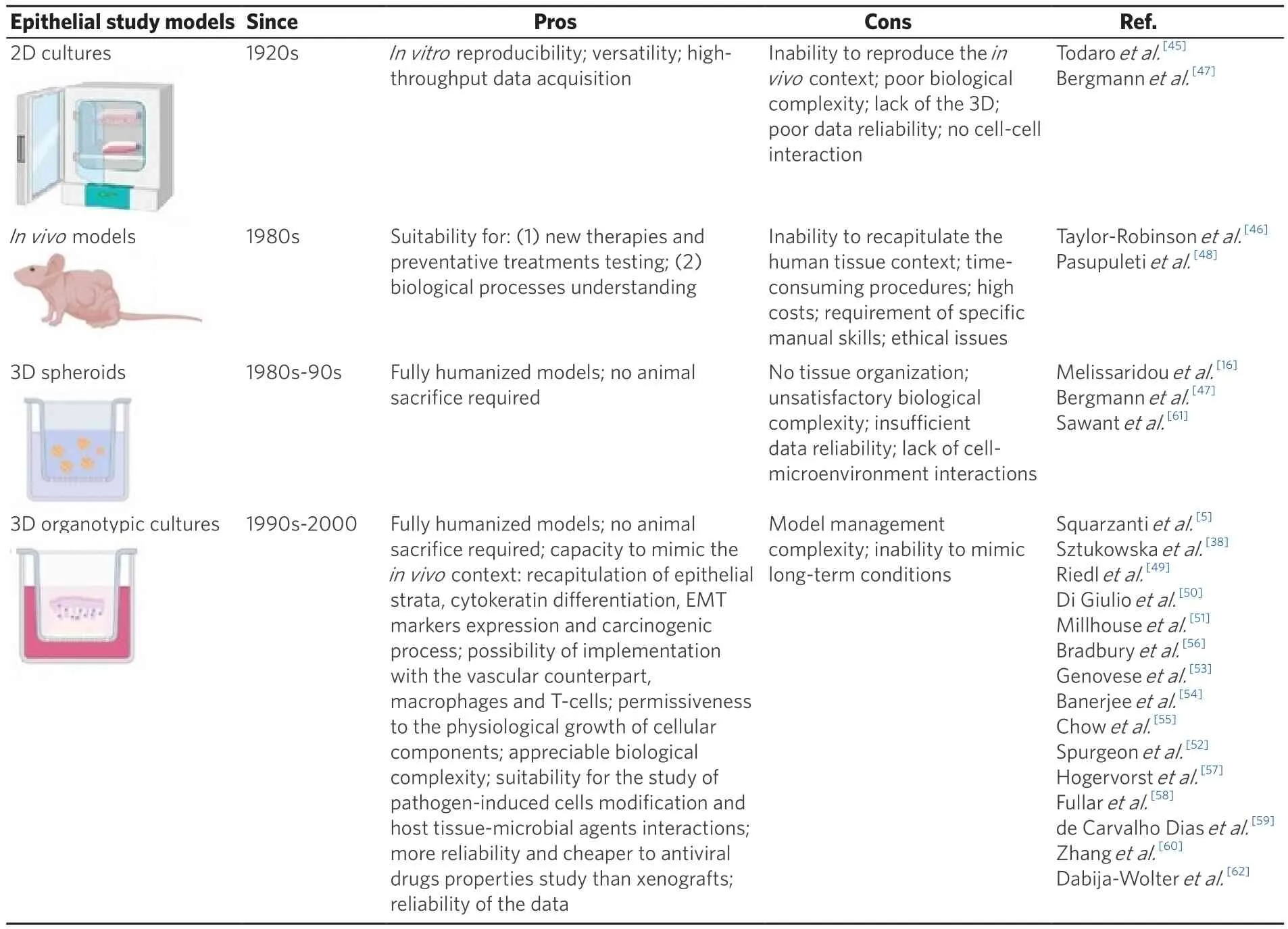
Table 1.Epithelial study models’ overview (images created with BioRender.com)
Despite these findings and the evidences on the cytoskeletal modifications, the impaired proliferation rate and the gene expression changes occurring in cells infected by bacterial or viral pathogens[50,63-66],some limitations arise when more complex evaluations are required.According to the literature, the microenvironment highly modifies cells behaviour; therefore, the lack of the “3D” in manyin vitromodels reduces the transferability potential of thein vitrodata[52].This is particularly true for epitheliotropic viruses, such as HPVs, for which the intracellular expression of viral proteins is conditioned by the epithelial renewal/stratification process that cannot be detected within 2D cultures.
