Increased pCO2 changes the lipid production in important aquacultural feedstock algae Isochrysis galbana, but not in Tetraselmis suecica
Susn C. Fitzer, Julien Plncq, Cmeron J. Floyd, Fith M. Kemp, Jime L. Toney
a Institute of Aquaculture, University of Stirling, Stirling FK9 4LA, UK
b School of Geographical and Earth Sciences, University of Glasgow, Glasgow G12 8QQ, UK
Keywords:Ocean acidification Algae Lipids Aquaculture Feedstock
A B S T R A C T Increased anthropogenic CO2 emissions are leading to an increase in CO2 uptake by the world's oceans and seas,resulting in ocean acidification with a decrease in global ocean water pH by as much as 0.3-0.4 units by the year 2100. The direct effects of changing pCO2 on important microalgal feedstocks are not as well understood. Few studies have focused on lipid composition changes in specific algal species in response to ocean acidification and yet microalgae are an indispensable food source for various marine species, including juvenile shellfish.Isochrysis galbana and Tetraselmis suecica are widely used in aquaculture as feeds for mussels and other shellfish.The total lipid contents and concentrations of I. galbana and T. suecica were investigated when grown under present day(400 ppm)and ocean acidification conditions(1000 ppm)to elucidate the impact of increasing pCO2 on an important algae feedstock. Total lipids, long-chain alkenones (LCAs) and alkenoates decreased at 1000 ppm in I.galbana.I.galbana produces higher lipids than T.suecica,and is perhaps as a result more impacted by the change in carbon available for lipid production under higher pCO2. I. galbana is an important feedstock,more easily assimilated for growth in juvenile shellfish and reductions in lipid composition may prove problematic for the growth of future shellfish aquaculture.Our findings suggest that higher pCO2 impacts on algal lipid growth are species specific and warrant further study.It is therefore vital to examine the impact of high CO2 on algal lipid production, especially those commercial shellfish feed varieties to predict future impacts on commercial aquaculture.
1. Introduction
Increased anthropogenic CO2emissions are leading to an increase in CO2uptake by the world's oceans and seas, resulting in a decrease in global ocean water pH by as much as 0.3-0.4 units by the year 2100(IPCC, 2013). This decrease in ocean pH, termed ocean acidification,will have severe ramifications for marine organisms, particularly calcifying organisms such as shellfish, mussels and corals, whose calcium carbonate constituents dissolve more readily and grow more slowly in increasingly acidic waters (Berge, Bjerkeng, Pettersen, Schaanning, &Øxnevad,2006;Tunnicliffe et al.,2009).It has been suggested that the current IPCC 2100 prediction could lead to a decrease in global calcification rates between 5% and 50% (Kleypas et al., 2005). The large variation in this estimate highlights the uncertainty surrounding the impacts of a pH decrease on marine calcifiers.
Studies on the impact of increased levels of partial pressure CO2(pCO2) and ocean acidification on mussel and shellfish have been undertaken in recent years.Gazeau et al.(2007)led an early study on the mussel Mytilus edulis and concluded that there was a 25% reduction in the calcification rate when it was exposed to a pCO2 of 740 ppm,a level predicted by the IPCC in next century. Further work by Fitzer et al.(2015) found that both the newest and older calcite of Mytilus edulis grown under high pCO2(1000 ppm) for a period of 6 months were harder and more brittle and hence fractured more easily. These shells would be more susceptible to breakage from industry or predation(Fitzer et al., 2015). Thus, increasing ocean CO2has the potential to significantly reduce global mussel and shellfish production. This could have major socio-economic impacts across the globe as it is currently estimated that shellfish account for approximately 23.6% of a $119.4 billion fish industry (Fitzer, Cusack, Phoenix, & Kamenos, 2014). Research has begun to investigate methods of mitigating against these impacts. It has been suggested by Melzner et al. (2011) that increasing the food supply available to mussels, can limit the potentially devastating effects of increasing ocean acidity. Indeed when mussels were reared in an environment with a high concentration of nutrients in the water column, inner shell corrosion was only visible in the specimens subjected to the very highest CO2concentrations far exceeding the IPCC predictions (2400 ppm and 4000 ppm). Similarly, Thomsen, Casties,Pansch, Körtzinger, and Melzner (2013) argued that when food supply is abundant, Mytilus edulis can withstand and thrive in higher pCO2 conditions.These studies suggest that supplementary feeding may have positive effects on mussel shell growth when mussels are reared in acidic water conditions, alleviating the negative impacts described by Hettinger et al. (2013) and Fitzer et al. (2015).
