Comparative age and growth of Uroteuthis chinensis and Uroteuthis edulis from China Seas based on statolith
Yue Jin, N Li, Xinjun Chen,b,c,d,e,*, Bilin Liu,b,c,d,e, Jinhu Li
a College of Marine Sciences, Shanghai Ocean University, Shanghai 201306, China
b Key Laboratory of Oceanic Fisheries Exploration, Ministry of Agriculture, Shanghai 201306, China
c National Engineering Research Center for Oceanic Fisheries, Shanghai Ocean University, Shanghai 201306, China
d Key Laboratory of Sustainable Exploitation of Oceanic Fisheries Resources, Ministry of Education, Shanghai Ocean University, Shanghai 201306, China
e Scientific Observing and Experimental Station of Oceanic Fishery Resources, Ministry of Agriculture, Shanghai 201306, China
f College of Marine Science, University of South Florida, Florida 33701, USA
Keywords:Uroteuthis chinensis Uroteuthis edulis Age Hatching date Growth
A B S T R A C T To determine the age and growth of Uroteuthis chinensis and U. edulis, growth increments of the lateral dome of the statolith were used in the present study. Specimens of the two species caught in the South China Sea (SCS)and East China Sea (ECS) were used to compare the age and growth differences between sexes. In the present study, we found the two species have similar lifespans of less than 200 days. We also found that there were significant differences in the relationships between mantle length and body weight for two species and for both sexes in the same species. The back-calculated hatching date for the two species was similar, i.e. October to January for specimens caught in spring, and March to June for specimens caught in summer (U. edulis) or autumn (U. chinensis). Exponential and logistic growth curves were fitted to build the age and mantle length relationship. Both growth curves showed that U. chinensis has a larger mantle length than U. edulis at the same age.The results of the growth rates also showed that U.chinensis grows faster than U.edulis.However,there are discrepancies between previous studies and the present study on the lifespan of the two species.Also,the sample size of the present study is small especially for U. edulis. Hence, future studies should be conducted to validate the daily periodicity of growth increment in the regions of statolith(except for the lateral dome which has been validated) and the sample size should be increased for age and growth studies.*Corresponding author. College of Marine Sciences, Shanghai Ocean University, Shanghai 201306, China.E-mail address: xjchen@shou.edu.cn (X. Chen).
1. Introduction
The age and growth of squid are critical parameters for not only understanding their basic biological characteristics, but also understanding the life history of squid and issues of ecosystem modeling and fisheries management(Jackson,2004).The length frequency method is considered to be inappropriate for age and growth analysis of squid because of the difficulties in the identification of discrete length-frequency modes (Natsukari, Nakanose, & Oda, 1988). Fortunately, the hard tissues of squid, such as statoliths, beaks, gladii and eye lenses bear periodical growth increments within their microstructures(Arkhipkin, 2005). Currently statoliths are the most widely used hard tissue for studying the age and growth of squid (Jackson, 1994). Statoliths are calcareous structures located in the equilibrium organs and are used for detecting body acceleration.They have been considered as‘black boxes' recording ecological information of the whole life history of the squid (Arkhipkin, 2005). In the 1980s, the daily periodicity of statolith growth increments of squid was validated by chemical marking for several species (Dawe, O'dor, Odense, & Hurley, 1985;Hurley, Odense, O'Dor, & Dawe, 1985; Lipinski, 1986; Nakamura &Sakurai, 1991).
Uroteuthis chinensis (the mitre squid) and U. edulis (the swordtip squid)are both loliginid squid that are distributed in shallow waters of the Indo-Pacific Ocean (Jereb, Vecchione, & Roper, 2010). They are a commercially important species and are abundant in the China Seas(Huang, 2006; Wang, 2009). The mitre squid and the swordtip squid constitute the majority of the Chinese neritic squid catch (Voss &Williamson, 1971, p. 138). U. chinensis is a large-sized squid that has a maximum reported size of 490 mm mantle length for males and 310 mm for females, while U. edulis is a medium to large-sized squid that has a maximum mantle length of 502 mm for males and 410 mm for females (Jereb et al., 2010). However, their size in commercial catches is more commonly around 200 mm for both species (Jereb et al., 2010).
The statolith daily increment has been validated in ten loliginid squid species including U. chinensis (referred as Loligo chinensis) (reviewed by Jackson, 2004). Jackson (1990) validated the daily periodicity of the rostrum and dorsal dome of the statolith of U. chinensis using tetracycline staining techniques. However, there has been no direct validation of daily growth increments of U.edulis.Natsukari et al.(1988) validated the daily periodicity of the statolith of U. edulis in an indirect way, i.e. “the maximum growth rate obtained for males of the warm-season brood(5 mm/day)agrees well with the value obtained by tag and recovery experiments on the present species”. The age of loliginids can be grouped into three categories (Jackson, 2004): less than 200 days (short lifespan), 200 days to 1 year (moderate lifespan) and more than 1 year (extended lifespan). Based on these categories,Jackson (2004) grouped U. chinensis into a short lifespan species and grouped U. edulis (Natsukari et al., 1988) into a moderate lifespan species. Sukramongkol, Tsuchiya, and Segawa (2007) and Bat et al.(2009)found that U.chinensis has a lifespan of less than 200 days using the lateral dome of the statolith, while Huang (2006) found that U.chinensis has a lifespan of around 1 year using the rostrum of the statolith.Natsukari et al.(1988)and Wang(2009)also found that U.edulis has a lifespan of around 1 year using the rostrum of the statolith. The age and growth of U. chinensis and U. edulis have been studied for decades (Natsukari et al., 1988; Chotiyaputta, 1990; Sukramongkol et al., 2007; Huang, 2006; Bat et al., 2009; Wang, 2009). However,there are still huge age differences between studies, especially for U.chinensis, with no appropriate explanation.
