Impact of conditioning regimen on peripheral blood hematopoietic cell transplant
Michael Burns, Anurag K Singh, Carrie C Hoefer, Yali Zhang, Paul K Wallace, George L Chen, Alexis Platek,Timothy B Winslow, Austin J Iovoli, Christopher Choi, Maureen Ross, Philip L McCarthy, Theresa Hahn
Abstract
Key words: Total body radiation; Peripheral blood hematopoietic cell transplant; Total nucleated dose; Neutrophil engraftment; Graft-versus-host-disease
INTRODUCTION
Peripheral blood hematopoietic cell transplant (PBHCT) is the most commonly used allogeneic hematopoietic cell source due to its faster rate of neutrophil engraftment[1-4].The optimal CD34+ cell dose range to minimize time to neutrophil and platelet recovery without increasing risk of acute graft-versus-host disease (GvHD) is 4-10 ×106/kg[5-11]. Some studies have reported a higher CD34+ cell dose yields improved overall survival (OS)[9,12-14], while others have found no significant association[7,10,15-18].
A higher total nucleated cell (TNC) dose has been reported to improve survival after PBHCT[14,16], but analyses of specific T-cell subsets (CD4+, CD8+, natural killer cells) have been inconsistent[17-19]. Factors such as T-cell depletion, conditioning regimen intensity, use of total body irradiation (TBI), and donor age may be interacting with graft cell doses to generate different effects on PBHCT outcomes. In addition, flow cytometric enumeration of cell doses are not standardized (except for CD34+ cell dose) and may also lead to differences in results between studies.
In our retrospective study, we explored whether the collected and infused CD34+,CD3+, CD4+, CD8+, or TNC dose influenced engraftment, OS, progression free survival (PFS), and incidence of acute and chronic GvHD, and whether the results were affected by conditioning regimen intensity or use of TBI.
MATERIALS AND METHODS
Study design
This retrospective cohort study included 247 consecutive adult (≥ 18 years old)patients receiving their first allogeneic PBHCT between January 2001 and September 2012. Patients receiving syngeneic, human leukocyte antigen (HLA)-mismatched, Tcell depleted, or bone marrow transplants were excluded from this analysis. This study was reviewed and approved by the Institutional Review Board of Roswell Park Cancer Institute.
Conditioning regimens
Four conditioning regimen groups were defined a priori as (1) myeloablative (MA)without TBI (MA-noTBI), (2) myeloablative with TBI (MA + TBI), (3) reduced intensity conditioning (RIC) without TBI (RIC-noTBI) and (4) RIC with TBI (RIC +TBI). These are described in Table 1. Conditioning regimens were assigned based on institutional standards including: (1) patients aged ≥ 60 years: received RIC regimens,(2) patients aged 41-59 years: a RIC regimen was preferred for patients with any of the following criteria: HLA mismatch, Karnofsky Performance Score (KPS) < 70, extensive co-morbidities, recent smoking history, (3) patients aged 19-40 years: a myeloablative regimen was preferred unless the patient had an HLA mismatched donor, KPS < 70,severe co-morbidity, and (4) patients aged ≤ 40 years with acute lymphoid leukemia:TBI regimen.
PBHC mobilization and collection
Donor marrow was stimulated with 10 mg/kg of granulocyte-colony stimulating factor for a minimum of 2 d and continued until white blood cell count was > 8000 ×109/L; the attending bone marrow transplant physician provided a target CD34+ cell dose to be collected and, for related donors, approved the final dose collected and the end of apheresis. Most donors underwent apheresis for 1 d.
Cell dose definitions
Apheresis product cell doses were determined using multi-parameter flow cytometry.CD34+ cell counts were obtained using the ISHAGE protocol[20], substituting 7-aminoactinomycin D with TO-PRO. CD3+, CD4+, and CD8+ cell counts used standard methodology[21]. TNC doses were determined by multiplying the white blood cell count (× 108/mL) on the day of apheresis by the volume of the product.Each cell count in the final infused product was divided by the actual recipient weight in kilograms measured within 2 d of the start of conditioning regimen to calculate the cell dose infused.
