Novel immunotherapeutic approaches in head and neck cancer
Molly E.Heft Neal, Catherine T.Haring, Jacqueline E.Mann, J.Chad Brenner,3,4, Matthew E.Spector,Paul L.Swiecicki
1Department of Otolaryngology Head and Neck Surgery, University of Michigan, Ann Arbor, MI 48103, USA.
2Department of Internal Medicine, Division of Hematology/Oncology, University of Michigan Medical School, Ann Arbor, MI 48103, USA.
3Department of Pharmacology, University of Michigan, Ann Arbor, MI 48103, USA.
4Rogel Cancer Center, University of Michigan, Ann Arbor, MI 48103, USA.
Abstract Unresectable recurrent or metastatic head and neck cancer is an incurable disease with survival of approximately 12 months.Head and neck tumors exhibit numerous derangements in the tumor microenvironment that aid in immune evasion and may serve as targets for future therapies.Pembrolizumab is now approved as a first line therapy.Despite the promise of currently approved immunotherapies there continues to be low response rates and additional strategies are needed.Here, alterations in the immune microenvironment and current therapeutic strategies are reviewed with a focus on novel immunologic approaches.
Keywords: Immunotherapy, head and neck, immune evasion, immune derangement, checkpoint inhibitors,recurrent/metastatic
INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) represents the 6th most common malignancy globally and accounts for 1%-2% of all cancer related deaths[1].HNSCC is comprised of a heterogenous group of tumors including those arising from the oral cavity, oropharynx, hypopharynx and larynx.Traditional riskfactors include tobacco, alcohol, and more recently human papillomavirus (HPV).High risk strains of HPV(HPV 16, 18) now are responsible for 70%-80% of oropharyngeal squamous cell carcinoma[2,3].
Treatment of HNSCC varies by tumor site and stage, however the mainstays of treatment include surgery,radiation, and cytotoxic chemotherapy.Despite advancements in surgical and radiation techniques,treatment failures occur in up to 50% of patients with HNSCC[4,5].In the unresectable recurrent or metastatic (R/M) setting, chemotherapy has previously been the main therapeutic option, with dismal outcomes and median survival times ranging from 6-10 months[6].Immunotherapy, particularly checkpoint inhibitors, have shown promising results in R/M HNSCC[7,8].In June of 2019, the United States Food and Drug Administration approved pembrolizumab as a 1st line treatment for patients based on PD-L1 expression in the tumor immune microenvironment[9].Despite these recent reports, overall response rates remain low with underwhelming improvements in long term survival.Hence there continues to be a need for novel therapeutic options.
Head and neck tumors display various derangements in anti-tumor immunity and detailed understanding of these changes has led to development of currently approved immunotherapies.Here, we discuss alterations in the tumor immune microenvironment, review the mechanism of current treatments and focus on approaches for development of novel immunologic therapies.
DERANGEMENT OF HEAD AND NECK TUMOR IMMUNE MICROENVIRONMENT
Tumor immunity cycle
Anti-tumor immunity requires a complex set of interactions between the tumor and host immune system.This process has been termed the cancer immunity cycle[10,11].Initial tumor cell lysis results in release of tumor specific antigens (TAs) and priming of antigen presenting cells (APCs).APCs then interact with host immune cells resulting in activation and trafficking of cytoxic T cells (CTLs) into the tumor.Once in the tumor, CTLs identify malignant cells displaying the specified tumor antigen and target them for cell death.Tumor antigens also referred to as neoantigens have become an area of intense research.These can be derived from either driver or passenger mutations and generation of TAs is thought to be closely linked to mutational burden, with a higher mutational load correlating to increased TAs[12,13].HNSCC has been found to have 1 of highest mutational burdens of all malignancies, likely due to their relationship with carcinogen exposure (i.e., tobacco smoke) which results in significant mutagenesis[13,14].As sequencing techniques have advanced, more sophisticated modeling has allowed for identification of specific mutational profiles including smoking and APOBEC signatures as well as prediction of neoantigen load[15].Detailed review of neoantigen prediction modeling has previously been published and is outside the scope of this review[16-19].Finally, with targeted tumor cell death by CTLs there is further release of tumor antigens resulting in perpetuation of the cycle.Head and neck tumors have evolved multiple mechanisms of immune escape which will be reviewed below in context of the cancer-immunity cycle.
