Biosorption of Ni(II)ions from aqueous solution using modif ied Aloe barbadensis Miller leaf powder
Shweta Gupta,S.K.Sharma,Arinjay Kumar*
University School of Chemical Technology,Guru Gobind Singh Indraprastha University,New Delhi 110078,India
Received 31 May 2018;accepted 11 December 2018 Available online 8 April 2019
Abstract This study aimed to investigate the biosorption potential of Na2CO3-modif ied Aloe barbadensis Miller(Aloe vera)leaf(MABL)powder for removal of Ni(II)ions from a synthetic aqueous solution.Effects of various process parameters(pH,equilibrium time,and temperature)were investigated in order to optimize the biosorptive removal.The maximum biosorption capacity of MABL was observed to be 28.986 mg/g at a temperature of 303 K,a biosorbent dose of 0.6 g,a contact time of 90 min,and a pH value of 7.Different kinetic models(the pseudo-f irst-order,pseudo-second-order,Elovich,and intraparticle diffusion models)were evaluated.The pseudo-second-order kinetic model was found to be the best f itted model in this study,with a coeff icient of determination of R2=0.974.Five different isotherm models(the Langmuir,Freundlich,Temkin,Dubinin-Radushkevich,and Brunauer-Emmett-Teller(BET)models)were investigated to identify the best-suited isotherm model for the present system.Based on the minimum chi-square value(χ2=0.027)and the maximum coeff icient of determination(R2=0.996),the Langmuir isotherm model was found to represent the system well,indicating the possibility of monolayer biosorption.The sticking probability(S*)was found to be 0.41,suggesting a physisorption mechanism for biosorption of Ni(II)on MABL.The biosorbent was characterized using Fourier-transform infrared spectroscopy(FTIR),scanning electron microscopy(SEM),zeta potential,and BET surface area,in order to understand its morphological and functional characteristics.
Keywords:Biosorption;A.barbadensis Miller leaf powder;Ni(II);Biosorption isotherm;Sticking probability;Kinetics
1.Introduction
As a result of rapid industrialization,the release of significant amounts of metals into the soil-water continuum has become one of the major global concerns.Non-biodegradable and toxic heavy metals(He and Chen,2014)can be a threat to human health and the environment if they are not removed from the wastewater before it is discharged into the environment.Nickel(Ni),one of the heavy metals,is being used as a primary component in various electroplating and mining industries.Nickel ion concentrations in electroplating industry eff luent vary from 10 to 1000 mg/L(Kadirvelu et al.,2001).The permissible limit prescribed by the U.S.Environmental Protection Agency for Ni(II)discharged with industrial eff luent is less than 1.0 mg/L(Levent et al.,2010).The Environmental Protection Act of 1986 allowed a limit of a concentration of 3 mg/L in eff luent released from electroplating industries in India(Ministry of Environment and Forests,2012).Nickel is one of the most common sources of heavy metal poisoning,and has carcinogenic,teratogenic,and mutagenic effects(Anoop Krishnan et al.,2011).Longterm exposure to nickel can cause cancer and lung f ibrosis,aswell asdermatitis(eczema)(Guo et al.,2015).Therefore,it has to be removed from industrial wastewater before the wastewater isdischarged.Several conventional processeshave been reported in the literature to remove Ni(II)from industrial eff luents,including solvent extraction(Babel and Kurniawan,2004),chemical precipitation(Ku and Jung,2001),membrane separation (Landaburu-Aguirre et al.,2009),coagulation(Lopez-Maldonado et al., 2014), electrocoagulation(Khandegar and Saroha,2014),and ion exchange(Alyu¨z and Veli,2009).Although signif icant metal removal has been observed when using the aforementioned conventional processes,the high cost,energy intensiveness,secondary pollution potential,high amount of sludge generation,and large amount of required chemicals limit the applicability of these methodsat alargescale.Recently,biosorption of heavy metals from wastewater with different types of agricultural waste has been gaining researchers’attention due to their high binding capacities,abundant availability in nature,and low cost(Singh et al.,2017a,2017b;Ngah and Hanaf iah,2008).The agricultural products most commonly used as biosorbents are peanut hulls(Aliaet al.,2016;Johnson et al.,2002),ricehusks(Sharma and Singh,2013;Verma and Rheal,1994),orange peels(Pellera et al.,2010),coconut husks(Abdulrasaq and Basiru,2010),hazelnut shells(Demirbas et al.,2008),Platanusorientalis leaf powder(Abadian et al.,2014),Neem leaves(Ibrahim and Sani,2015),and Cinnamomum camphora leaf powder(Chen et al.,2010).
Recently,due to growing interest in the medical f ield in Aloe vera as a wonder drug and cosmetic,many industries using A.vera have emerged throughout theworld,including in India.In turn,they produce considerable solid residue throughout the year from processing the A.vera leaves.
Aloe barbadensis Miller,popularly known as A.vera,is a perennial,shallow-rooted,and xerophytic plant of 30-60 cm in height.Each leaf is composed of three layers:an inner clear layer of gel that contains 99%of water with the remainder being glucomannans,amino acids,lipids,sterols,and vitamins;a middle layer of latex that contains bitter yellow sap,anthraquinones,and glycosides;and an outer layer that covers these two layers.A.vera originates from warm and dry climates in Africa.It grows mainly in the dry regions of Africa,Asia,Europe,and America.In the 16th century it reached India and inspired growers to begin commercial cultivation in many parts of the country due to its multifarious uses as a medicinal plant,as a vegetable,for pickling purposes,etc.In India,it is cultivated in Rajasthan,Andhra Pradesh,Gujarat,Maharashtra,and Tamil Nadu.
The major components of A.vera leaf waste are aloin(containing the hydroxyl group) and its derivatives(Giannakoudakis et al.,2018).Application of aloin and its derivatives has been banned by the U.S.Food and Drug Administration since 2002,so this residue is waste for these industries and can be used as an adsorbent.The waste can be used as it is or after modif ication.Recently,several studies havereported on theuseof A.vera asabiosorbent for removal of Pb(II)(Reena et al.,2015),Cu(II)(Abediet al.,2016;Singh et al.,2017a,2017b),Zn(II)(Moosa et al.,2016),Cr(III)(Abedi et al.,2016),As(Tripathi et al.,2016),Cr(VI)(Pragathiswaran et al.,2013),and Cd(II)(Gupta and Jain,2017b).The raw waste biomass can be modif ied using both acid and alkaline chemicals.Gupta and Jain(2017a)treated A.vera waste using various acid and alkaline chemicals and concluded that Na2CO3-modif ied A.vera leaf waste shows strong metal adsorption capacity.The present research work envisages the use of Na2CO3-modif ied A.barbadensis Miller leaf powder as a biosorbent to remove Ni(II)from a synthetic aqueous solution.Data were analyzed to determine best f itted kinetic and isotherm models.The thermodynamics of the system were also investigated.Characterization studies of this biosorbent were also done in terms of zeta potential analysis,scanning electron microscopy (SEM),Brunauer-Emmett-Teller(BET)analysis,and Fourier-transform infrared spectroscopy(FTIR).
2.Materials and methods
2.1.Preparation of metal solution
A standard stock solution of 1000 mg/L was prepared by dissolving the desired amount of Ni(NO3)2⋅6H2O in double distilled water. A solution of desired concentration(20-200 mg/L)was prepared by diluting the stock solution.The pH of the solution was adjusted using HCl or NaOH solution.
2.2.Preparation of biosorbent
A.barbadenesis Miller leaf residue was procured from Juneja Ayurveda and Herbal Industry,in New Delhi,India.Raw material was washed two to three times with distilled water to remove dust and other impurities.A.barbadenesis Miller leaf residue was steam-heated for 20-30 min under a pressure of 40-50 kPa.After the steam-heating,the sample was washed three times with distilled water to remove color and then dried in an oven at 80°C for 24 h.The dried material was then pulverized by a grinder and sieved to 100 mesh size(150μm).Aloe barbandensis Miller leaf powder samples weighing 10 g each were mixed with 100 mL of 0.1 mol/L aqueous solution of Na2CO3and kept in an orbital shaker working at 250 rpm at room temperature for 12 h.The treated samples were f iltered and washed with distilled water several timesuntil the pH reached aconstant value,followed by ovendrying at 80°C for 24 h.
2.3.Biosorption study
Batch biosorption experiments were conducted for removal of Ni(II)from synthetic solution.Treated biosorbent weighing 0.2-2 g was mixed with 100 mL synthetic aqueous solutions containing 20-200 mg/L of Ni(II).The solution was maintained at a pH value of 7.0 by addition of NaOH(0.1 mol/L)or HCl(0.1 mol/L).The solution was kept in an orbital shaker for a predetermined time at 150 rpm in order to provide eff icient mixing and to minimize the external mass transfer resistance.This solution was then f iltered,and a concentration of Ni(II)in the f iltrate was determined by an atomic absorption spectrophotometer(ECIL AAS 4141)at a wave length of 217 nm using the air-acetylene f lame.All chemicals used in this study were of analytical grade.The biosorption capacity and percentage removal eff iciency of Ni(II)from the solution are calculated according to Eqs.(1)and(2),respectively.