In the present study, we try to give reasoned explanations for the age differences in the two species (U. chinensis and U. edulis) among studies.Also,the other objective of this study is to compare the age and growth of these two commercially important loliginid species between sexes,i.e.establishing the relationship between mantle length and age,back-calculating hatch dates, fitting growth curves and calculating growth rates.
2. Materials and methods
2.1. Sampling
Squid specimens of U. chinensis and U. edulis were collected by commercial bottom-trawling in the East China Sea(ECS)and the South China Sea (SCS) during September 2015 to August 2016 (Table 1). Sex was determined and mantle length (ML) and body weight (BW) were measured to an accuracy of 1 mm and 1 g,respectively,after specimens were thawed in the laboratory. Sagittal statoliths were extracted from the head and washed with freshwater, and then stored in 75% ethanol for further processing (Wang, 2009). A total of 253 specimens were measured and were used to estimate age.
2.2. Statolith processing

Fig. 1. Growth increments of U. chinensis in the lateral dome of the statolith.Mantle length of 107 mm, age of 128 days.
The longitudinal section was chosen to read the increments. The statoliths were mounted into rectangular blocks with a mix of epoxy and hardener and left for 24 h to harden(Liu et al.,2017).Grinding was then operated on waterproof sandpaper(using successive grades of 120,600 and 1200 grit).After the nucleus had appeared under the surface of the cross-section, this side was polished using alumina powder. The same grinding process was repeated for the second side. It was important to periodically check the nucleus under a light microscope in order not to over-grind the section.Digital images of the statoliths were taken at×400 magnification with an Olympus light microscope using a Charge Coupled Device (CDD) and then photo-merged with Photoshop CS 5.0.The lateral dome of the statolith was used to determine age as the daily periodicity has been validated for this part(Jackson,1990)and has been used in several studies (Sukramongkol et al., 2007; Bat et al.,2009)(Fig.1).Since the increments around the edge of the lateral dome could not be recognized in many statoliths, the number of increments for this part was extrapolated (Natsukari et al., 1988). The increments in the statolith were counted three times by a skilled person.For each statolith, the mean value of the readings was kept if the differences among the readings were less than 10% of the mean value(Jackson, Forsythe, Hixon, & Hanlon, 1997).
2.3. Data analysis
The ML (mm) and BW (g) relationship was expressed as a power equation: BW=aMLb. The age (day) and ML relationship was expressed as an exponential equation: ML=aebt. The differences of regression slopes and intercepts of the ML-BW relationship and Age-ML relationship between sexes for each species were linearized by log transformation (lnML-lnBW and Age-lnML) (Bat et al., 2009;Sukramongkol et al.,2007).Analysis of covariance(ANCOVA)was used for testing the homogeneity of slopes and intercepts. If the slopes were not significantly different from each other, the intercepts were then tested (Bat et al., 2009). The hatching date was back-calculated from the capture date and estimated age of specimens.
The averages of ML for every 5-day class were used for calculating logistic growth curves. For those classes that lacked data, the ML averages were calculated by interpolation based on adjacent known averages of ML (Natsukari et al., 1988). Logistic growth curves werethen used to predict the relationship between ML and age in the whole life history based on the present data (Huang, 2006; Natsukari et al.,1988).
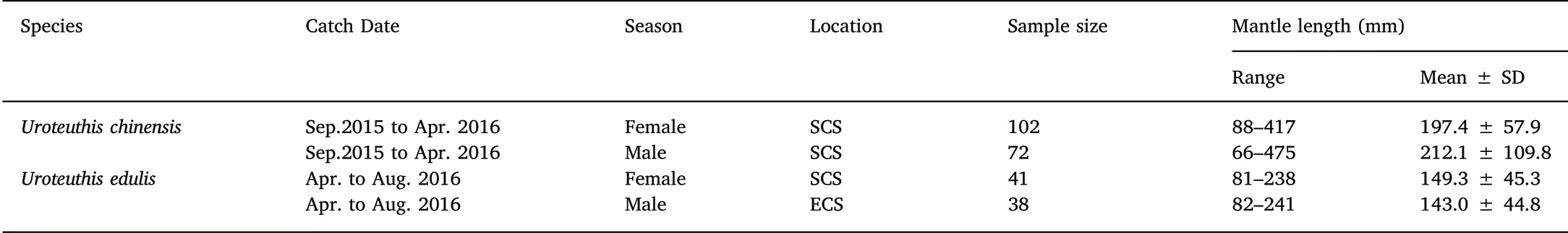
Table 1Specimens of information of U. chinensis and U. edulis from China Seas.
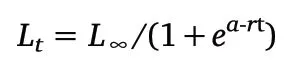
Where Ltis the ML at age t,L∞is the infinite ML,a and r are parameters.
Daily growth rate (DGR, mm/day or g/day) and instantaneous growth rate (G, %) were calculated for each 20-day class using the following equations (Forsythe & Van Heukelem, 1987).
