Loss of the Krüppel-like factor 4 tumor suppressor is associated with epithelial-mesenchymal transition in colorectal cancer
Kimberley C.Agbo, Jessie Z.Huang, Amr M.Ghaleb, Jennie L.Williams, Kenneth R.Shroyer,Agnieszka B.Bialkowska, Vincent W.Yang,4
1Department of Medicine, Stony Brook University School of Medicine, Stony Brook, NY 11794, USA.
2Department of Pathology, Stony Brook University School of Medicine, Stony Brook, NY 11794, USA.
3Department of Family, Population and Preventive Medicine, Stony Brook, NY 11794, USA.
4Department of Physiology and Biophysics, Stony Brook University School of Medicine, Stony Brook, NY 11794, USA.
#Authors contributed equally.
Abstract Aim: Colorectal cancer (CRC) is the third leading cancer-related cause of death due to its propensity to metastasize.Epithelial-mesenchymal transition (EMT) is a multistep process important for invasion and metastasis of CRC.Krüppel-like factor 4 (KLF4) is a zinc finger transcription factor highly expressed in differentiated cells of the intestinal epithelium.KLF4 has been shown to play a tumor suppressor role during CRC tumorigenesis - its loss accelerates development and progression of cancer.The present study examined the relationship between KLF4 and markers of EMT in CRC.
Methods: Immunofluorescence staining for KLF4 and EMT markers was performed on archived patient samples after colorectal cancer resection and on colonic tissues of mice with colitis-associated cancer.
Results: We found that KLF4 expression is lost in tumor sections obtained from CRC patients and in those of mouse colon following azoxymethane and dextran sodium sulfate (AOM/DSS) treatment when compared to their respective normal appearing mucosa.Importantly, in CRC patient tumor sections, we observed a negative correlation between KLF4 levels and mesenchymal markers including TWIST, β-catenin, claudin-1, N-cadherin, andvimentin.Similarly, in tumor tissues from AOM/DSS-treated mice, KLF4 levels were negatively correlated with mesenchymal markers including SNAI2, β-catenin, and vimentin and positively correlated with the epithelial marker E-cadherin.
Conclusion: These findings suggest that the loss of KLF4 expression is a potentially significant indicator of EMT in CRC.
Keywords: Krüppel-like factor 4, colorectal cancer, epithelial-mesenchymal transition
INTRODUCTION
Colorectal cancer (CRC) is the third leading cause of the cancer-related deaths often with metastasis to the liver, lung, and bone[1,2].Epithelial-mesenchymal transition (EMT) is a transdifferentiation process that allows a polarized epithelial cell to assume a mesenchymal cell phenotype, characterized by enhanced migratory and invasiveness capacity, elevated resistance to apoptosis, and increased synthesis of extracellular matrix (ECM) components[3].During the EMT process, epithelial cells lose apical-basal polarity that is accompanied by reorganization of cytoskeleton and reprogramming of the signaling pathways that allow for an increase in motility and the development of an invasive phenotype.This multistep complex process is characterized by modifications in the expression of a host of transcription factors and specific cell-surface proteins, as well as reorganization and expression of cytoskeletal proteins, and production of enzymes that degrade the ECM[3].A change in some of these factors, such as upregulation of TWIST, SNAI1, SNAI2, ZEB1, vimentin, and N-cadherin, and downregulation of E-cadherin and tight junction proteins such as ZO-1 is indicative of progression of EMT[4,5].In CRC, EMT has been strongly associated with the invasive and metastatic phenotype, thereby generating the lifethreatening manifestations of metastatic disease cancer.The activation of the EMT program has been suggested to be the critical mechanism for the acquisition of malignant phenotypes by epithelial cancer cells[3].
Krüppel-like factor 4 (KLF4) belongs to the family of zinc-finger transcriptions factors that play critical roles during development, proliferation, differentiation, and homeostasis, as well as development and progression of many diseases including inflammation and carcinogenesis[6-8].In the digestive tract,KLF4 is predominantly expressed in differentiated cells of the villus and surface epithelium of the small intestine and colon, respectively[9-13].Importantly, evidence indicates that KLF4 functions as a tumor suppressor that inhibits progression of CRC[12].It has been shown that loss of KLF4 expression is associated with the early stage of CRC development and that KLF4 is a prognostic indicator for CRC survival and recurrence[14,15].Recently, we demonstrated that KLF4 also plays a protective role against tumor formation during inflammation-induced colorectal tumorigenesis[16-18].The biochemical mechanisms triggering the acquisition of the invasive phenotype and the subsequent systemic spread of the cancer cell have been areas of intensive research.Here, we demonstrate that KLF4’s role in colorectal tumorigenesis extends to its ability to regulate EMT.