where qeis the amount of Ni(II)adsorbed on the biosorbent at equilibrium(mg/g),Ciis the initial concentration of Ni(II)(mg/L),Ceis the equilibrium concentration of Ni(II)(mg/L),M is the amount of biosorbent(g),V is the volume of Ni(II)solution(mL),and ECis the removal eff iciency of Ni(II).
2.4.Characterization of biosorbent
Zetapotential analysiswasperformed using azetapotential analyzer(Microtrac Zetatrac™,NPA 152)at room temperature to study the charged behavior at thesolid-liquid interface.The specif ic surface area,pore volume,and pore diameter of the Na2CO3-modif ied A.barbadensis Miller leaf(MABL)biosorbent were determined using a BET analyzer(Sorptomatic,1990 series,Thermo-Finnigan).Morphological changes in the biosorbent during the biosorption process were identif ied using a SEM(ZEISS EV018).Gold coating was done on non-conducting biosorbent with the aid of vacuum evaporation to obtain a uniform thickness of the specimen during analysis.FTIR analysis(Varian 600 UMA)was performed to investigate any changes in the functional groups of the biosorbent before and after the biosorption of Ni(II).Pellets were prepared by mixing 1-2 g of biosorbent powder with a suff icient amount of potassium bromide(KBr)powder.FTIR spectra of native and Ni(II)-loaded biosorbent were measured in the wavenumber range of 4000 to 500 cm-1.
3.Results and discussion
3.1.Characterization of biosorbent
the isoelectric point,at which the net surface charge becomes zero,liesat apH valueof 4.6.Therefore,thesurfaceof MABL has a negative charge above the pH value of 4.6 that favors biosorption of Ni(II)with a positive charge.
3.1.1.Zeta potential
Zeta potential was used to measure the surface charge of the biosorbent.The surface charge at the interface between a solid surface and an aqueous solution is generated by two major mechanisms:(1)acid-base reactions of surface functional groups,and(2)biosorption of ions.Zeta potential is the most important factor and it is affected by change in the solution pH.In the present study,0.2 g of MABL powder was dispersed in 5 mL of distilled water and the pH was adjusted using HCl or NaOH solution.The zeta potential wasmeasured with variation in pH valuesranging from 2 to 10.A plot of pH versus zeta potential is given in Fig.1.The f igure shows that