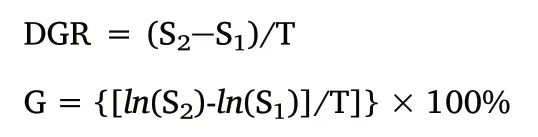
Where S1and S2are the mean ML (or BW) at the beginning and end of the time interval T (T=20 days).
All statistical analyses were conducted with R (Mangiafico, 2015)and all plots were drawn using Microsoft Excel 2013.
3. Results
3.1. Body weight and mantle length relationship
The BW and ML equations of U.chinensis and U.edulis between sexes are as follows (Fig. 2):
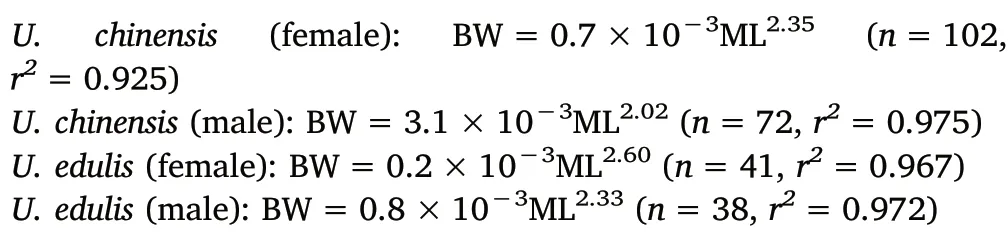
For U. chinensis, the slopes (F(1,170)=18.613, P <0.01) and intercepts (F(1,171)=27.001, P <0.01) of the log-transformed equations between sexes showed significant difference based on ANCOVA.For U.edulis,the slopes of the log-transformed equations between sexes showed significant difference based on ANVOCA (F(1,75)=7.013,P <0.01), while the intercept showed no significant difference(F(1,76)=4.323,P >0.01).Also,ANCOVA showed that both the slopes(F(1,258)=14.380, P <0.01) and intercepts (F(1,259)=24.653,P <0.01) had a significant difference between the two species.
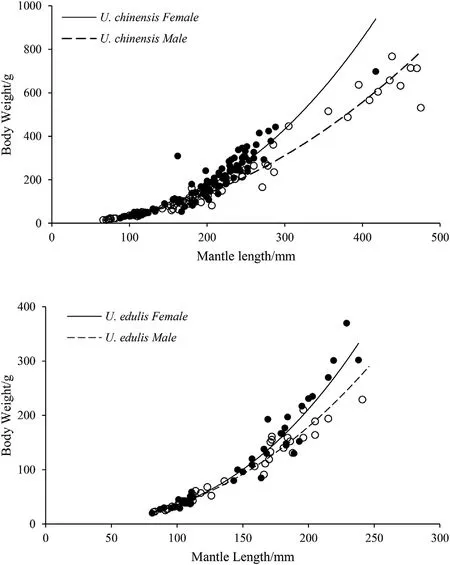
Fig. 2. Mantle length and body weight relationships of U. chinensis and U.edulis.
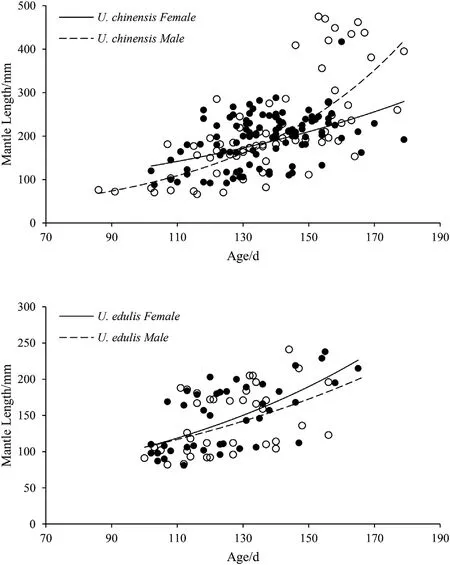
Fig. 3. Exponential growth curves of U. chinensis and U. edulis.
3.2. Age and hatching date
3.2.1. U. chinensis
The estimated age of U. chinensis ranged from 86 to 179 days [102(ML in 87 mm)to 179(ML in 395 mm)days for squid caught in autumn,86 (ML in 76 mm) to 169 (ML in 381 mm) days for squid caught in spring)]. The relationships between ML and age are as follows (Fig. 3):
U. chinensis (female): ML=47.99e0.0098Age(n=102, r2=0.202)
U. chinensis (male): ML=12.65e0.0196Age(n=72, r2=0.558)
According to ANCOVA, the slopes (F(1,170)=11.705, P <0.01) of the log-transformed equations showed significant difference between sexes, while intercepts (F(1,171)=0.004, P >0.01) showed no significant difference.For U.chinensis collected in spring(April 2016),the back calculation of the hatching date showed that hatching occurred from October 2015 to January 2016 (Fig. 4). November and December are the dominant hatching months.For U.chinensis collected in autumn(September 2015), the results showed that hatching occurred from March to June 2016 (Fig. 4). April is the dominant hatching month.