METHODS
Samples from patients
Surgical specimens of resected colorectal cancer specimen obtained from Stony Brook University and State University of New York Downstate were used in this study.In total, 12 specimens were processed for immunofluorescence.All samples were of Caucasian origin, with 2 female and 10 male.One sample was qualified as stage I, one as stage 2, two as stage 3, and eight as stage IV [Table 1].The protocol for the sample collection was originally approved by the Institutional Review Board by the State University ofNew York at Stony Brook on 17 October 2014 (CORIHS 2014-2821-F) and qualified for a waiver under the Federal Law of Department of Health and Human Services per article 45CFR46.116.d.
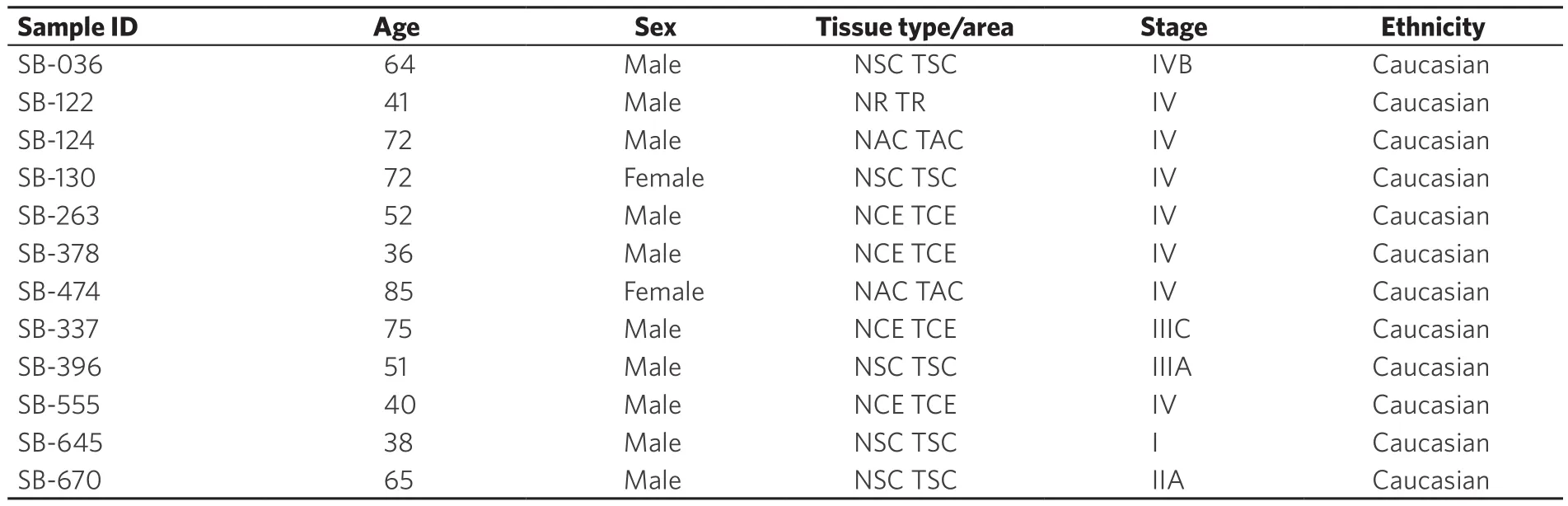
Table 1.The characteristics of human samples
Mice
All animal studies were approved by the Stony Brook University Institutional Animal Care and Use Committee and performed in accordance with institutional policies and NIH guidelines.Mice with the floxedKlf4gene (Klf4fl/fl) were described previously[12].These mice were derived from a C57BL/6 background and are indistinguishable from the wild-type mice.
Azoxymethane and dextran sodium sulfate treatment
Azoxymethane (AOM) and dextran sodium sulfate (DSS) treatment was performed as described previously[18].Briefly, adult gender- and age-matchedKlf4fl/flmice (n= 16) were injected intraperitoneally with 10 mg/kg of AOM working solution.After seven days, normal water was replaced with 2.5% DSS in the drinking water for five days, followed by two weeks of recovery (with normal water).This was followed by a second cycle of 2.5% DSS for five days, with two weeks of recovery (with normal water).The mice were euthanized at the end of the last recovery treatment, and samples were collected for pathologic analysis.