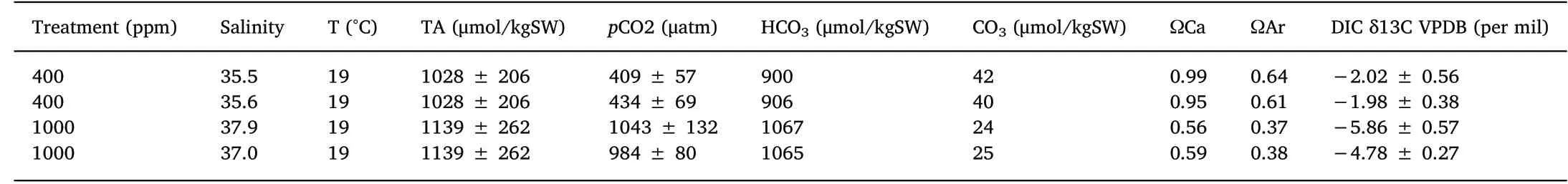
Table 1Seawater TA, salinity, temperature and pCO2 were used to calculate other seawater parameters using CO2Sys (Riebesell et al., 2010).
While these studies measured the direct effect of changing pCO2on important edible shellfish,other important impacts,for instance,on the microalgal feedstocks are not as well understood. Microalgae are an indispensable food source for various marine species,including juvenile molluscs and crustaceans (Dörner, Carbonell, Pino, & Farias, 2014),providing key unsaturated fatty acids that most higher organisms cannot produce themselves. A few studies have focused on lipid composition changes in specific algal species in response to ocean acidification(Riebesell,Revill,Holdsworth,&Volkman,2000;Rossoll et al.,2012; Tsuzuki, Ohnuma, Sato, Takaku, & Kawaguchi, 1990). For instance,the diatom Thalassiosira pseudonana has been shown to decrease the proportion of long-chain polyunsaturated fatty acids relative to saturated fatty acids in response to high pCO2(Rossoll et al., 2012).Fatty acids were also shown to be depleted in13C in the coccolithophore Emiliania huxleyi (Riebesell et al., 2000) and depleted fatty acids in cells of Chlorella vulgaris was observed in response to high pCO2(Tsuzuki et al., 1990). Similar results were found in other algae with respect to a shift to higher proportions of saturated fatty acids,but also accompanied by a shift from nano-plankton (i.e., chlorophytes and haptophytes) to favoring pico-plankton biomass (Bermúdez, Riebesell,Larsen, & Winder, 2016). Typically, aquaculture feedstocks contain a mix of algal species and sizes to satisfy both adult and juvenile molluscs(Beck & Neves, 2003; Gatenby, Parker, & Neves, 1997), hence it is important to understand changes in size classes induced by increasing pCO2.
Algae size has been linked to improved juvenile growth, however algae in the size range of 2.8-8.5 μm is recommended for best results(Beck & Neves, 2003), although perhaps more importantly higher polyunsaturated fatty acids are required for newly metamorphosed juvenile mussels (Gatenby et al., 1997). A varied diet of mixed algae is thought to improve retention time and greater assimilation efficiency in bivalves, however, there is no evidence to relate particle size to assimilation of energy in M. edulis (Shumway, Cucci, Newell, & Yentsch,1985;Wang&Fisher,1996).It is suggested that cell wall structure and biochemical composition of algae cells may influence assimilation with a lower efficiency in bivalves from green algae cells due to the difficulty in breaking down sporopollenin(Wang&Fisher,1996).Therefore there is potential for the juveniles to more easily digest a non-sporopollenin containing Isochrysis sp. chosen as a preferential food source for juveniles such as the Veligers of Mercenaria mercenaria (Shumway et al.,1985).Isochrysis galbana(~5 μm)and Tetraselmis suecica(~10 μm)are widely used in aquaculture as feeds for mussels and other calcifying organisms (Cripps, Lindeque, & Flynn, 2014; Fabregas, Herrero,Cabezas, & Abalde, 1985; Parker et al., 2012; Priyadarshani, Sahu, &Rath,2012;Zittelli,Rodolfi,Biondi,&Tredici,2006).Limited research has been done on the effects of pCO2change on the lipid content of these commercially important algae for shellfish aquaculture and the potential effect in mussel feeding. Here, the total lipid contents and concentrations of I. galbana and T. suecica were investigated when grown under present day(400 ppm)and ocean acidification conditions(1000 ppm)to elucidate the impact of increasing pCO2on an important algae feedstock.