Fig.1.Zeta potential of MABL.
3.1.2.Surface area
The specif ic surface area of MABL,the average pore diameter,and the pore volume were analyzed using a BET surface area analyzer.The surface area and some other physico-chemical characteristics are provided in Table 1.
3.1.3.SEM
The characterization of a biosorbent through SEM offers morphological and elemental information.Fig.2 shows SEM of MABL before and after Ni(II)ion adsorption.Fig.2(a)indicates the porous and irregular surface structure of the MABL due to the presence of longitudinal tissue.Fig.2(b)shows that after Ni(II)ion adsorption the matrix layers of the biosorbent surface shrunk.This structural change is attributable to the strong cross-linking of Ni(II)ions.
3.1.4.FTIR
FTIR spectra show the change in the functional groups and surfacepropertiesof thebiosorbentafter biosorption.Thespectra of native and Ni(II)-loaded biosorbents were measured in the wavenumberrangeof 4000to500cm-1.Fig.3presentsthe FTIR spectra before and after Ni(II)adsorption.The intensity of the peaksfor Ni(II)-loaded biosorbentwaseither increased or shifted slightly.Thebiosorption peak wasshifted fromwavenumbersof 3550.32-3600.62cm-1,exhibitingthestretchof the O-Hgroup because of the complex vibration modes due to the mixed stretching vibration bands of the-OH group in hydrogenbonding and chemisorbed water(Lim et al.,2014).Another peak shown at 2879.71 cm-1was shifted slightly to 2912.50cm-1,indicatingthe C-Hstretchthatincludesaromaticcarboxyl group of conjugated carbonyl,i.e.,mainly ketonesand esters(Ding et al.,2012).The peaks appear in the range of 1598.98-1662.63 cm-1,which is attributed to C=O and C-O-Cstretching of acetyl groupspresentin thesample(Kiran and Rao,2014).It can be concluded that the main group of modif ication is(O-H,C=O,C-N)(Shouman and Khedr,2015).Several peaksdue to hydroxyl and carboxyl groupsafter biosorption of Ni(II)were also noted.These phenomena highlighted thefact that carboxyl,hydroxyl,and amidegroupswere all engaged in thebiosorption of thismetal.