CD34+ cell dose was analyzed using previously published categories of < 4, 4-8, > 8× 106/kg. CD3+, CD4+, and CD8+ cell doses were analyzed above and below the respective median cell doses in the study population. TNC dose was analyzed as above and below the median cell doses and also with various doses ranging from 7-10× 108cells/kg to determine an optimal TNC dose threshold.
Post-transplant outcome definitions
Neutrophil engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count > 0.5 × 109/L. Platelet engraftment was defined as the first date with a platelet count > 20 × 109/L after 7 consecutive days with no platelet transfusions. PFS was calculated as the time from PBHC infusion to date of first disease progression post-PBHCT or date of death from any cause; survivors without disease progression were censored at date of last follow-up. OS was calculated as the time from PBHC infusion to date of death from any cause with survivors censored at date of last follow-up. Acute and chronic GvHD were graded using standard definitions[22-23].
Statistical analysis
The statistical methods of this study were reviewed by Yali Zhang from Roswell Park Comprehensive Cancer Center. Correlations between TNC dose and CD3+ dose,CD4+ dose, CD8+ dose, and CD34+ dose were calculated using the Pearson productmoment correlation coefficient. The cumulative incidence of acute and chronic GvHD was analyzed adjusting for the competing risk of disease relapse. Univariable analysis of OS and PFS were analyzed as time-to-event; survival curves were generated using the Kaplan-Meier method and were compared using the log-rank test. Multivariable analyses tested each cell dose while adjusting for significant factors in the univariate analysis, first in all patients and then stratified by the four conditioning regimen groups. Variables included in the multivariable analyses were age (≥/< 40 years), KPS(≥/< 80) at time of transplant, and BMI (≥/< 35 kg/m2). All analyses were performed using SAS version 9.4.
RESULTS
The cohort consisted of 135 sibling and 112 unrelated donor transplant recipients.Sibling donors were 6/6 HLA-matched at HLA-A, -B, and -DRB1. Unrelated donors were 10/10 HLA-matched at HLA-A, -B, -C, -DRB1, and DQB1 (3 patients were 8/8HLA-matched at HLA-A, -B, -C, -DRB1). Patients who received a MA regimen were significantly younger, had a higher KPS, more commonly had a sibling donor,tacrolimus/methotrexate GvHD prophylaxis regimen, and were treated for different diseases than patients who received a RIC regimen (Table 2).

Table 1 Conditioning regimen descriptions
Peripheral blood apheresis cell doses
Median (range) cell doses for the whole cohort were 264.3 (10.4-1137.5) × 106/kg for CD3+, 166.2 (8.3-590.9) × 106/kg for CD4+, 103.7 (2.2-590.9) × 106/kg for CD8+, 6.5(0.9-27.6) × 106/kg for CD34+, and 8.3 (1.4-21.4) × 108/kg for TNC. Graft composition for conditioning subgroups are detailed in Supplementary Table 1.
Neutrophil engraftment
The cumulative incidence of neutrophil engraftment was 99% at day 28 post-PBHCT.Six patients died on days 3, 5, 12, 20, 26, and 36 before neutrophil engraftment.Overall, patients who received a CD34+ cell dose > 4 × 106/kg experienced faster neutrophil engraftment (median 13 dvs15 d,P= 0.05) as compared to patients who received a CD34+ cell dose < 4 × 106/kg. Analysis by conditioning regimen demonstrated significantly faster neutrophil engraftment for an infused CD34+ cell dose > 4 × 106/kg in the RIC + TBI group (median 15 dvs18 d,P= 0.01) and no statistically significant differences by CD34+ cell dose for the other three conditioning regimen groups (Table 3). There were no significant differences in time to neutrophil engraftment by CD3+, CD4+, CD8+, and TNC dose either overall or in any conditioning subgroup (Supplementary Table 2).