2. Methods
2.1. Algae cultures
I. galbana and T. suecica cultures were grown from stock algae obtained from the Culture Collection of Algae and Protozoa (CCAP)(Strains 927/1 and 66/4 CCAP, respectively), Oban. Both species were cultured at two different levels of pCO2; 400 ppm representing present day conditions and 1000 ppm representing predicted levels by the year 2100 (IPCC, 2007; Doney, Fabry, Feely, & Kleypas, 2009). To create these mesocosms, CO2was bubbled into the samples from gas lines at the desired concentrations. CO2was mixed into air lines supplying all experimental cultures and gas concentrations were logged continuously using LI-COR®Li-820 CO2gas analysers at 400 and 1000 μatm pCO2(Table 1). The concentrations of pCO2were recorded daily and the temperature remained constant at 19°C throughout the experimental period, controlled via air-conditioned laboratory. Seawater samples were collected at the start and end of the experimental culture in air tight rubber-septum glass exetainers and spiked with mercuric chloride(50 μl in 12 ml seawater) (Dickson, Sabine, & Christian, 2007). Total alkalinity (TA) was determined using standard semi-automated titration (Metrohm 848 Titrino plus) of 0.1 Molar (M) hydrochloric acid(HCl) in 0.6 Molar (M) sodium chloride (NaCl) (Dickson et al., 2007),combining the spectrometric analysis using bromocresol indicator(Yao&Byrne,1998)and the absorbance read through a spectrometer(Hach DR 5000™UV-Vis) at wavelengths of ʎ444 and ʎ616. Certified seawater references materials for oceanic CO2(Batch 156, Scripps Institution of Oceanography, University of California, San Diego) were used as standards to quantify the error of analysis (Measured 2186.39 ± 4.56 μmol/kg, CRM value 2234.07 ± 0.39 μmol/kg)(Dickson et al., 2007). Dissolved inorganic carbon (DIC) was measured in triplicate for seawater sampled at the start, end of the experimental culture, and reported as δ13C VPDB (per mil) (Table 1). The DIC was analysed using an AP 2003 continuous flow automated carbonate system giving results as VPDB δ13C (Vienna Peedee Belemnite) Marine Carbonate Standard obtained from a Cretaceous marine fossil, Belemnitella Americana (Scottish Universities Environment Research Centre, SUERC). Seawater TA, salinity, temperature and pCO2were used to calculate other seawater parameters using CO2Sys (Riebesell,Fabry, Hansson, & Gattuso, 2010) (Table 1).
Six replicates were used for the different combinations of controlled variables (400 ppm and 1000 ppm pCO2, T. suecica and I. galbana species); hence there were 24 samples in total. The samples were each placed in a 250 ml conical flask containing f/2 medium, connected to air supplies using 0.2 μm filters to prevent contamination. To replicate conditions in the field,a light source was set up above the samples and was placed on a timer from 8 a.m.to 6 pm to represent daylight cycles.In order to enable continuous growth in a closed system culture the algae samples were diluted with sterilized media every week, the dilution frequency was determined from growth rate during the first week.F/2 media was prepared and autoclaved(Priorclave TACTROL®2)for sterile preparation following the CCAP f/2 recipe (https://www.ccap.ac.uk/media/documents/f2.pdf).50 ml media was used to replace 50 ml of concentrated algae,which was added to each culture per week in a sterile laminar flow cupboard(Laminar Air Flow Cabinet GELAIRE CLASS 100).