Table 1Physico-chemical characteristics of biosorbent.

Fig.2.SEM of native and Ni(II)-loaded MABL biosorbents.

Fig.3.FTIR spectra of native and Ni(II)-loaded MABL biosorbents.
3.2.Effect of process parameters on Ni(II)biosorption
3.2.1.Effect of contact time
The effect of contact time on the extent of biosorption of Ni(II)is shown in Fig. 4. It is observed that the removal efficiency ofNi(II) from the synthetic solution increased from 38.6%to 79.2%when the contact time increased from 20 to 180 min, due to theNi(II) ion reaching sites available in the interior of the biosorbentwith time. However, no significant change in removal efficiencywas observed beyond 90 min due to the fact that the biosorptionprocess slowed downlater because of exhaustion of the remainingsurface sites and repulsive forces between solute molecules and bulk phase (Nasrullah et al., 2015). Therefore, a period of 90 min was considered the optimum contact time for Ni(II) removal.Results were found to be in agreement with Aloma et al. (2012).Therefore, the period of 90 min was seen as sufficient for biosorptionto attain equilibrium and was used in further experiments.
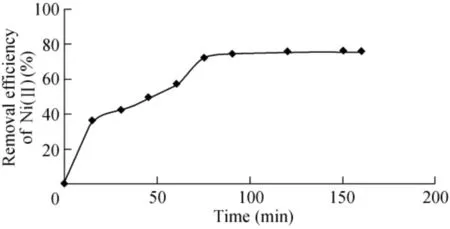
Fig.4.Effect of contact time on Ni(II)removal eff iciency(initial Ni(II)concentration is 100 mg/L,agitation speed is 150 rpm,MABL dose is 0.6 g,pH value is 7,and temperature is 303 K).

3.2.2.Effect of pHThe solution pH has a strong inf luence on the biosorption of metal ions by affecting surface charge density of the biosorbent and charge of the metallic species.In this study,the extent of Ni(II)biosorption was investigated in the pH range of 1-10 with a constant amount of MABL powder(0.6 g)in 100 mL of Ni(II)solution.It can be observed from Fig.5 that removal eff iciency increased from 30.2%to
Fig.5.Effect of pH value on Ni(II)removal eff iciency(initial Ni(II)concentration is 100 mg/L,MABL dose is 0.6 g,agitation speed is 150 rpm,temperature is 303 K,and contact time is 90 min).90.9%with an increase in solution pH.A similar trend was observed for the biosorption capacity of MABL that increased from 5.0 to 15.1 mg/g,as shown in Fig.5.The observed biosorption capacity could be related to electrostatic interaction between the biosorbent and metal cations(Ponnusami et al.,2008).At a lower pH value,the positive surface charge of biosorbent restricts the approach of metal cations due to the competitive effects of protons with metal ions.Moving towards a higher solution pH (above 5),removal eff iciency increased due to interaction between positively charged metal ions and the MABL biosorbent.These observations can be explained on the basis of the isoelectric point.It can be inferred from Fig.1 that the surface charge approaches zero at a pH value of 4.6,which suggests that the MABL surface charge becomes negative when the pH value is larger than 4.6.It is also suggested in the literature that biosorption of cations is favored at pH values larger than the isoelectric point while biosorption of anions is considered favorable at pH values less than the isoelectric point(Srivastavaand Hasan,2011).Thus,a strong negative surface charge with pH values larger than the isoelectric point intensif ied the possible biosorption of positivecharge Ni(II)ions on the negatively charged biosorbent in the proposed biosorption study.Beyond a pH value of 7,the increase in removal eff iciency occurs due to other possible mechanisms such as precipitation and the ion exchange process.It has been illustrated in the literature that during the ion exchange process,H3O+leaves the biosorbent surface at a higher pH value and thus the number of available sites for biosorption of Ni(II)ions increases(Wang et al.,2010).Higher removal eff iciency of Ni(II)ions at alkaline pH can also be explained on the basis of the formation of nickel hydroxide precipitate at alkaline pH(Kandah and Meunier,2007).Further experiments were performed at a pH value of 7 to exclude the effect of removal mechanisms other than adsorption,e.g.,precipitation.Nearly 75.3%biosorption of Ni(II)ions and a biosorption capacity of 15.1 mg/g were observed at an optimum pH value of 7.