Inhibition of antigen processing and presentation and immune cell activation
While HNSCC is thought to be highly antigenic, further steps are required for activation of a TA-specific adaptive immune response.After being released from tumor cells, TAs are degraded, processed, and presented by professional APCs including dendritic cells.Normal processes allow for extracellular protein presentation through major histocompatibility complex (MHC) class II and CD4 interaction, however, for TA to activate a CD8 response, cross presentation occurs, requiring an additional set of processing machinery[20].Large scale sequencing studies as well as analysis of TCGA data have revealed that up to 20% of HNSCCs contain alterations in antigen processing machinery (APM) or downregulation of MHC class I[20,21].In HPV positive tumors, the latter is thought to be mediated through viral oncoproteins, E5 and E7, which have been shown to downregulate both MHC class I and class II[22-24].Additional studies show that patients withalterations in these pathways had both decreased CD8 T cell infiltration and worse survival outcomes[25,26],indicating inhibition of antigen presentation may play a key role in head and neck tumor immune escape.
Once primed, APCs interact with and activate CTLs.Activation of CTLs occurs through contact between the T cell receptors and MHC class I bound to TA.This process requires a co-stimulatory signal between CD80 (present on the surface of the APC) and CD28 (a surface receptor on the CTL).Conversely, CD80 may instead bind CTL associated antigen 4 (CTLA-4) leading to CTL inhibition[11,27,28].While in normal physiologic conditions, the CTLA-4 immune checkpoint prevents an exaggerated immune response, in the setting of malignancies, it is thought to be a major mechanism of immune escape[27,28].
Immune cell trafficking and infiltration
After priming and activation, CTLs infiltrate the tumor where they identify malignant cells displaying the specific TAs.In order for successful immune cell trafficking to occur, there must be appropriate cytokine signaling as well as an optimized physical environment.Physical blockade of immune cell infiltration has been suggested to play a role in immune escape and is thought to be mediated through elevated vascular endothelial growth factor (VEGF) signaling.This results in increased angiogenesis and increased oncotic pressure within the tumor creating a physical barrier to infiltration[29].In vivostudies evaluating antiangiogenic tyrosine kinase inhibitors have shown an increase in tumor infiltrating lymphocytes correlating with reduced angiogenesis[30,31].Tumors may also promote an immune deplete environment through recruitment of suppressive and regulatory immune cells.This is achieved through direct secretion of suppressive cytokines such as tumor growth factor beta (TGF-β), or through secretion of CCL, CXCL, or VEGF which recruit myeloid derived suppressor cells (MDSC)[32-34].MDSCs are able to directly induce T cell tolerance through arginase, nitric oxide synthase[35]and indoleamine 2,3-dioxygenase (IDO)[36]dependent mechanisms[32,37,38].In HNSCC, increased tumor lymphocyte infiltration is linked to improved prognosis[39-41], while elevated levels of MDSCs have been linked worse prognosis[38,42].
PD-1/PD-L1 axis inhibition
Once in the tumor, CTLs induce tumor cell death.However, tumor cells may inhibit cell killing via costimulatory signals through the PD-1/PD-L1 axis.PD-1 is a member of the CD28 superfamily and is expressed on dendritic cells, regulatory T cells (Treg), CD8 and CD4 T cells, MDSCs, and natural killer cells (NK)[43].PD-L1 is expressed on APCs and causes T cell anergy and apoptosis upon binding with PD-1 receptors, thereby serving as a check to prevent an overactive immune response[44,45].However,upregulation of PD-L1 or additional PD-1 ligands such as PD-L2 can also be seen on tumor cells and acts as a mechanism of immune escape in various malignancies[46].The interaction between PD-L1 and PD-1 is complex and has been previously explored in multiple reviews[47,48].In HNSCC, PD-1/PDL-1 expression has been reported in 46%-100% of tumors and with higher expression in HPV-positive tumors compared to HPV negative tumors[46,49-51].Multiple currently approved therapeutics have been developed to target the PD-1/PD-L1 axis, including Pembrolizumab and Nivolumab as well as additional therapeutics that are currently undergoing clinical investigation such as Atezolizumab, and Durvalumab among others [Figure 1].Both Pembrolizumab and Nivolumab inhibit PD-1, while the latter 2 (Atezolizumab and Durvalumab) target PD-L1.As PD-1 can be activated by additional ligands such as PD-L2 there is a theoretical advantage to targeting PD-1 over PD-L1, however anti-PD-1 and anti-PD-L1 therapies have demonstrated similar response rates and toxicity profiles in clinical trials as discussed below.