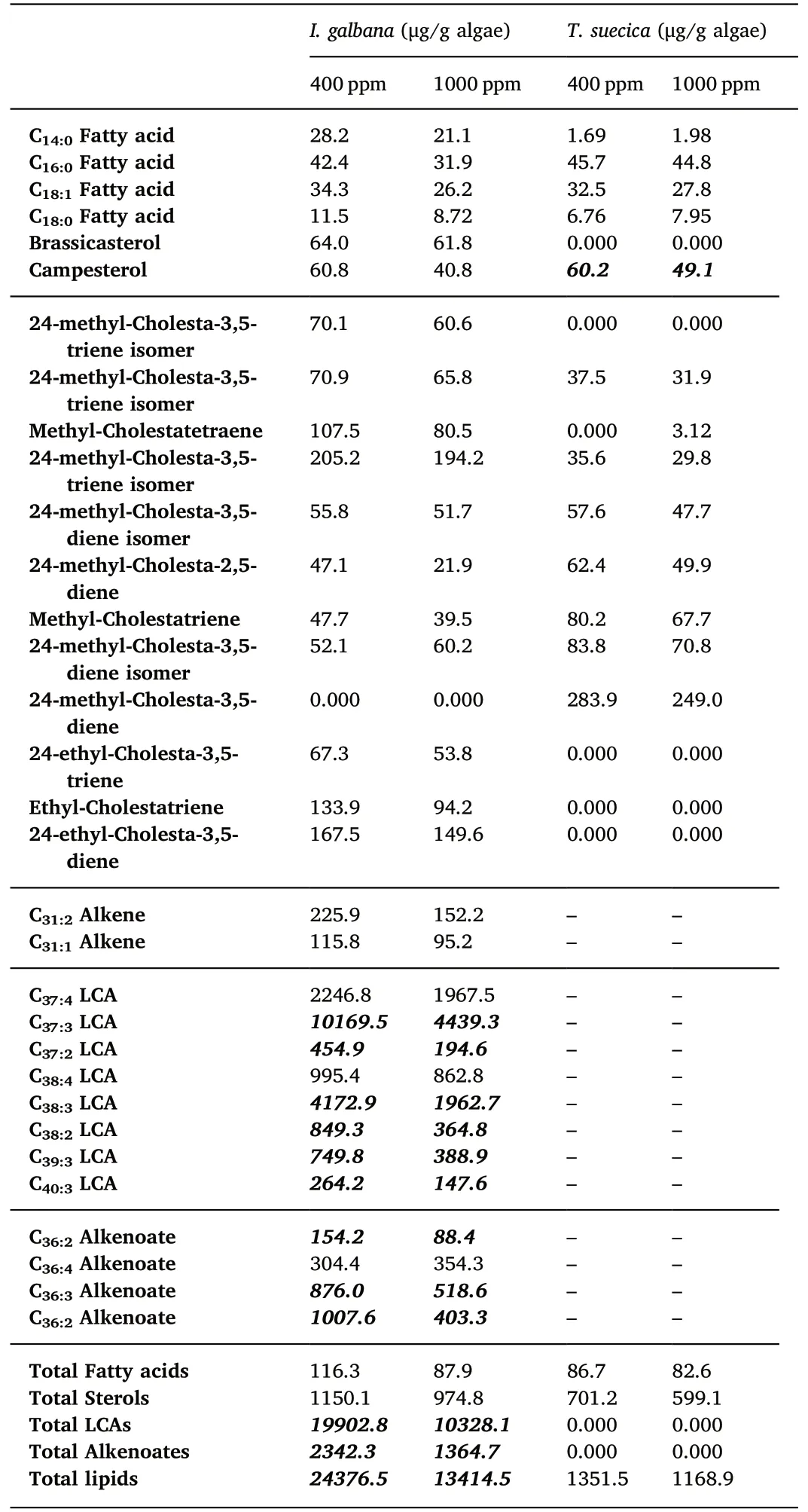
Table 2Average concentrations of the main lipids identified in Isochrysis galbana and Tetraselmis suecica for each pCO2 treatment. Values in bold and italic indicate that differences between the two pCO2 treatments are statistically significant(ttest, p <0.05).
After the 9-week period of experimental culture,microscopic counts were undertaken in order to determine the cell concentrations. A Hausser haemacytometer was used and 4 counts were done for each algae sample.The algal cells in five boxes of the haemacytometer were counted and an average taken, this was then divided by 4×10-6to obtain the concentration of cells/ml.This was repeated for the 4 counts and a mean concentration for each sample was noted.