In fact, most 3D models enable not only viral life cycle, but also virus induced cellular modifications.They are generally constituted by HPV infected keratinocytes, cultivated onto fibroblast repopulated matrices and let to stratify at the air-liquid interface in specific culture media.They can be easily analysed by western blot, histology, high throughput sequencing and protein-protein interaction assays.
These models made possible to identify novel pro-oncogenic co-factors, such as p130[53]or 53BP1[5],important for investigating novel antiviral targets.
3D RAFT (real architecture for 3D tissue) cultures also allowed to elucidate the effect of HPV chronic infection onto eukaryotic cells mitosis; as showed by Banerjeeet al.[54], HPV DNA amplification occurs during host’s cell G2 phase, despite DNA eukaryotic amplification normally occurs in the S phase; in particular, they elucidated the key role of E7 in forcing host cells permanence in the G2 phase via cyclin B1 cytoplasmic accumulation.
Additionally, RAFT cultures are a more reliable and cheaper solution to study antiviral drugs respect to xenografts in SCID mice, since they are more suitable to be analysed byin situhybridization and RT-qPCR techniques[55].
Moreover, they can allow the study of host-virus interactions in absence of other cell types or tissues that could hide fundamental pathways promoted by the pathogen, especially when the studies require miRNA or EMT analysis.
As previously described, HR-HPVs E6 and E7 proteins interact and inhibit host p53 and pRb oncosuppressors respectively.Recently, the idea that E6 and E7 can also bind host miRNA or miRNA regulators has been evaluated[67].Indeed, despite HPV inability to encode for miRNAs, HPV mediated indirect regulation of both onco-suppressive or tumorigenic miRNAs may offer the opportunity to identify novel therapeutic targets.
RaFT CULTRes, DRUG ResIsTaNCe aND emT ReseaRCH
Regarding drug resistance and EMT markers expression analysis, RAFT cultures can overcome both 2D and spheroid cultures limitations.Melissaridouet al.[16], working with 5 HNSCC-derived cell lines grown in both 2D and spheroids assays, observed that, while 2D eukaryotic cells and spheroid conformation changes the receptor topography and gene expression, and erases the drug resistance pathways that occurin vivo, 3D cultures can better mimic what happens duringin vivotherapies and allow to study EMT expression markers such as CDH1, NANOG, SOX and EGFR.
However, when epithelial models are necessarily to be used, spheroids utility is limited, because of the less reliability of the data they produce respect to those obtained with the organotypic models.In fact, although they are both made up of epithelial cells, spheroids morphology and structure are too much simplified and do not reproduce the mucosal epithelium in such a representative way as the 3D epithelial RAFTs do.Therefore, compared to 3D RAFTs, the spheroids are less performant for studies on the specific interactions that occur in a well-definedin vivoenvironment.
On the other hand, RAFT cultures can be built with both isolated healthy or tumour/cancer associated keratinocytes and fibroblasts, able to better elucidate the interactions between stromal and epithelial compartments that are at the basis of the initial steps of the carcinogenetic process[56].Moreover, they recapitulate all the epithelial layers, the physiological cytokeratin differentiation and the carcinogenic process.
By usingin vitroreconstructed epithelial systems, Hogervorstet al.[57]highlighted the importance of the papillary-reticular fibroblasts switch.In fact, when epithelial cultures are grown in presence of switched fibroblasts, the expression of CAF associated markers (α-SMA and vimentin) and EMT markers (SNAIL2,N-cadherin and ZEB1) increase, thus suggesting that the fibroblasts switch could be considered an indicator of squamous cell carcinoma (SCC) progression.Similarly, Fullaret al.[58]proved that HPV16 induces the modification of connective matrix that, by the action of epithelial Matrix Metalloproteinase MMP-7 and fibroblast-produced MMP-2, loses collagen and fibronectin in favour of laminin, thus facilitating EMT and carcinogenic events.Comparable effects on MMP super-families were also observed in other HR-HPV positive models[68].