3.2.2. U. edulis
The estimated age of U. edulis ranged from 100 to 165 days [100(ML in 91 mm) to 156 (ML in 123 mm) days for squid from the ECS caught in summer,102(ML in 110 mm)to 165(ML in 215 mm)days for squid from the SCS caught in spring]. The relationships between ML and age are as follows (Fig. 3):

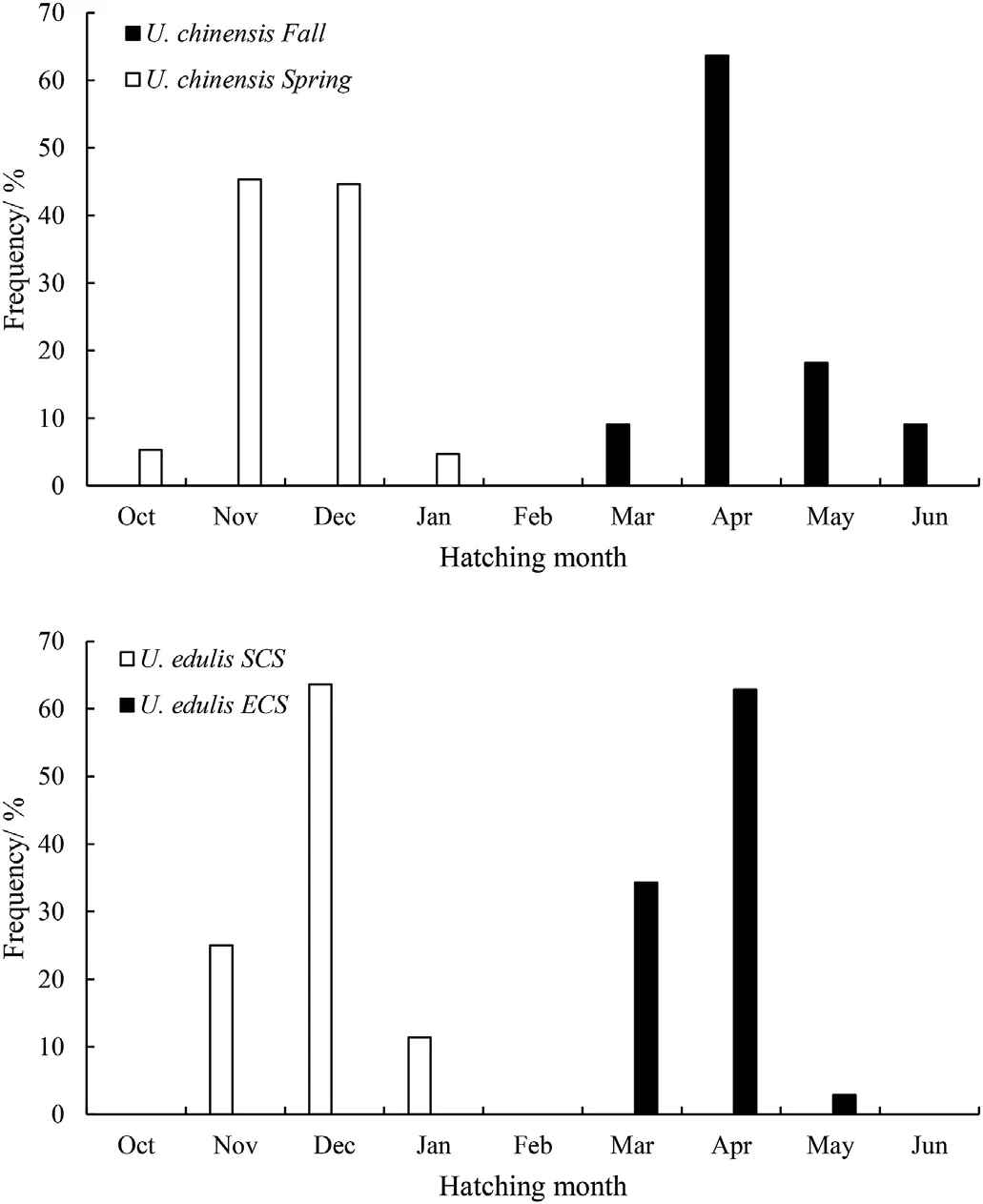
Fig. 4. Hatching date of U. chinensis and U. edulis based on back-calculation.
According to ANCOVA, the slopes (F(1,75)=0.241, P=0.625) and intercepts (F(1,76)=0.6037, P=0.440) of the log-transformed equations showed no significant difference between sexes. For U. edulis collected in spring (April 2016), the back calculation of the hatching date showed that hatching occurred from November 2015 to January 2016 (Fig. 4). December is the dominant hatching month. For U. edulis collected in summer (August 2016), the results showed that hatching occurred from March to May 2016 (Fig. 4). April is the dominant hatching month.
According to ANCOVA,the slopes(F(1,258)=2.336,P=0.128)and intercepts (F(1,259)=6.322, P >0.01) of ML-age equations between the two species showed no significant difference.
3.3. Logistic growth curve
The following logistic growth equations (Fig. 5) of U. chinensis and U. edulis between sexes were calculated by the least squares method from the averages of ML for each 5-increment class (Table 2 and Table 3).