2.2. Lipid extraction and analysis
Between 25 and 40 ml of each sample was filtered on a pre-weighed glass fibre filter paper and freeze-dried at -55°C and 0.006 Pa. The freeze-dried samples were extracted with dichloromethane (DCM):methanol (MeOH) (9:1, v:v) using a Thermoscientific Dionex ASE350 accelerated solvent extractor (ASE). Prior to analysis by gas chromatography, the total lipid extracts (TLEs) were derivatised with 30 μl of O-Bistrifluoroacetamide (BSTFA) and 40 μl of Pyridine at 80°C for 2 h.Lipids were detected and quantified using gas chromatography with a flame ionization detector (GC-FID). Analyses were performed on an Agilent 7890B Series GC system configured with a Restek RTX-1 fused silica column (60 m length, 0.25 mm i.d., 0.1 μm film thickness).Helium was used as the carrier gas at a flow pressure of 20.8 psi. The GC method used splitless injection, and the oven temperature was programmed from 60°C(hold for 2 min)to 120°C at 30°C/min,then to 330°C at 5°C/min and held for 15 min. Samples were run with the same temperature program on an Agilent 7890B Series GC coupled with a 5977A GC-EI mass spectrometer (GC-MS) to confirm the identity of the lipids using the known ion chromatograms and by comparison of mass spectral data and GC retention times with published data. Lipids(fatty acids, sterols, alkenes, long-chain alkenones, alkenoates) were quantified from the TLEs using Eicosane (n-C20alkane) and Hexatriacontane (n-C36alkane) as external standards. Calibration curves had R2values of 0.99 and 0.96 for n-C20alkane and n-C36alkane,respectively.
To improve fatty acid identification, TLEs were separated into neutral and acid fractions by elution through a LC-NH2SPE column using DCM:isopropyl alcohol (1:1, v:v), followed by ether with 4%acetic acid(v:v).Acid samples were methylated with a Boron trifluoride(BF3)- methanol solution (14% BF3in MeOH) at 70°C for 1 h. The methylated samples were then eluted through silica-gel columns with nhexane to remove impurities from the methylation reaction, followed by DCM to collect the fatty acid methyl esters(FAMEs).The hexane and DCM fractions were then analysed in GC-FID and GC-MS as described above. All the FAMEs were contained in the DCM fractions.
3. Results
3.1. Algal concentrations
Average concentrations ranged from 7.69×10-6cells/ml to 9.58×10-6cells/ml for I. galbana, and from 2.88×10-5cells/ml to 9.88×10-5cells/ml for T. suecica. No significant differences were observed between the concentration of algae cultured at 400 ppm pCO2and 1000 ppm pCO2.
3.2. Lipid composition and concentrations
The lipid profiles(from the TLEs)for each species of algae were not affected by the change of pCO2level,which means that the same lipids were observed at 400 ppm pCO2and 1000 ppm pCO2for I.galbana and T. suecica.
For I. galbana, the main lipids identified were the fatty acids C14:0(myristic acid),C16:0(palmitic acid),C18:0(stearic acid),and C18:1(oleic acid), the sterols brassicasterol and campesterol, and the 24-methyl-Cholesta-3,5-triene and 24-ethyl-Cholesta-3,5-diene. In addition, I.galbana presented C31:2and C31:1alkenes,long-chain alkenones(LCAs),and alkenoates. These latter lipids are specific to the Isochrysidale haptophyte clade to which it belongs (Marlowe et al., 1984). For T.suecica, the main lipids identified were also the fatty acids C14:0(myristic acid), C16:0(palmitic acid), C18:0(stearic acid), and C18:1(oleic acid). Only the sterol campesterol was identified, whereas the 24-methyl-Cholesta-3,5-diene prevailed.Additional fatty acids were identified in both I. galbana and T. suecica through acid/neutral separation including the C12:0, C13:0, C15:0, C17:0, C18:2, C20:0and C24:0fatty acids,however these were too low in concentration to be quantified and therefore have not been compared between treatments.