IMMUNOMODULATORY MECHANISMS OF TRADITION THERAPIES
Prior to review of current immunotherapeutics, it is prudent to discuss the immunomodulatory role of traditional therapies.Both platinum based chemotherapy and radiation have been shown to alter the tumor immune microenvironment.In vivostudies revealed that cisplatin treatment increases expression of MHC class I and antigen presentation machinery in patient derived HNSCC cell lines[52].While thesechanges may improve the anti-tumor immune response, this same study also demonstrated increased PDL1 expression, decreased IFN-γ release by Tregs, and increased antigen specific T cell death with cisplatin treatment[52,53].These alterations in the tumor immune microenvironment suggest potential for dual treatment with cisplatin and anti-PD-1/PD-L1 immunotherapeutics, andin vivostudies have shown benefit of combination therapy[52].While cisplatin leads to increased Treg populations in HNSCC, similar studies performed in other types of malignancies have found that cisplatin treatment can actually lead to decreased Treg function and CTL activation by dendritic cells[53,54].These contradicting results suggest a complex mechanism by which cisplatin may affect the tumor microenvironment.
Radiation therapy (RT) is commonly used in the treatment of head and neck cancer and has also been shown to play a regulatory role in the tumor immune microenvironment.The specific mechanism by which radiation enhances the immune system is multifactorial.RT has been shown to increase production of TA[55], increase expression of MHC class I and APM components[56-58], and increase the number of tumor infiltrating lymphocytes[59].Similar to chemotherapy, RT can also suppress the immune response through recruitment of MDSCs and other suppressive immune cells[60].Further indication of the immunoregulatory effect of radiation is evident by the fact that radiation has been reported to induce tumor cell death in tumor deposits outside of the irradiated field.This is termed the abscopal effect and is thought to be an immune mediated response[61,62].
MECHANISMS OF CURRENTLY APPROVED IMMUNOTHERAPEUTICS
In addition to traditional therapies, there are 3 currently approved biologics for HNSCC including Cetuximab, Pembrolizumab, and Nivolumab, which have also been shown to have immunomodulatory mechanisms.
Cetuximab, a monoclonal antibody against epidermal growth factor receptor (EGFR), was designed as a targeted therapy.However, recent studies have suggested that it may be tumoricidal via immune modulation[63].Accumulating evidence suggests that cetuximab increases antibody dependent cellular cytotoxicity (ADCC) of NKs[63-65].As Tregs inhibit NK mediated ADCC, through a TGF-β dependent pathway, it is postulated Cetuximab’s mechanism of action may be through reduction or inhibition of Tregs.A recent study demonstrated a decrease in the number of Treg in peripheral blood samples in HNSCC patients treated with Cetuximab.This observed decrease in Treg population also correlated with improved overall survival in this cohort[63].Additional mechanisms of cetuximab have also been suggested, including increased crosstalk between dendritic cells and NK cells with further activation of the adaptive immune response, specifically targeting EGFR expressing cells[66,67]and activation of the complement system[68].More recent studies have also indicated that cetuximab may increase CTLA-4 and PD-1/PD-L1 expression and suggest a need for combination therapies[64,69].
Both Pembrolizumab and Nivolumab which target PD-1 have proven to have clinical benefit in patients with progressive disease after platinum based therapy[7,70].Initial approval for these drugs occurred in 2016 after the published results from multiple clinical trials were released.The phase III clinical trial, CHECKMATE-141, evaluated nivolumabvs.standard of care (SOC) chemotherapy in patients with platinum refractory R/M HNSCC.The overall response rate (ORR) for the nivolumab group was 13.3% compared to 5.5% in the SOC arm.Furthermore, nivolumab demonstrated an improved survival [7.5 monthsvs.5.1 months (HR = 0.7, 97.7%CI: 0.51-0.96)] which was the 1st time a 2nd line agent demonstrated survival benefit[7].Similar results were seen in the KEYNOTE 040 trial, comparing Pembrolizumab to SOC in patients with R/M HNSCC who had failed platinum therapy.In this trial there was an ORR of 14.6% in the pembrolizumab group compared to 10.1% in the SOC group and a median OS of 8.4 monthsvs.6.9 months (95%CI: 0.65-0.98)[70].Both studies had secondary endpoints evaluating survival outcomes stratified by PD-L1 status and revealed a greater benefit in patients with positive PDL1 expression although not statistically significant.These results supported the use of nivolumab or pembrolizumab as a standard of care treatment for patients with platinum refractor R/M HNSCC.