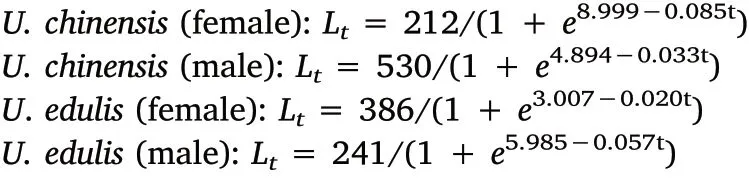
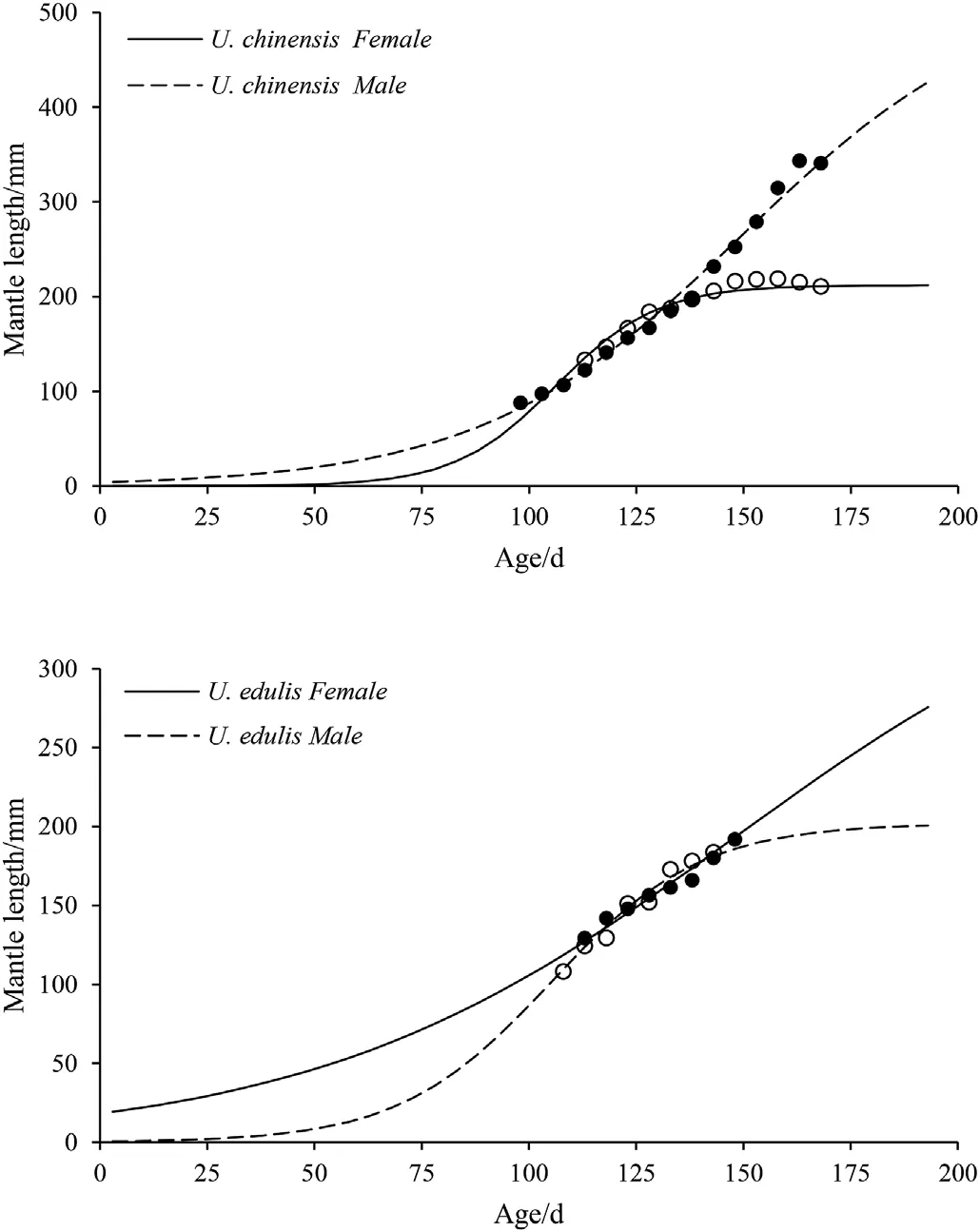
Fig. 5. Logistic growth curves of U. chinensis and U. edulis.
3.4. Growth rates
For DGR of ML, the highest growth rates were 2.46 mm/d in 121-140 d, 5.17 mm/d in 141-160 d, 2.05 mm/d in 141-160 d and 1.71 mm/d in 101-120 d for female U. chinensis, male U. chinensis, female U. edulis and male U. edulis, respectively. For DGR of BW, the highest growth rates were 3.62 g/d in 121-140 d, 9.48 g/d in 141-160 d,5.27 g/d in 141-160 d and 2.57 g/d in 141-160 d for female
U. chinensis, male U. chinensis, female U. edulis and male U. edulis, respectively. See Table 4.
For G of ML,the highest growth rates were 1.48 in 121-140 d,2.33 in 141-160 d, 1.20 in 141-160 d and 1.59 in 101-120 d for female U.chinensis, male U. chinensis, female U. edulis and male U. edulis, respectively. For G of BW, the highest growth rates were 2.68 in 121-140 d, 5.89 in 101-120 d, 3.34 in 141-160 d and 5.07 in 101-120 d for female U. chinensis, male U. chinensis, female U. edulis and male U. edulis, respectively. See Table 4.
Negative growth rates were found in ML at the 161-180 d class of female U. chinensis (Table 4).