Platelet engraftment
Five patients did not nadir their platelet count below 20000/mm3post-PBHCT and were excluded from the analysis of platelet engraftment. The cumulative incidence of platelet engraftment was 89% at day 40 post-PBHCT. One patient failed to engraft platelets and had a second transplant on day 44. Ten patients died before day 40, three patients died between days 41 to 100, and one patient died 6 mo post-PBHCT without platelet engraftment. Overall, patients who received a CD34+ cell dose > 4 × 106/kg experienced significantly faster platelet engraftment (median 16 dvs20 d,P= 0.001)as compared to patients with a CD34+ cell dose < 4 × 106/kg. Analysis by conditioning regimen demonstrated significantly faster platelet engraftment in patients with a CD34+ cell dose > 4 × 106/kg for the MA + TBI group (median 20 dvs34 d,P= 0.001), and the RIC-noTBI group (median 17 dvs22 d,P= 0.01), but no statistically significant differences in time to platelet engraftment by CD34+ cell dose for the other two conditioning regimen groups (Table 3). Platelet engraftment was significantly faster in patients who received a higher CD3+ or CD8+ cell dose in the RIC-noTBI group, but not in any of the other conditioning regimen groups. CD4+ and TNC cell doses were not significant (Supplementary Table 2).
Graft-versus-host disease
In the MA + TBI conditioning regimen group, there was a higher incidence of grade II-IV acute GvHD in patients who received a TNC dose > 8 × 108/kg, however there was no difference in grade III-IV acute GvHD (Figure 1A and 1B). Conversely, there was a higher incidence of grade III-IV acute GvHD in patients who received a lower CD34+ cell dose (≤ 8 × 106/kg), however there was no difference in grade II-IV acute GvHD by CD34+ cell dose (Figure 1C and 1D). These effects with TNC and CD34+dose in MA + TBI were not seen in any of the other conditioning regimen groups.There were no statistically significant associations of CD3+, CD4+, or CD8+ dose with acute GvHD overall or in any conditioning regimen subgroup.
There was no significant association of chronic GvHD incidence with a TNC dose of > 8 × 108/kg either overall or by conditioning regimen. There was a significantlyhigher incidence of moderate to severe chronic GvHD in all patients who received aTNC dose > 9 × 108/kg (P= 0.004) but was not statistically significant in any conditioning regimen subgroup. There was no association of CD34+, CD3+, CD4+, or CD8+ cell dose with chronic GvHD either overall or in any conditioning regimen group.

Table 2 Patient characteristics for each of four conditioning regimen groups, n (%)

Table 3 Time to neutrophil and platelet engraftment by CD34+ dose for each conditioning regimen group
Overall and progression-free survival
Median follow-up in all patients was 4.8 years (range 1.6-12 years). CD34+, CD3+,CD4+, and CD8+ cell doses were not associated with either OS or PFS in all patients or stratified by conditioning regimen. TNC dose showed no significant difference in OS or PFS when analyzed in all patients (Figure 2A). However, a significant improvement in OS was seen in patients with TBI-based conditioning regimens who received higher (> 8 × 108/kg) TNC doses (Figure 2B). Further analysis showed this effect was restricted to the RIC + TBI (Figure 2D) group with no significant difference in the MA + TBI group (Figure 2C). Similar results were found with PFS: a higher (> 8× 108/kg) TNC dose was associated with improved PFS in patients who received TBIbased conditioning regimens, which was driven by the RIC + TBI subgroup.
Multivariate analysis
Based on the univariate analysis, age, KPS, and BMI were included as covariates in the multivariable analysis of each cell dose with OS, and KPS and BMI were included as covariates in the multivariable analysis of each cell dose with PFS (Table 4). Similar to the univariate analysis, TNC dose > 8 × 108cells/kg was associated with improved OS and PFS in patients who received TBI-based conditioning regimens. However,upon further stratification, this finding was statistically significant only in the RIC +TBI conditioning group.