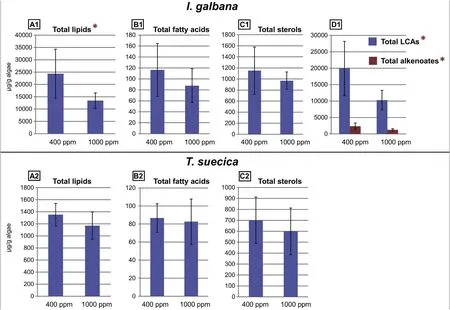
Fig.1. Variations in the content of total lipids and major lipid groups between the two pCO2 treatments for Isochrysis galbana(A1-D1)and Tetraselmis suecica(A2-C2).Error bars represent standard deviation. Differences in concentrations that are statistically significant (t-test, p <0.05) between the two pCO2 treatments are indicated by the red asterisks. Note that the scales are different for each diagram. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The concentrations of the total lipid content (determined from the TLEs) and the major lipids for each species at the different pCO2levels are presented in Table 2(averages of the six replicates for each species and each treatment).In general,I.galbana had higher lipid content than T.suecica,which is easily explained by the high concentrations of LCAs and alkenoates (Table 2).When comparing between the different pCO2levels, a decrease in concentrations is observed at 1000 ppm for each species for the total lipids, fatty acids, sterols, LCAs and alkenoates(Fig. 1). Since the concentrations showed large standard deviations(error bars in Fig. 1), a t-test was performed to evidence changes in concentrations that are statistically significant between 400 ppm and 1000 ppm.Only the concentrations of total lipids,LCAs and alkenoates for I. galbana proved to be significantly (p <0.05) lower at 1000 ppm(Fig. 1; Table 1). When specifically looking at lipids, a decrease in concentrations is also observed at 1000 ppm for each species (Fig. 2).However,for I.galbana,only most of the LCAs and alkenoates showed a significant (p <0.05) concentration decrease (Fig. 2; Table 2). For T.suecica, only the campesterol significantly decreased (Fig. 2; Table 2).
In summary,for I.galbana,a significant concentration decrease was observed at 1000 ppm for the total lipids, LCAs and alkenoates, while for T. suecica, only the campesterol significantly decreased.
3.3. Seawater chemistry
The seawater chemistry data outlines changes to the pCO2and resulting changes to the DIC and carbonate (CO32-). The carbonate and DIC is lighter in the seawater at 1000 ppm pCO2compared with the 400 ppm pCO2(Table 1).
4. Discussion
The nutritional value of algae is related to its fatty acid and lipid compositions, with the most important aspect being the proportion of polyunsaturated fatty acids (PUFAs) (Fidalgo, Cid, Torres, Sukenik, &Herrero, 1998). It has been stated in previous research that the profile and quantity of fatty acid algal lipids is crucial to the development and growth of certain marine fauna (Zhu, Lee, & Chao, 1997). While in the present study we identified fatty acids not previously reported (for example C12:0, C13:0, C15:0, C17:0, C20:0and C24:0fatty acids), other studies have reported more fatty acids and especially PUFAs (such as C18:3, C18:4, C20:4, C20:5and C22:6PUFAs) for I. galbana and T. suecica(e.g., Custódio et al., 2014; Servel, Claire, Derrien, Coiffard, & De Roeck-Holtzhauer, 1994). These discrepancies in the fatty acid composition are certainly due to the study of different algal strains coming from different Culture Collections and/or different culture conditions.Fatty acid content and distributions can be strongly affected by environmental conditions such as differing nutrient, irradiance, salinity conditions, and growth stage as shown by culture studies (Dunstan,Volkman, Barrett, & Garland, 1993; Martinez-Roldan, Perales-Vela,Canizares-Villanueva,&Torzillo,2014;Pal,Khozin-Goldberg,Cohen,&Boussiba,2011).We note that Servel et al.(1994)studied I.galbana and T. suecica strains from an aquaculture centre of Loire Atlantique(France), whereas our strains are from the Culture Collection of Oban(Scotland). However, since we studied the same algae grown under different CO2levels, we do believe that these discrepancies with previous studies in the fatty acid composition do not affect the interpretations/conclusions of the present work.