4. Discussion
The specimens of U. chinensis were collected from the northern SCS at almost the same locations in spring and autumn. Although female and male U.chinensis have overlapping habitats and life histories and share a great proportion of the same living environment, there was significant difference in the BW-ML relationship of U.chinensis between sexes as shown in the results (Fig. 2). After U. chinensis reaches the mantle length of around 150 mm,female is fatter than male.Individuals over 400 mm were usually male. We found the same growth pattern between sexes in U.edulis(Fig.2).Similarly,Bat et al.(2009)compared the BW-ML relationship of U. chinensis between sexes, they got the similar result as in the present study. However, Wang (2009) found that male is fatter than female with subtle difference in U. edulis. The difference should be related to gonad development between female and male.According to the slope of the BW-ML equation,U.edulis appeared to be heavier than U.chinensis at the same mantle length,which means that U. edulis matures earlier than U. chinensis.
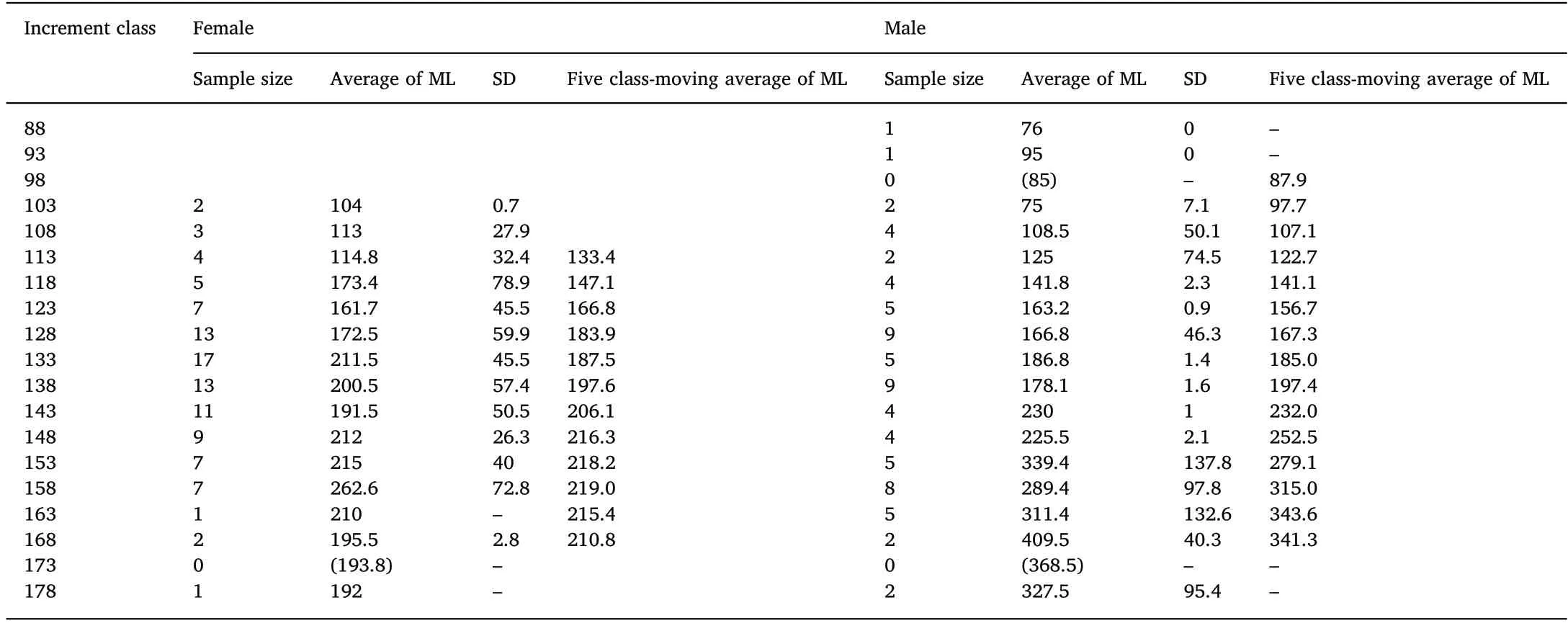
Table 2Five class-moving averages of mantle length of U. chinensis for each 5-increment class based on statolith.
Although U. edulis has a small sample size and narrow ML range,both species showed a similar age range.In the present study,they both have a lifespan of less than 200 days according to the growth increments in the lateral dome of the statolith. Bat et al. (2009) and Sukramongkol et al. (2007) found similar results for U. chinensis.However, other studies found that U. chinensis had a lifespan of approximately 1 year (Huang, 2006), and U. edulis had a lifespan of approximately 1 year (Natsukari et al.,1988) or around 270 days (Wang,2009).These might be caused by determining age using different domes of the statolith. For those studies using the lateral dome for age determination (Bat et al., 2009; Sukramongkol et al., 2007; the present study), a lifespan less than 200 days was found. However, for studies using the rostrum for age determination(Natsukari et al.,1988;Huang,2006;Wang,2009),a lifespan of much more than 200 days was found.However, Jackson (1990) validated the daily growth periodicity in the dorsal dome and the rostrum of U.chinensis,and Natsukari et al.(1988)used an indirect way to prove the daily growth periodicity of U. edulis,i.e.the tag-based maximum growth rate agreed with their study.In the paper of Jackson (1990), although he claimed that growth increments of the dorsal dome and the rostrum deposit daily (Table 2 in Jackson,1990), he only presented a figure showing growth increments in the lateral dome in his paper (Fig. 1 in Jackson, 1990). Hence, we believe that the daily periodicity of growth increments in the lateral dome have been validated. In the paper of Natsukari et al. (1988), the daily periodicity of growth increment based on their indirect method cannot be proved because of the allometric growth characteristic of squid (see below). Therefore, we inferred that the lateral dome and rostrum have different numbers of growth increment in these two species. In the future new experiments should be offered to validate the daily periodicity of growth increments in other domes to verify this inference.