Tissue harvesting and tumor assessment, preparation, and immunostaining
Tissues were collected and prepared for immunofluorescence as described previously[18].Briefly, tissue sections were baked in a 65 °C oven overnight, deparaffinized in xylene, and rehydrated by incubation in a decreasing ethanol bath series (100%, 95%, and 70%), followed by antigen retrieval in citrate buffer solution(10 mM sodium citrate and 0.05% Tween-20, pH 6.0) at 120 °C for 10 min using a decloaking chamber(Biocare Medical) and 30 min incubation at 4 °C.The histological sections were incubated with blocking buffer (5% bovine serum albumin and 0.01% Tween 20 in 1 × Tris-buffered phosphate-buffered saline) for 1 h at 37 °C.The primary antibodies goat anti-KLF4 (1:200 for human sections and 1:300 for mice sections;R&D: AF3158), mouse anti-PanCK (1:200 for human sections; Biocare Medical: AE1/AE3), rabbit anti-βcatenin (1:500 for human sections and 1:150 for mice sections; Cell Signaling: 8480), rabbit anti-TWIST(1:500; Abcam: ab49254), rabbit anti-Claudin-1 (1:500; Cell Signaling: 13255), rabbit anti-N-cadherin(1:500; Cell Signaling: 13116), rabbit anti-E-cadherin (1:300 for mice sections, Cell Signaling: 3195), rabbit anti-Vimentin (1:500 for human sections and 1:100 for mice sections; Cell Signaling: 5741), and rabbit anti-SNAI2 (1:500 for human sections and 1:400 for mice sections, Cell Signaling: 9585) were added and incubated at 4 °C overnight.For KLF4, secondary unconjugated bovine anti-goat antibody was added at 1:500 dilution in blocking buffer for 30 min at 37 °C.For human sections to stain for PanCK, secondary unconjugated chicken anti-mouse antibody was added at 1:500 dilution in blocking buffer for 30 min at 37°C.Appropriate Alexa Fluor-labeled antibodies (Molecular Probes) were added at 1:500 dilution in blocking buffer for 30 min at 37 °C.For mice sections, mouse anti-PanCK antibodies conjugated with Alexa 488(1:100; ThermoFisher Scientific: 53-9003-82) were used.All slides were counterstained with Hoechst 33258(ThermoFisher Scientific: H3569) and mounted with Fluoromount Aqueous Mounting Medium (Sigma-Aldrich: F4680).Slides were analyzed using a Nikon eclipse 90i microscope (Nikon Instruments Inc.)equipped with DS-Qi1Mc and DS-Fi1 CCD cameras (Nikon Instruments Inc.).
Immunofluorescence quantification
For each EMT/KLF4 co-stain, we quantified slides of 4-6 human specimen.For normal adjacent mucosa and tumor sections, we quantified four separate fields and counted 250-400 cells per each field.Each cell was labeled as positive or negative for KLF4 and positive or negative for each of the specific EMT markers.The statistical analysis was performed using a two-tailed Studentttest.
Cell culture
SW480 colorectal cancer cell line (ATCC® CCL-228) was maintained in RPMI640 medium supplemented with 10% FBS and 1% penicillin/streptomycin at 37 °C in atmosphere containing 5% CO2.To overexpress Klf4-GFP and GFP-control in SW480 cell line, cells were transiently transfected with 3 μg plasmid DNA(per well in a six-well plate) using Lipofectamine 2000 reagent (ThermoFisher Scientific) according to manufacturer’s instructions.The cell lysates were collected using Laemmli buffer and subjected to Western blot analysis with the following antibodies: rabbit anti-KLF4 (MBL: PM057), rabbit anti-ZEB1 (Cell Signaling: 3396), rabbit anti-SNAI1 (Cell Signaling: 3879), rabbit anti-SNAI2 (Cell Signaling: 9585), and mouse anti-actin (SigmaAldrich: A1978).Then, they were developed using secondary antibodies goat antirabbit HRP-conjugated (JacksonImmuno Research: 111-035-144) and goat anti-mouse HRP conjugated(SigmaAldrich: AP200P), respectively.
Statistical analysis
Student’s paired or unpairedttest was used for statistical analyses.Differences between values were considered significant whenP< 0.05.This analysis was performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA).