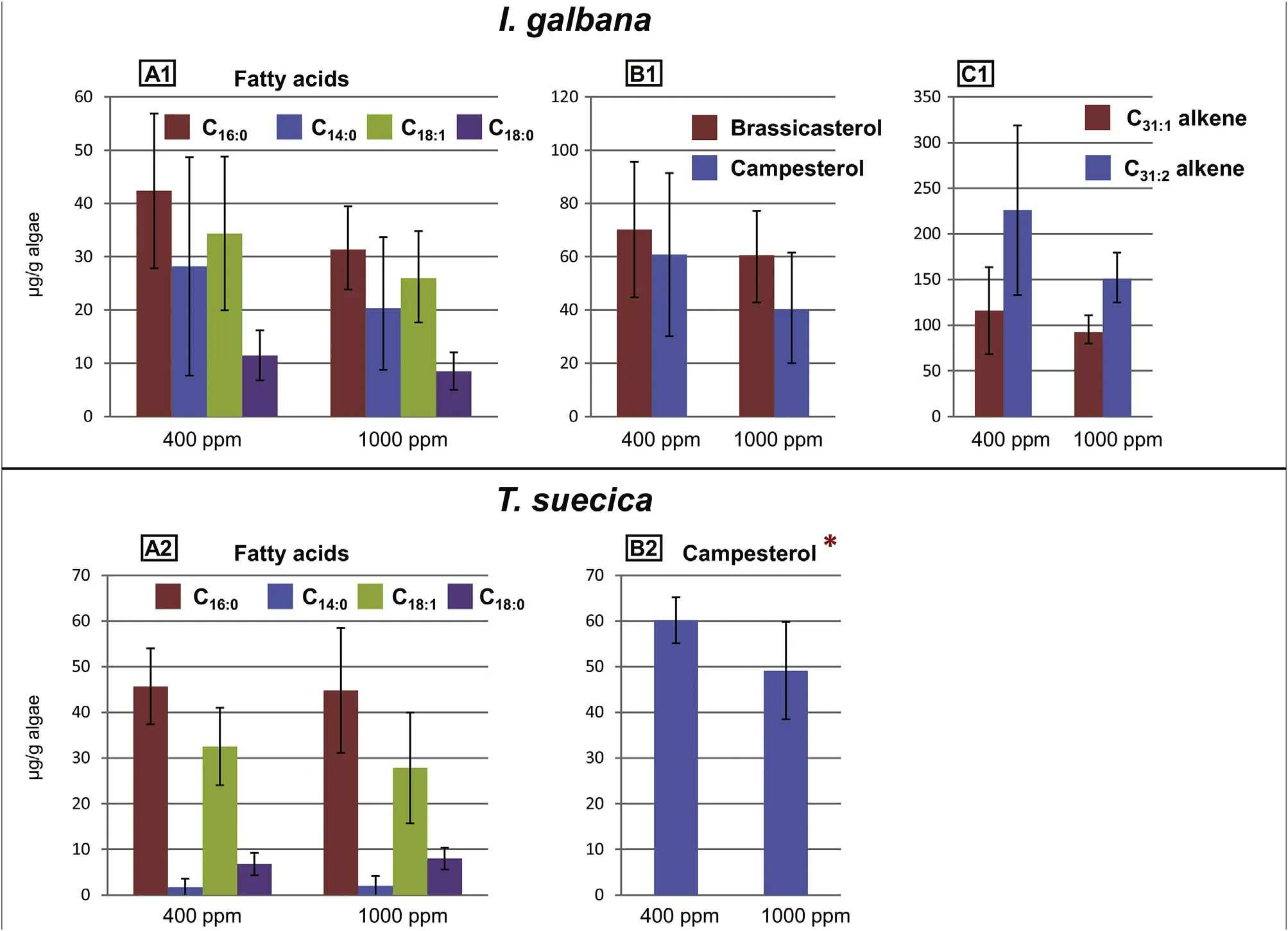
Fig.2. Variations in the content of specific lipids between the two pCO2 treatments for Isochrysis galbana(A1-C1)and Tetraselmis suecica(A2-B2).Error bars represent standard deviation. Differences in concentrations that are statistically significant (t-test, p <0.05) between the two pCO2 treatments are indicated by the red asterisks. Note that the scales are different for each diagram. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The correlation between increased lipid content and growth rate in calcifiers has been documented in numerous studies. Parrish, Wells,Yang,and Dabinett(1999)found that there was a significant increase in growth of scallop juveniles, Placopecten magellanicus, when fed I. galbana with a higher lipid content with the addition of the algae Chaetoceros muelleri compared to I. galbana with a lower lipid content along with C. muelleri. The importance of PUFAs is also reiterated as the higher lipid I. galbana alone did not affect the growth rate of the juvenile scallops (Parrish et al., 1999). Parrish et al. (1999) suggest that because C. muelleri had much more of the essential PUFA C20:4than I.galbana this supplement was needed to reach the threshold required for growth.