In the present study, the back-calculated hatching dates of U. chinensis were similar to that of U. edulis. The results showed that U. chinensis caught in spring had hatched in late autumn to winter, U. chinensis caught in early autumn had hatched in spring,U.edulis caught in spring had hatched in winter and U. edulis caught in summer had hatched in spring. Therefore, we can reasonably infer that both species hatch continuously throughout the whole year. Continuous breeding and spawning throughout the year have been reported in several loliginid species, such as U. duvauceli (Chotiyaputta, 1990; Roongratri,1989, p. 20), U. chinensis (Chotiyaputta, 1990; Huang, 2006;Roongratri, 1989, p. 20), U. edulis (Wang, 2009), Loligo vulgaris (Sauer& Smale, 1993) and L. forbesi (Pierce, Boyle, Hastie, & Key, 1994). Bat et al. (2009) used U. chinensis caught in the Tokin Gulf from April to October to back-calculate the hatching date and found U. chinensis hatched in October to December and March to August. Similarly,Sukramongkol et al.(2007)used U.chinensis caught near Thailand from April to August and found they hatched from November to June.

Table 3Five class-moving averages of mantle length of U. edulis for each 5-increment class based on statolith.
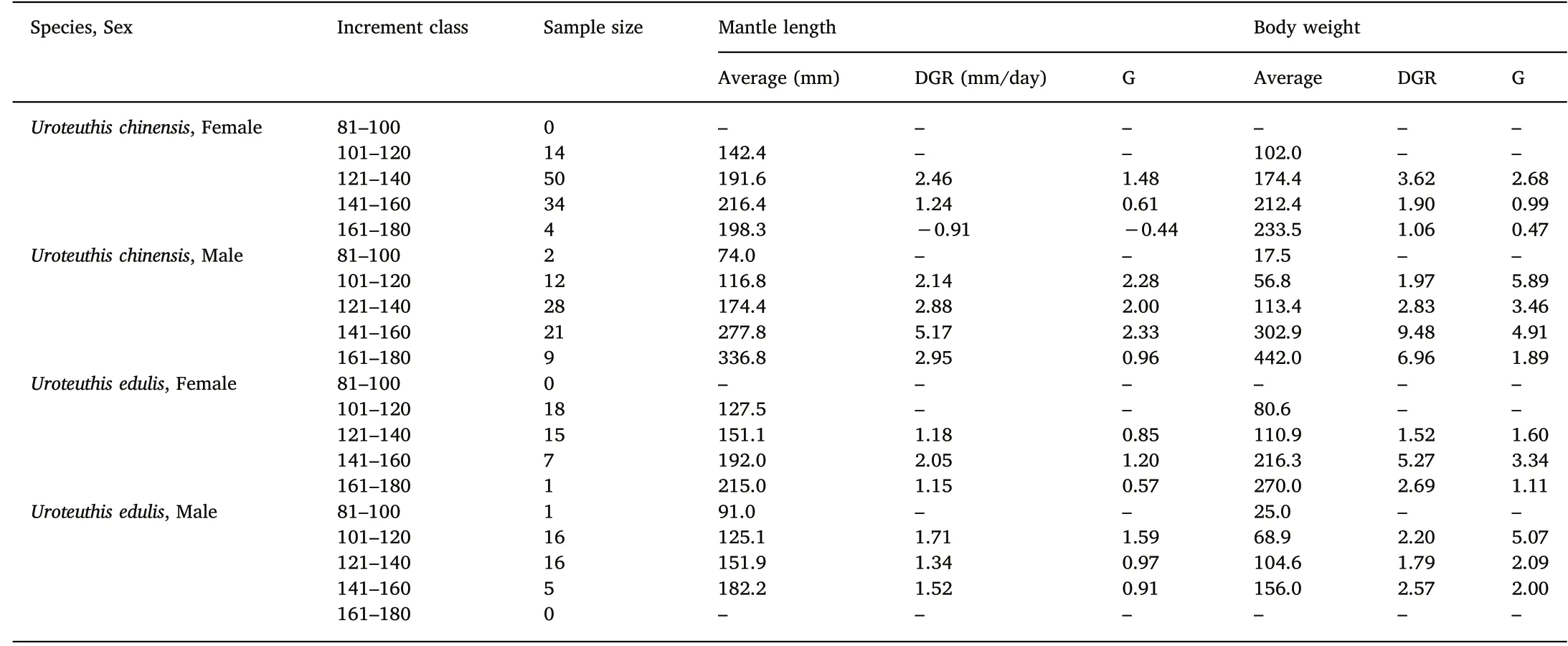
Table 4Growth rates of U. chinensis and U. edulis.