Until recently, there has been no data supporting use of PD-1 inhibitors as a first line treatment option for R/M HNSCC.The results of the KEYNOTE 048 trial were presented at the 2019 American Society of Clinical Oncology meeting[71].This study evaluated the use of pembrolizumab alone, pembrolizumab with platinum and fluorouracil,vs.the EXTREME regimen in patients with R/M HNSCC.Results revealed an improvement in OS in all patients treated with pembrolizumab with a PD-L1 combined positive score ≥ 1%by the 22C3 assay[9].
While there are no mature data evaluating the effect of anti-PD-L1 therapeutics, such as Atezolizumab or Durvalumab, ongoing phase I and II trials have demonstrated excellent safety profiles with promising responses.A phase I trial published by Colevaset al.[72]in 2018 enrolled 32 patients with advanced unresectable or incurable HNSCC who underwent treatment with Atezolizumab.The overall response rate in this cohort was 22% with a median progression free survival of 2.6 months and median overall survival of 6 months.Responses were found to be independent of PD-L1 expression level or HPV status.While 66%of patients experienced treatment related adverse events, only 4 patients (13%) had grade 3 or 4 toxicity.Given these results, a phase III randomized control trial is currently underway investigating the use of Atezolizumab in HNSCC (NCT03452137).Durvalumab has also been evaluated in Phase I/II trials in HNSCC.A study by Segalet al.[73]published in 2019 examined 62 patients with unresectable and previously treated HNSCC treated with Durvalumab and found an ORR of 6.5% with a median overall survival of 8.4 months.Unfortunately, the results of the phase III EAGLE trial evaluating Durvalumab as single modality therapy in patients with progressive disease after platinum therapy was did not reveal any survival benefit alone or in combination with CTLA-4 inhibitor, Tremelimumab[74].An ongoing trial (NCT02551159)is evaluating Durvalumab with and without Tremelimumab in patients with R/M disease who have not received previous treatment for recurrent disease.A summary of select ongoing clinical trials investigating these therapies in addition to other checkpoint inhibitors is shown in Table 1.
DEVELOPMENT OF NOVEL IMMUNOTHERAPEUTICS
Despite these encouraging results and updated treatment guidelines, it should be noted that overall response rates to these immunotherapies remains low for HNSCC and ongoing clinical trials are evaluating novel immunotherapeutic strategies [Table 2].
Oncolytic viruses provide potential for direct tumor cell lysis with further activation of an immune specific response.One of the most promising trials involving oncolytic vaccines in HNSCC to date evaluates the use of pembrolizumab in combination with Talimogene laherparepvec in patients with R/M disease.Final study results are pending, however initial updates suggest at least partial response in some patients (NCT02626000).Additional oncolytic viruses are also under early phase studies (NCT00625456,NCT03740256, NCT01584284).
Vaccinations represent a promising novel therapeutic strategy for various malignancies including HNSCC.Vaccines currently under investigation in HNSCC target patient specific TA (NCT03633110,NCT03548467), or TAs that are expressed in the majority of HNSCC such as mutant p53 (NCT02432963,NCT02955290, NCT02544880, NCT03946358).Additional vaccines are designed to enhance a general immune response such as those using the fowlpox-TRICOM vaccine (NCT00021424).For patients with HPV-mediated disease, the potential for vaccines targeting HPV specific peptides has gained enthusiasmand there are multiple ongoing trials investigating vaccines in this subset (NCT03418480, NCT03260023,NCT00257738, NCT02002182).