RESULTS
Expression of KLF4 in human colorectal cancer is negatively correlated with markers of EMT
EMT is a precisely orchestrated multistep process regulated by several transcriptional factors including TWIST, SNAI1, SNAI2, and ZEB1[3].To determine the correlation between KLF4 expression and EMT in CRC, we performed immunofluorescence analysis of matched pairs of archived samples from patients after tumor resections.Firstly, we analyzed the expression pattern of KLF4 and TWIST.As shown in Figure 1A(SB396N), KLF4 is expressed in the nucleus of epithelial cells in the normal-appearing mucosa adjacent to the cancer tissues.These cells are positive for PanCK, an epithelial marker, and negative for the nuclear expression of the biomarker of EMT, TWIST.In contrast, in the tumor samples from the same patient[Figure 1A, SB396T], the expression of KLF4 is downregulated in the epithelial cells, which is accompanied by a significant increase in the expression of TWIST.Our statistical analysis showed that there is a negative correlation between KLF4 and TWIST expression in normal-appearing mucosa and tumor tissues (P< 0.001).Several common signaling pathways regulate factors involved in EMT including HH,WNT, NOTCH, and TGF-β[19].WNT signaling plays an important role in the homeostasis of the intestinal epithelium and its deregulation leads to cancer formation which is accompanied by modification of the pattern and level of expression of its major effector, β-catenin[20-22].In the normal-appearing mucosa, β-catenin is predominantly localized to the membrane of the epithelial cells with a modest nuclear staining [Figure 1B,SB474N].Upon loss of KLF4 in the colorectal tumor [Figure 1B, SB474T], the levels of the cytoplasmic andnuclear β-catenin are significantly increased while its membranous expression is decreased (P< 0.001).Loss of cell polarity and cell-cell junctions is another hallmark of EMT and is characterized by increased expression of claudin-1 and N-cadherin[23-27].Immunofluorescence staining of claudin-1 [Figure 2A,SB474N] and N-cadherin [Figure 2B, SB378N] within the normal-appearing mucosa shows little or no staining while there is pronounced nuclear KLF4 staining.In matching colorectal cancer tumor tissues with lack of KLF4 expression, both claudin-1 and N-cadherin levels are significantly increased [Figure 2A,SB474T and Figure 2B, SB378T, respectively].Our analysis showed that there is a significant negative correlation between expression of KLF4 and claudin-1 and N-cadherin between the normal-appearing mucosa and tumor tissues (P< 0.001).Furthermore, staining for vimentin, another mesenchymal marker,showed a lack of expression within the epithelial component of the normal-appearing mucosa [Figure 3,SB474N], but a slight increase within the epithelial compartment upon loss of KLF4 in the tumor tissues[Figure 3, SB474T, white arrowheads].Statistical analysis showed that the expression levels of KLF4 and vimentin are negatively correlated in normal mucosa (P< 0.05) and in tumor sections (P< 0.001).
KLF4 expression is negatively correlated with markers of EMT in a mouse model of colitisassociated cancer
Recently, in a mouse model, we demonstrated that KLF4 plays a protective role against progression of colitis-associated cancer and that decrease in KLF4 levels is associated with increased aggressiveness of the disease[18].To test if KLF4 expression levels correlate with the expression pattern of EMT markers,we performed immunofluorescence staining of KLF4 and select EMT markers on the mouse tissues(Klf4fl/flmice) after treatment with AOM/DSS, as described in the Material and Methods Section.Immunofluorescence staining showed that in normal mucosa KLF4 is expressed in the nuclei of epithelial cells defined by staining for PanCK.Co-staining for SNAI2 showed that SNAI2 was predominantly expressed in the stromal section of the normal-appearing mucosa and tumor section of mice after AOM/DSS treatment [Figure 4A] and was absent from epithelial compartment.However, in the tumor sectionwhere KLF4 loss was observed, we noticed increased levels of SNAI2 in the nuclei of the epithelial cells that were defined by positive staining for PanCK [Figure 4A].As in human specimens, we performed immunostaining analysis for β-catenin.However, we did not observe an increase in nuclear β-catenin staining in the tumor sections in comparison to the normal adjacent mucosa [Figure 4B].With respect to adherens junction complexes, we observed a reduction in the expression levels of E-cadherin in the tumor section as compared to the normal adjacent mucosa [Figure 5A].Expression of the mesenchymal marker vimentin in normal adjacent mucosa of mice after AOM/DSS treatment is confined to the stroma[Figure 5B] and does not correlate with KLF4 expression.However, in the tumor of mice after AOM/DSS treatment, we observed a slight increase in the staining of vimentin within the epithelial section, which suggests a change in the characteristics of these cells from epithelial toward mesenchymal phenotype[Figure 5B].Furthermore, overexpression of KLF4 in SW480 CRC cell line resulted in decreased levels of the mesenchymal markers of EMT, namely ZEB1, SNAI1, and SNAI2 [Figure 6], confirming a suppressive role in the regulation of the epithelial-to-mesenchymal transition.