An exponential equation was used to establish the relationship between ML and age,which was fitted better in the present study than in the paper of Sukramongkol et al.(2007),while Bat et al.(2009)used a power equation to establish the relationship.The variation of ML at the same age increases with age, which means growth rates vary considerably among individuals, especially in the second half of their life history(Natsukari et al.,1988).According to ANCOVA,the age and ML of U. chinensis showed significant differences of slopes between sexes,while there were no significant difference of both slopes and intercepts between sexes in U. edulis. The growth of male U. chinensis is slower than that of female, and then surpass female at about 140 days. While the growth of male edulis is slower than that of female all the time.Both Bat et al. (2009) and Huang (2006) found that male grow faster than female all the time in U. chinensis. and Wang found the same growth pattern in U. edulis. Chen compared the ML-Age relationships of U.edulis between torch-light fishery and trawl fishery, and found that female grow faster than male in torch-light fishery while male grow faster than female in trawl fishery.Whereas,it is inconsistent with the present study since we used the trawl net for sampling.
The predicted maximum MLs from the present data were used in this study to fit the logistic growth curves. Natsukari et al. (1988)calculated the maximum MLs that were inconsistent with the actual maximum MLs, which is the same case in the present study. Generally,U. chinensis had a larger maximum ML than U. edulis in this study.Nevertheless,the reported maximum ML has the opposite tendency,i.e.U. edulis having a larger maximum ML than U. chinensis (Jereb et al.,2010). Apparently, sample size should be increased to get a more precise logistic growth curve in the future. According to the logistic curve(Fig. 5), in the present study, the ML of U. chinensis and U. edulis have different growth tendencies between sexes. For U. chinensis, male ML has a tendency to grow after ca. 200 days, while female ML becomes stable at ca. 200 mm after ca. 150 days. However, U. edulis has a contrary tendency between sexes, i.e. male becomes stable at ca. 200 mm after ca. 175 days, while female has a tendency to grow after ca. 200 days. The growth rates are various in different life stage between sexes for both U. chinensis and U. edulis. For U. chinensis, male grows faster than female before ca.100 days,female grows faster than male between ca. 100 days and ca. 130 days, and then male keeps growing while female stops growing. For U. edulis, however, male and female have opposite grow trends compared with U. chinensis, which means that female grow faster than male before ca.120 days,and male grow faster than female between ca.120 days and ca. 140 days, and then female keeps growing while male stops growing. Huang (2006) found that male U. chinensis have faster growth and larger maximum ML than females based on logistic growth curve. Natsukari et al. (1988) had similar results with U. edulis, where they also found that warm season broods had a higher ML than cold season broods. Based on the five class-moving averages of ML, data points of female U. chinensis were limited to ages of 113-168 days and ML of 133-211 mm, and those of male U. chinensis were limited to ages of 98-168 days and ML of 88-341 ML, while those of female U. edulis were limited to ages of 113-148 days and ML of 129-192 mm,and those of male U.edulis were limited to ages of 108-143 days and ML of 108-183 mm.Therefore,we had unusual growth trend for both female and male of U.edulis,which can be explained by small sampling ML range (81-238 mm for female and 82-238 mm for male). Again, sample size and ML range should be increased to get a more precise logistic growth curve in the future.
According to growth rates, both ML and BW of U. chinensis grow faster than U. edulis for DGR and G. However, some unreasonable values were found in the present study (Table 5), e.g. female U. chinensis had negative growth rates at 161-180 days age class, which might be caused by small sample size. Natsukari et al. (1988) found that growth rate varied considerably among individuals, especially at the adult life stage.In the present study,some age classes only have a few individuals(less than 10 individuals for each class). Therefore, a large sample size and ML range can be used to offset part of the individual variation effect.
In conclusion, studies that used different regions to determine the age of U. chinensis and U. edulis had significantly different results.Future studies should be conducted to standardize the ageing process.U.chinensis grow faster than U.edulis.Generally,male grows faster than female and male has maximum size for U. chinensis based on logistic growth curve which is reasonable, while U. edulis has an opposite growth trend compared with U. chinensis in the present study and U.chinensis and U. edulis in the previous studies, which is unreasonable.The unreasonable growth trends for U. edulis in the present study are caused by small sample size and ML range.The specimens from this study were assigned to a spring hatching cohort and an autumn hatching cohort for both U.chinensis and U. edulis.Sample size and ML range should be enlarged to get more accurate growth curves and growth rates in the future.
Conflicts of interest
All the authors, YJ, NL, XJC, BLL, JHL, have approved the manuscript submitted and declared they have no conflicts of interest in regard to this work.
Acknowledgments
This work was funded by the National Science Foundation of China(NSFC41306127 and NSFC41276156). We thank G B Chen of South China Sea Fisheries Research Institute of Chinese Academy of Fishery Sciences for sample collection.We thank F L Lin for helping us grind the statoliths.
 Aquaculture and Fisheries2019年4期
Aquaculture and Fisheries2019年4期
- Aquaculture and Fisheries的其它文章
- Natural versus synthetic anesthetic for transport of live fish: A review
- Methane and nitrous oxide emissions in rice-crab culture systems of northeast China
- Increased pCO2 changes the lipid production in important aquacultural feedstock algae Isochrysis galbana, but not in Tetraselmis suecica
- Effects of fermented protein feed on the growth performance of pond-raised crab
- Assessing recent gradual upsurge of marine captured Hilsa stock (Tenualosa ilisha) in Bangladesh