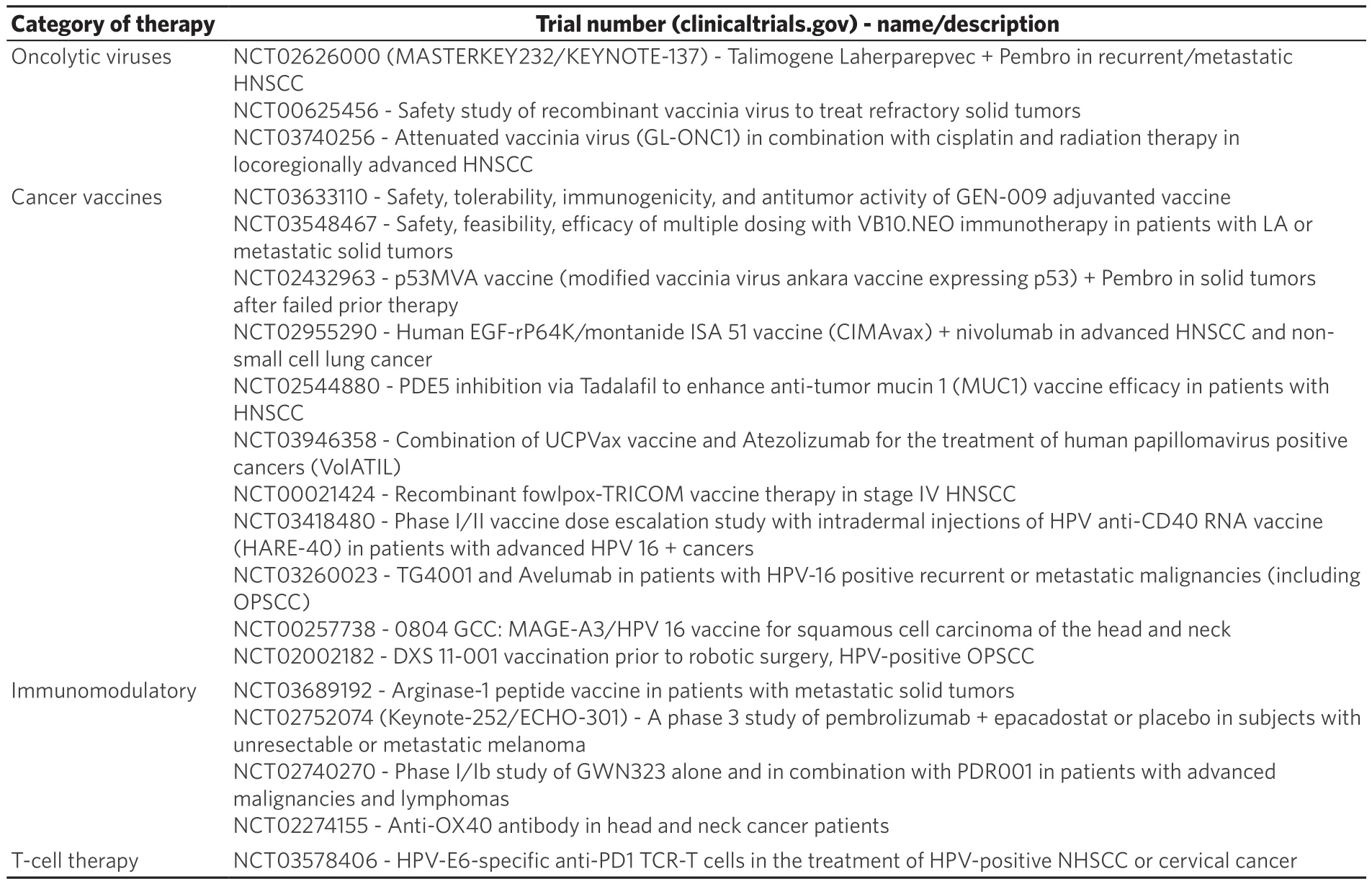
Table 2.Ongoing clinical trials
Both oncolytic viruses and vaccine therapy aim to promote a tumor specific adaptive immune response.Additional novel therapeutics have also been developed to try to remove the inhibitory signals that suppress an already active immune response.