DISCUSSION
In this study, we investigated the correlation in expression between KLF4 and EMT markers in tissues obtained from patients with CRC and from a mouse model of colitis-associated cancer.The results from studies of epidermal cancer, hepatocellular carcinoma, breast cancer, pancreatic cancer, and prostate cancer with data predominantly originating fromin vitroexperiments show that KLF4 negatively regulates EMT[28-33].On the other hand, KLF4’s ability to regulate the stemness of cancer cells has been shown as an important factor in stimulating EMT in pancreatic, ovarian, endometrial, nasopharyngeal, prostate,and non-small cell lung cancers[34-40].We demonstrated that KLF4 expression is positively correlated withepithelial markers of EMT in normal mucosa and negatively with mesenchymal markers in CRC.These results are in agreement with previous observations that KLF4 is a suppressor of EMT[28-31,41].This could be accredited to the role of KLF4 in the regulation of differentiation along the crypt-luminal axis in the intestinal epithelium[10].Importantly, KLF4’s suppressive role in EMT regulation is not limited to the colonic epithelium but has been shown to play a crucial role in corneal epithelial cell fate.It was shown that downregulation of KLF4 promotes expression of mesenchymal markers and decreases the expression of epithelial markers[42-45].Furthermore, studies using conditional ablation of KLF4 from the intestinal epithelium showed a deficiency in goblet cell differentiation, thereby demonstrating that KLF4 plays a role in maintaining intestinal epithelial morphology and homeostasis[9,12].Additionally, KLF4 has been shown to regulate apical-basolateral polarity in intestinal epithelial cells and to enhance their polarity by transcriptional regulation[13].Thus, loss of KLF4 expression during development and progression of CRC may lead to loss of cell polarity with progression toward EMT.Furthermore, it has been previously demonstrated that E-cadherin (Cdh1), N-cadherin (Cdh2), vimentin (Vim), and β-catenin (Ctnnb1) genes are direct transcriptional targets of KLF4[30].Yoriet al.[29,41]demonstrated that KLF4 is necessary for the maintenance of E-cadherin expression, repression of SNAI1, and prevention of EMT in mammary epithelial cells.These results are consistent with our observations.We demonstrated a positive correlation between KLF4 and E-cadherin and a negative one between KLF4 and N-cadherin and vimentin in human and mouse tissues.However, we were not able to show increased nuclear levels of β-catenin in mouse tissues after AOM/DSS treatment[18].This could be due to technical differences, as we previously used immunohistochemical staining and the current analysis was based on immunofluorescence studies.In addition, we showed that KLF4 expression is negatively correlated to the expression of TWIST, SNAI2,and claudin-1.Taken together, these results suggest that KLF4 may regulate EMT at several different stages of CRC progression, including suppression of expression of transcription factors (TWIST and SNAI2),transcriptional co-activator (β-catenin), and regulation of cell polarity (E-cadherin, N-cadherin, and claudin-1).In conclusion, we showed a negative association between KLF4 and mesenchymal EMT markers in both human and mouse CRC tissues.Future studies are necessary to identify the mechanism by which KLF4 regulates EMT progression in CRC.
DECLARATIONS
Acknowledgments
We thank the Department of Pathology at Stony Brook University for technical assistance in histopathological analysis.
Authors’ contributions
Made substantial contributions to conception and design of the study and performed data analysis and interpretation: Yang VW, Ghaleb AM, Bialkowska AB
Performed data acquisition and analyzed data: Agbo KC, Huang JZ, Ghaleb AM
Provided tissues samples: Williams JL
Provided assistance with histopathological analysis: Shroyer KR
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by grants from the National Institutes of Health awarded to Yang VW (CA084197).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
The protocol for the sample collection was originally approved by the Institutional Review Board by the State University of New York at Stony Brook on October 17th, 2014 (CORIHS 2014-2821-F) and qualified for a waiver under the Federal Law of Department of Health and Human Services per article 45CFR46.116.d.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2019.
 Journal of Cancer Metastasis and Treatment2019年11期
Journal of Cancer Metastasis and Treatment2019年11期
- Journal of Cancer Metastasis and Treatment的其它文章
- High-risk HPVs, microbiota and epithelial carcinogenesis: state of the art and research contribution of in vitro 3D models
- Novel immunotherapeutic approaches in head and neck cancer
- Monoclonal antibody pharmacogenomics in cancer treatment
- CXCR4 signalling, metastasis and immunotherapy:zebrafish xenograft modeI as transIationaI tooI for anti-cancer discovery
- Histone chaperone FACT and curaxins: effects on genome structure and function
- AUTHOR INSTRUCTIONS