Fig.6.Effect of temperature on Ni(II)removal eff iciency(initial Ni(II)concentration is 100 mg/L,MABL dose is 0.6 g,agitation speed is 150 rpm,pH value is 7,and contact time is 90 min).
3.2.3.Effect of temperature
Ni(II)biosorption on MABL powder is also affected by temperature.The effect of temperature on the metal biosorption was investigated at f ive different temperatures:293,303,313,323,and 333 K.As shown in Fig.6,the maximum equilibrium uptake of 78.9%for Ni(II)was found at 293 K.A similar trend for biosorption capacity was observed,and the biosorption capacity was found to be 13.15 mg/g at 293 K.At higher temperatures,removal percentage and biosorption capacity decreased due to the weakening of biosorptive forces between the active sites of the biosorbent and adsorbate and also between the adjacent molecules of the adsorbed phase(Karami,2013).Results indicated the exothermic nature of the biosorption process.The decrease in biosorption capacity with the rise in temperature may also be enhanced by the increasing tendency of metal ion desorption from the interface to the aqueous solution(Sarıet al.,2007).
3.3.Equilibrium studies
An isotherm relatestheequilibrium metal ion concentration at the solid surface to that in the solution.In this study,the Langmuir(1918),Freundlich(1926),BET(Brunauer et al.,1938),Dubinin-Radushkevich(D-R)(Dubinin,1960),and Temkin(Temkin and Pyzhev,1940)isotherms were f itted to the experimental data.These models are tabulated in Table 2.The best f it of the appropriate model to the experimental data waschecked with the coeff icient of determination(R2),aswell as the sum of the square of the errors(SSE),the sum of the absolute errors(SAE),and the chi-square(χ2),shown as follows:
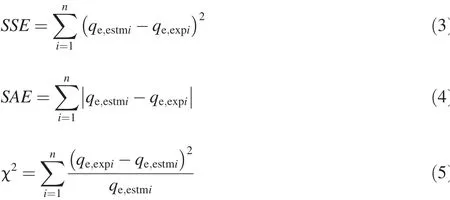
where qe,estmiis the i th estimated equilibrium biosorption capacity(mg/g),qe,expiis the i th experimental value of the equilibrium biosorption capacity(mg/g),and n is the number of data points.
Valuesof isotherm constantsobtained from Fig.7 aregiven in Table 2 based on the values of R2,χ2,SSE,and SAE.It can be seen in Table 2 that the Langmuir isotherm model was found to be the best f it for this system.The Langmuir maximum biosorption capacity(qm)was 28.986 mg/g,and the equilibrium constant(KL)was 0.0246 L/mg.RLwas the dimensionless constant separation factor of Langmuir isotherm,def ined by the following equation:εis the Polanyi potential,andε=RT ln(1+1/Ce),with R being the ideal gas constant(8.314(J/(mol⋅K))and T being the temperature(K);Csis the adsorbate monolayer saturation concentration(mg/L);and CBis the BET biosorption isotherm relating to the energy of surface interaction(L/mg).

Table 2Biosorption isotherm constants for Ni(II)onto MABL.

Fig.7.Langmuir isotherm for biosorption of Ni(II)(initial Ni(II)concentration is from 20 to 200 mg/L,MABL dose is 0.6 g,pH value is 7,and temperature is 303 K).

RLprovides important information about the nature of biosorption.The value of RLindicates the type of Langmuir isotherm that is irreversible(RL=0),favorable(0<RL<1),linear(RL=1),or unfavorable(RL>1).All the RLvalues were found to be less than one and greater than zero,indicating favorable biosorption of Ni(II)onto MABL in the concentration range studied.
3.4.Biosorption kinetics
Kinetic studies play an important role in the design of biosorption systems and lead to understanding of the adsorption rate control mechanism of the solute uptake at the solid-liquid interface.Kinetic behavior of Ni(II)biosorption on MABL was analyzed using kinetic models,namely,the pseudo-f irst-order model(Lagergren and Svenska,1898),pseudo-second-order model(Ho and McKay,1999),and Elovich model(Aharoni and Ungarish,1976),as shown in Table 3.
The linearized plots of lg qeversus t of the pseudo-f irstorder kinetic model for the biosorbent were found to have good coeff icients of determination(greater than 0.9),indicating the applicability of a pseudo-f irst-order model in the present study.Thepseudo-f irst-order rateconstant(K1)and the qevalues for the biosorbent were determined from the slope and the corresponding intercept of linearized plots.
The plot of t/qtversus t for the biosorbent are shown in the pseudo-second-order form.The qevalues and K2for the biosorbent were determined from the slope and intercept of the corresponding plot and are compiled in Table 3.R2for the pseudo-second-order adsorption model had a high value,as shown in Fig.8.The results follow the Elovich equation.Conformation to this equation alone might be taken as evidence that the rate-determining step is diffusive in nature and this equation should be applied under conditions in which the desorption rate can be ignored(Shukla et al.,2005).αandβ parameters of the Elovich equation can be calculated from the slope and intercept of the linear plot of qtversus ln t.The kinetic curve of sorption demonstrated a good f it with the model(R2=0.912),which may indicate that the diffusion is rate-limiting in biosorption of Ni(II)onto MABL.