One example of this strategy is the targeting of MDSCs, as multiple studies have shown these cells to be present in high abundance in HNSCC[38,75].In vivostudies have shown that elimination of MDSCs from the tumor microenvironment results in decreased Tregs and increased activity of CTLs[76].As discussed above,Arg, NOS, and IDO are thought to play a role in MDSC mediated Treg function; thus, drugs targeting these pathways are of particular interest.Phosphodiesterase-5 inhibitors are known to reduce both NOS and Arg production and a recent clinical trial leveraged the already FDA approved drug Tadalafil for use in HNSCC.In this randomized, double blinded, placebo controlled trial, patients with previously untreated (primary or recurrent) HNSCC received either tadalafil or placebo for 10 or more days prior to definite treatment.This study revealed decreased MDSC and Treg populations in the tadalafil treated cohort compared to placebo controls as well as increased CTL activity.Subgroup analysis of patients with available tumor specimens(n= 6) demonstrated increased tumor specific immunity in the Tadalafil treated group[37].No measures of survival outcomes were reported for this study.An additional clinical trial (NCT03689192) evaluating a vaccine targeting arginase is also currently underway.
Inhibition of IDO in combination with Pembrolizumab has also been under intense study in various malignancies[77].In HNSCC, a phase III clinical trial evaluating IDO inhibitor, Epacadostat in combinationwith Pembrolizumab (NCT02752074), was unfortunately halted after a similar trial in melanoma revealed no improvement in overall or progression free survival with the addition of Epacadostat to pembrolizumab compared to the control arm[36].
Additional therapies targeting immunosuppressive cytokines have also been developed[78].Current trials investigating antagonists of TNF receptor aims to inhibit Treg activity and are being tested both as monotherapy and in combination with anti-PD-1 therapies in HNSCC (NCT02740270, NCT02274155).
The final step in the cancer immunity cycle involves tumor cell death induced by activated CTLs.The ideal immunotherapy would bypass the preceding steps and provide T cells already primed to patient specific antigens.This has been successful in hematologic malignancies with therapies such as CAR-T and multiple ongoing clinical trials are investigating the use of T-cell therapies in solid malignancies as well[79].A clinical trial investigating the use of adoptive T cell transfer in HPV mediated disease in currently ongoing(NCT03578406).A previous study has shown some promise in seven patients with head and neck cancer (5 with SCC, 1 with melanoma and 1 with spindle cell sarcoma)[80].
There are also ongoing trials assessing unique approaches that target multiple aspects of the immune cycle.MVX-ONCO-1 is a treatment that involves subcutaneous injection of capsules containing immunemodulatory granulocyte-macrophage colony stimulating factor and irradiated tumor cells with the aim of stimulating a tumor specific immune response (NCT02999646).
CONCLUSION
HNSCC represents a diverse group of diseases and exhibits varying degrees of immune dysregulation.Traditional therapeutic approaches are curative in 50% of patients and have proven to have immunomodulatory effects.Currently approved immunotherapies have shown some promise but unfortunately only a small fraction of patients benefit.This review summarizes the most common immune disruptions identified in head and neck cancer and discusses ongoing approaches aimed at targeting the tumor immune microenvironment.
DECLARATIONS
Authors’ contributions
Conceptualization, data curation, writing, review/editing: Heft Neal ME
Writing, review/editing: Haring CT
Writing, review/editing: Mann JE
Supervision, review/editing: Brenner JC
Conceptualization, writing, review/editing: Spector ME
Conceptualization, writing, review/editing: Swiecicki PL
Availability of data and materials
Not applicable.
Financial support and sponsorship
Heft Neal ME was supported in part by the NIH Grant (T32 DC005356).Brenner JC was supported in part by American Cancer Society Grant: 132034-RSG-18-062-01-TBG.Brenner JC was supported by F31: DE-027600-01.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2019.
 Journal of Cancer Metastasis and Treatment2019年11期
Journal of Cancer Metastasis and Treatment2019年11期
- Journal of Cancer Metastasis and Treatment的其它文章
- High-risk HPVs, microbiota and epithelial carcinogenesis: state of the art and research contribution of in vitro 3D models
- Monoclonal antibody pharmacogenomics in cancer treatment
- CXCR4 signalling, metastasis and immunotherapy:zebrafish xenograft modeI as transIationaI tooI for anti-cancer discovery
- Loss of the Krüppel-like factor 4 tumor suppressor is associated with epithelial-mesenchymal transition in colorectal cancer
- Histone chaperone FACT and curaxins: effects on genome structure and function
- AUTHOR INSTRUCTIONS