In agreement with the above, the last trend is to reproduce tissue models with the whole organ complexity[69]; in fact, the models have been implemented with the vascular component, with macrophages and T-cells, thus allowing to further improve the knowledge in the microbiology field[59-61].
In line with the technological progress, in the last few years several authors have focused their attention on the bacterial-induced EMT.For instance, Sztukowskaet al.[38]showed thatP.gingivalis, associated with OPSCC development, is able in a gingival 3D equivalent to induce the expression and the nuclear relocalization of ZEB1, a well-known EMT transcriptional factor.This effect is reduced by the lack of the bacterial fimbrial FimA protein, while it is enhanced by the co-infection withF.nucleatumorS.gordonii.A similar study conducted by Leeet al.[37]showed that the carcinogenic properties ofP.gingivalisare unrelated to the presence of chronic inflammation periodontitis.In this study, the authors showed that in addition to ZEB1, also glycogen synthase kinase-3 beta (p-GSK3β), Slug, Snail, and vimentin usually increase during EMT under the influence ofP.gingivalis.Moreover, the co-infection withF.nucleatuminduces the loss of adhesive molecules such as E-cadherin and β-catenin in cancer-committed cells and the overexpression of MMP-2, -7 and -9 as showed for HPV infection[69].These findings confirm those obtained in an established periodontitis-associated oral tumorigenesis murine model used to demonstrate that bothP.gingivalisandF.nucleatuminduce tumorigenesis independently from the inflammatory status[70].In this latter study, the interaction of bacteria with the keratinocytes Toll-like receptor and the consequent activation of the IL-6-STAT3 axis was possibly involved in the tumorigenesis process.
The above cellular models can be also used to quantify the microorganisms’ migration potential, as made by Dabija-Wolteret al.[62]whoin vitroreconstructed a gingiva to evaluate the migration ofF.nucleatumthrough a keratinized squamous epithelium without destroying it, while establishing a chronic infection[Figure 1].
Summarizing, in the last few years, thanks to their suitability to exceed the limitations of monolayer adherent cell cultures, as well as ofin vivomodels and spheroids, 3D epithelial co-cultures have become indispensable tools for investigating the interactions between microbial agents and their hosts.
Although several questions are still open, soon further improvements might help to generate microenvironments that also mimic the bacteria and virus migratory potential.
CONCLUsION
Overall this review highlights the importance of thein vitro3D modeling research field for the study of microbial induced epithelial carcinogenesis.Yet, further improvements are still needed to reach the level of complexity of the real tissue.These models will hopefully help to better understand the respective roles of virus and bacteria in HR-HPV-related cancers.
DeCLaRaTIONs
Acknowledgments
Authors thank Michelle Schoeman for the linguistic revision.
Authors’ contributions
Wrote part of the paper and prepared Table 1: Squarzanti DF
Wrote part of the paper and prepared Figure 1: Sorrentino R
Designed the review article and wrote part of the paper: Azzimonti B
Availability of data and materials
Not applicable.
Financial support and sponsorship
DFS was partially supported by BioLab Srl/Probiotical SpA.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2019.
 Journal of Cancer Metastasis and Treatment2019年11期
Journal of Cancer Metastasis and Treatment2019年11期
- Journal of Cancer Metastasis and Treatment的其它文章
- Novel immunotherapeutic approaches in head and neck cancer
- Monoclonal antibody pharmacogenomics in cancer treatment
- CXCR4 signalling, metastasis and immunotherapy:zebrafish xenograft modeI as transIationaI tooI for anti-cancer discovery
- Loss of the Krüppel-like factor 4 tumor suppressor is associated with epithelial-mesenchymal transition in colorectal cancer
- Histone chaperone FACT and curaxins: effects on genome structure and function
- AUTHOR INSTRUCTIONS