Table 3Kinetic parameters for biosorption of Ni(II)onto MABL.
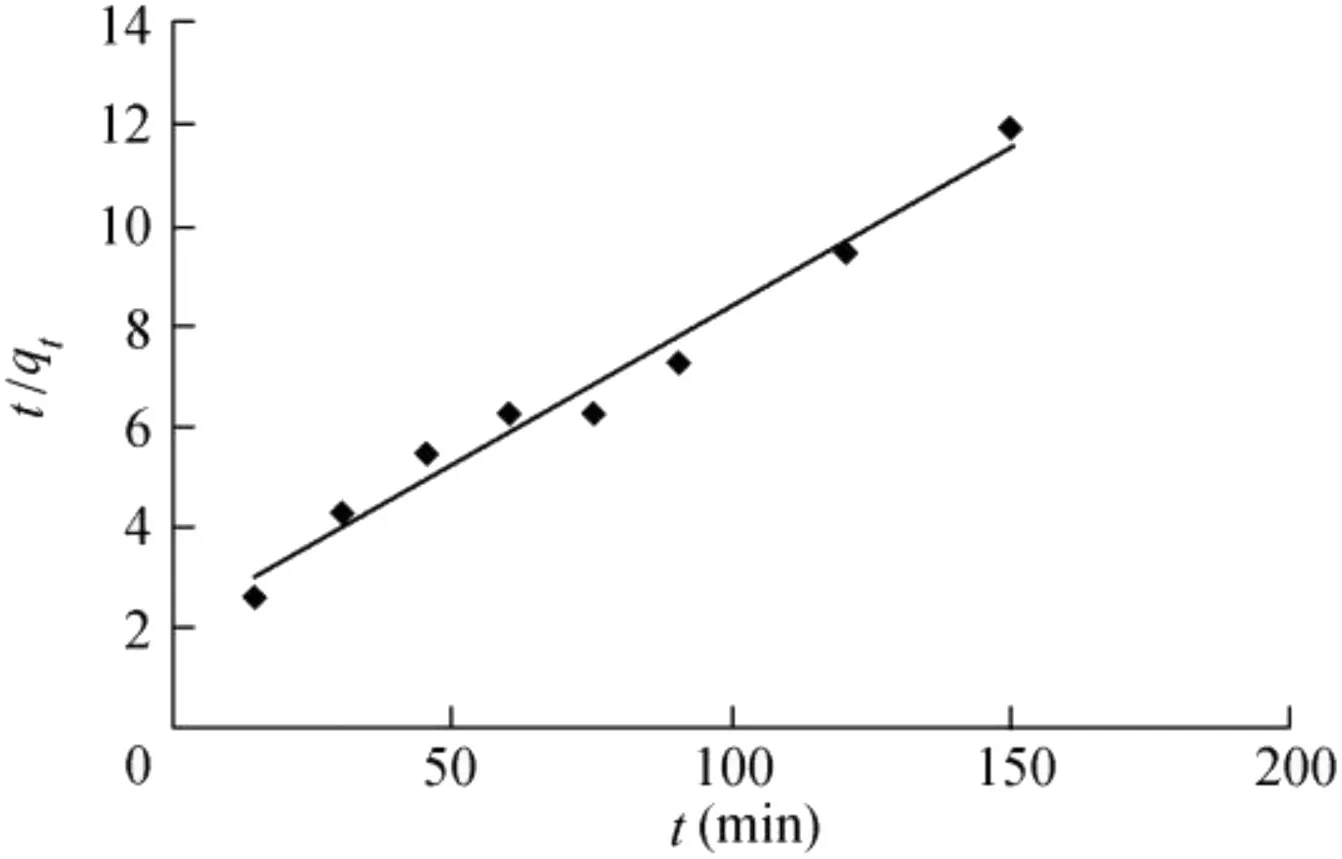
Fig.8.Pseudo-second-order kinetic model for biosorption of Ni(II).
3.5.Intraparticle diffusion model
The intraparticle diffusion model is commonly used to analyze the nature of the rate-determining step,which is represented by the following equation(Weber and Morris,1963):