The fact that concentrations of the total lipids, fatty acids, sterols,LCAs and alkenoates decreased at 1000 ppm in I. galbana contradicts biofuel studies examining lipid composition under experimental rapid growth in higher CO2(Nakanishi et al., 2014; Singh & Singh, 2014;Tanadul,Vandergheynst,Beckles,Powell,&Labavitch,2014).The role of sterols and alkenones for bivalve growth are not well understood(Soudant et al., 1998). For the Scallop Pecten maximus, ingested sterols cholesterol and stigmasterol were preferentially incorporated by the larvae, potentially indicating the sterols as essential for growth(Soudant et al., 1998). Ocean acidification research into the diatom T.pseudonana has similarly observed a decrease in the proportion of longchain polyunsaturated fatty acids relative to saturated fatty acids in response to high pCO2(Rossoll et al., 2012), similarly to the depleted fatty acids in the E.huxleyi under high pCO2(Riebesell et al.,2000),and in agreement with the I. galbana lipids of this study. Examining the carbon isotopes of the seawater might explain why lower lipid production occurs when higher carbon-13 (δ13C) incorporation has been observed in monosaccharides and lipids of aquatic plants(van Dongen,Schouten, & Sinninghe Damsté, 2002), where the δ13C is lighter as a result of higher pCO2at 1000 ppm, the algae are unable to incorporate the δ13C for lipid production.When comparing the two studied species,I. galbana produces higher lipids than T. suecica, linked in part to the production of alkenones, and is perhaps as a result more impacted by the change in carbon available for lipid production under higher pCO2.Higher lipid contents (as total lipid content as percentage of dry mass;TLDM)have been observed previously in I.galbana compared T.suecica(Servel et al.,1994).The two species of microalgae were chosen for this study as commercially important species for shellfish aquaculture, and are widely used in aquaculture as feeds for mussels and other calcifying organisms(Cripps et al.,2014;Fabregas et al.,1985;Parker et al.,2012;Priyadarshani et al.,2012;Zittelli et al.,2006).I.galbana is particularly important as it is more easily assimilated for growth in bivalves, particularly juveniles such as the clam Veligers of M.mercenaria(Shumway et al., 1985). We show in this study that higher pCO2reduces the lipid production,specifically total lipids,fatty acids,LCAs and alkenoates for I. galbana, which may affect shellfish growth in aquaculture. Our findings, and the findings of others (Rossoll et al., 2012) suggest that higher pCO2impacts on algal lipid growth are species specific and warrant further study. In commercial hatchery shellfish aquaculture algae are mass produced often using high CO2to enhance growth rates.Additionally, in field shellfish aquaculture where climate change is increasing the CO2within seawater naturally available algae species may alter their lipid composition affecting shellfish growth. The findings of this study suggest that lipid production in I. galbana is significantly reduced under higher pCO2.Further studies are therefore necessary to examine the impact of high CO2on algal lipid production,especially those commercial shellfish feed varieties which are used in 6 mix algal feeds for aquaculture (e.g. Shellfish Diet 1800®a mix of six marine microalgae - Isochrysis, Pavlova, Tetraselmis, Chaetoceros calcitrans,Thalassiosira weissflogii and Thalassiosira pseudonana http://www.reedmariculture.com/product_instant_algae_shellfish_diet_1800.php).
5. Conclusions
In this study, total lipids, LCAs and alkenoates decreased at 1000 ppm in I.galbana.I.galbana produces higher lipids than T.suecica,and is perhaps as a result more impacted by the change in carbon available for lipid production under higher pCO2.I. galbana is an important feedstock, more easily assimilated for growth in juvenile shellfish and reductions in lipid composition may prove problematic for the growth of future shellfish aquaculture. Our findings suggest that higher pCO2impacts on algal lipid growth are species specific and warrant further study.It is therefore vital to examine the impact of high CO2on algal lipid production, especially those commercial shellfish feed varieties to predict future impacts on commercial aquaculture.
Acknowledgments
We thank Mohammad Ali Salik for laboratory and technical assistance. SF would like to acknowledge funding from NERC Independent Research Fellowship [NE/N01409X/1]. We thank two anonymous reviewers for their constructive comments that helped to improve the quality of the manuscript.
 Aquaculture and Fisheries2019年4期
Aquaculture and Fisheries2019年4期
- Aquaculture and Fisheries的其它文章
- Natural versus synthetic anesthetic for transport of live fish: A review
- Methane and nitrous oxide emissions in rice-crab culture systems of northeast China
- Effects of fermented protein feed on the growth performance of pond-raised crab
- Assessing recent gradual upsurge of marine captured Hilsa stock (Tenualosa ilisha) in Bangladesh
- Comparative age and growth of Uroteuthis chinensis and Uroteuthis edulis from China Seas based on statolith