where Kipistheintraparticlediffusionrateconstant(mg⋅min/g),and I is the intercept of the plot ref lecting the boundary layer effect.
According to this model,if biosorption of a solute is controlled by the intraparticle diffusion process,a plot of qtversus t1/2gives a straight line(Fig.9).The Kipvalue from Fig.9 is 1.0845 and the I value is 0.47.It is evident from the plot that there are two separate stages:the f irst linear portion(Stage 1)and the second path followed by a plateau(Stage 2).In Stage 1,this is attributed to the immediate utilization of the most readily available adsorbing sites on the biosorbent surfaces.In Stage 2,very slow diffusion of adsorbate from the surface site into theinner poreswasobserved.Thus,the initial portion of nickel biosorption by the MABL biosorbent may be governed by the initial intraparticle transport of nickel controlled by the surface diffusion process,and thelater part is controlled by pore diffusion(Mohanty et al.,2005).

Fig.9.Intraparticle diffusion model(MABL dose is 0.6 g,agitation speed is 150 rpm,pH value is 7,and temperature is 303 K).
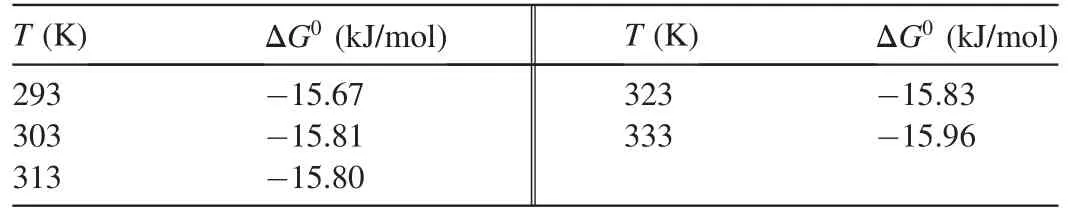
Table 4Gibbs free energy values for biosorption of Ni(II)onto MABL.
3.6.Biosorption thermodynamics
Adsorption of Ni(II)onto MABL was investigated as a function of temperature.The batch experiments were performed by varying the temperature from 293 to 333 K with a f ixed initial Ni(II)concentration of 100 mg/L at a pH value of 7 and an MABL dose of 0.6 g.The equilibrium metal ion biosorption capacity of the MABL is greater at a lower temperature.The change in thermodynamic parameters such as the Gibbs free energy(ΔG0),enthalpy(ΔH0),and entropy(ΔS0)with biosorption are determined using the following equations:

where Kdis the distribution factor of biosorbent(dimensionless).
The values ofΔG0at different temperatures are shown in Table 4.
The values ofΔH0andΔS0were calculated from the slope and intercept of the Van't Hoff plot.The Van't Hoff plot of ln Kdversus 1/T(Fig.10)gives a straight line with an acceptable coeff icient of determination.The values ofΔH0andΔS0are-0.1624 and 0.04592 kJ/mol,respectively.
The values of activation energy(Ea)and sticking probability(S*)were calculated from the experimental data.They were calculated using a modif ied Arrhenius-type equation related to surface coverage(θ)as follows(Das et al.,2013):
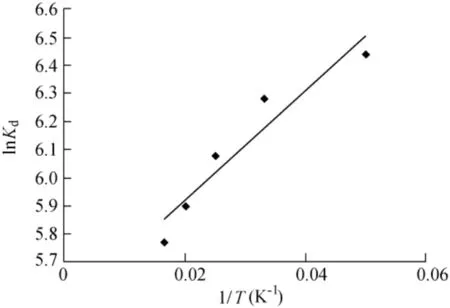
Fig.10.Van't Hoff plot of ln K d versus 1/T.
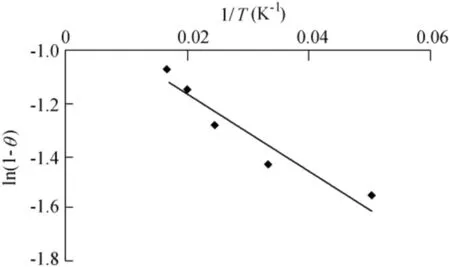
Fig.11.Graph of ln(1-θ)versus 1/T.
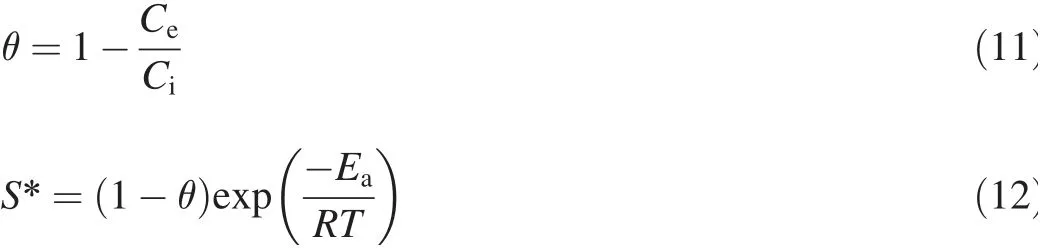
S*is a function of the adsorbate/biosorbent system under consideration but must satisfy the condition 0<S*<1 and is dependent on the temperature of the system.The values of Eaand S*can be calculated from the slope and intercept of the plot of ln(1-θ)versus 1/T,respectively(Fig.11).They were determined to be-0.118 kJ/mol and 0.41 J⋅K/mol,respectively,for the biosorption of Ni(II)onto biosorbent.Negative Eavalues indicate that a lower temperature is favorable.It is clear from Table 4 that the reaction is spontaneous,since the values ofΔG0are negative.A high negative value ref lects more energetically favorable biosorption(Duffus,2002).A negative value ofΔH0=-0.1624 conf irms that the biosorption isexothermic in nature.The positivevalue of entropy(0.04592 kJ/mol)conf irms that the degree of randomness is increased at the solid-liquid interface during biosorption.The values of Eawere determined to be-0.118 kJ/mol.
The sticking probability of the Ni(II)ions on the surface of the biosorbent is S*≪1.This value conf irms that the biosorption ref lectstheaff inity of theprocessto physisorption and a measurement of S*of less than 1 indicates that the treated biosorbent used in this study was excellent(Horsfall Jnr and Spiff,2005).
3.7.Comparison of adsorption capacity values
The comparison of the adsorption capacity of MABL for nickel ions with previously reported biosorbent studies is presented in Table 5.The adsorption capacity of MABL for nickel ionswasfound to becomparableand moderately higher than thoseof several corresponding biomass-based biosorbents reported in theliterature.Accordingly,it can beshown that the MABL biosorbent has considerable potential for the removal of nickel ions from aqueous solutions.
3.8.Application foreground and recycle strategy
The outcome of this study demonstrates the adsorption potential of MABL for the removal of Ni(II)from wastewater.Thissuggests the applicability of MABL for eff luent treatment units of electroplating,Ni-Cd battery industries,etc.The preliminary studies in the laboratory indicate that adsorbent can beregenerated using 0.1 mol/L of HCl.Alternatively,with Ni(II)being amicronutrient,thesludgeof theabsorber may be added to the soil as fertilizer(Hansch and Mendel,2009).
4.Conclusions
Na2CO3was the best pre-treatment of MABL.Optimum conditionsfor the MABL biosorption processwere found to be a pH value of 7 and an MABL dose of 0.6 g,with an equilibrium time of 90 min at an initial Ni(II)concentration.The Langmuir isotherm was the best-f it model for the equilibrium experiment data.For the separation factor,RLvalues are between 0 and 1,indicating the favorable biosorption of Ni(II)ions onto the MABL.Pseudo-second-order kinetics f itted well to experimental data,with an R2value of 0.974.Thermodynamic parameters indicate that the process was spontaneous,feasible,and exothermic.Sticking probability indicates that the MABL was an excellent biosorbent for Ni(II)ions.The FTIR showed that the main groups involved in the binding process were carboxyl,hydroxyl,and amide groups.Thus,MABL is a technically feasible biosorbent with an ecological advantage(being eco-friendly in nature).The MABL biosorbent can be used for the removal of Ni(II)ions from industrial wastewater.
With growing awareness of the medicinal and cosmetic uses of A.vera-based products,there has been a spurt of farm and manufacturing activities related to this plant.As a result,a lot of solid waste is being generated throughout the year.As entailed in this research,it has been established that A.vera leaf residue powder can be an extremely ecofriendly alternative to traditional adsorbents based on,for example,activated carbon,in removing heavy metals from industrial waste,thus protecting the environment in more than one way.

Table 5Adsorption capacity of different biosorbents for nickel ions.
 Water Science and Engineering2019年1期
Water Science and Engineering2019年1期
- Water Science and Engineering的其它文章
- Application of power law to vertical distribution of longshore currents
- Computational investigation of hydraulic performance variation with geometry in gabion stepped spillways
- Treatment of hydroxyquinone-containing wastewater using precipitation method with barium salt
- Modeling river water quality parameters using modif ied adaptive neuro fuzzy inference system
- Evaluation of copper removal eff iciency using water treatment sludge
- Modeling water resources under competing demands for sustainable development:A case study of Kaligandaki Gorge Hydropower Projectin Nepal