Correlations between cell populations
To investigate potential correlations between cell types, Table 5 summarizes the matrix of Pearson correlations between cell doses. While most cell doses are significantly and positively correlated with the others (P< 0.001), most correlation coefficients were low. Pearsonr2< 0.5 means < 50% of the difference between cell doses can be explained by the linear relationship between the two. CD3+ cell dose is correlated with CD4+, CD8+, and TNC cell doses (r2: 0.5-0.83, Table 4), however CD34+ cell dose is not correlated with any of the other cell types (r2< 0.05) and is thus an independent cell type.
DISCUSSION
The effect of infused cell dose on post-transplant outcomes is complex. Our single center study is the first to analyze the relationship of conditioning regimen intensity and use of TBI with infused cell doses. A recent study demonstrated that in reduced intensity transplant without TBI, TNC dose was associated with improved PFS and OS similar to our results in reduced intensity conditioning with or without TBI[24].Martinet al[24]reported that higher TNC dose was also associated with decreased relapse and increased incidence of chronic GvHD.
Our results indicate that overall CD34+ cell dose is not associated with OS or PFS in our patient population as observed in other studies[9,11-13,24]. This differs from a study in T-cell depleted transplants after myeloablative TBI conditioning, which reported CD34+ doses between 4-8 × 106/kg were optimal for OS, and anything above or below this range resulted in increased mortality[25]. Gorinet al[16]demonstrated RIC + TBI patients receiving a TNC dose > 9.1 × 108/kg had improved PFS, which was similar to our results.
档案材料流失的问题。作为档案材料应该是系统的永久存放资料,应该为其发展而服务,作为众多的科技人员所创造的成果只能说的在职期间为国家和集体做的工作,不应该看出是自己的私人材料。在我们单位就存在着这样的问题,当时一些老科技人员他们确实有一种忘我的钻研精神,不分单位和家里都是埋头苦干地钻研畜牧局的档案管理工作。但是当他们退休后就将其材料占为己有,视为自己的私有财产,这样以一个集体而凝结成的智慧结晶却难以得到集体的传承发展,为此使畜牧局档案管理工作造成了不小的麻烦,出现了一定的断空,更影响了下一步的管理工作。
There was no association in patients with higher CD3+, CD4+, CD8+, or CD34+doses and OS or GvHD, however recent studies indicated that an optimal CD34+ cell dose can lead to improved survival, less GvHD, and improved engraftment[5-11]. Our results demonstrated an association with TNC dose, which could indicate there is another graft cell subset that may better predict these outcomes. One candidate is the natural killer (NK) cell. A low donor NK cell dose was associated with significantly longer time to engraftment and worse OS[16]. NK cells have also been implicated as an important modulator of GvHD and the graftvsleukemia effect[26]. It is possible that increasing the donor NK cell dose could allow for a more robust graftvsleukemia effect without increasing risk of GvHD[27]. Focusing on the recipient, previous work demonstrated that host NK cells are relatively radiation-resistant and may decrease the incidence and severity of GvHD[28-30]. Thus, in the setting of low dose TBI, host NK cells could be preserved and mediate a decrease in GvHD while allowing for an improved graftvsleukemia effect, translating into an improved PFS/OS.
Further confirmation of our results in a larger, multi-center registry study could be performed. In addition, analysis of other cell populations (i.e., NK cell dose) could explain our findings. Further understanding of the impact of graft composition on post-transplant outcomes, and their potential interactions with conditioning regimens could allow physicians to better target certain cell doses in order to improve posttransplant survival outcomes.

Table 4 Multivariable analysis shows no association of cell doses with overall survival or progression free survival, except for total nucleated cell dose in the reduced intensity conditioning + total body irradiation group
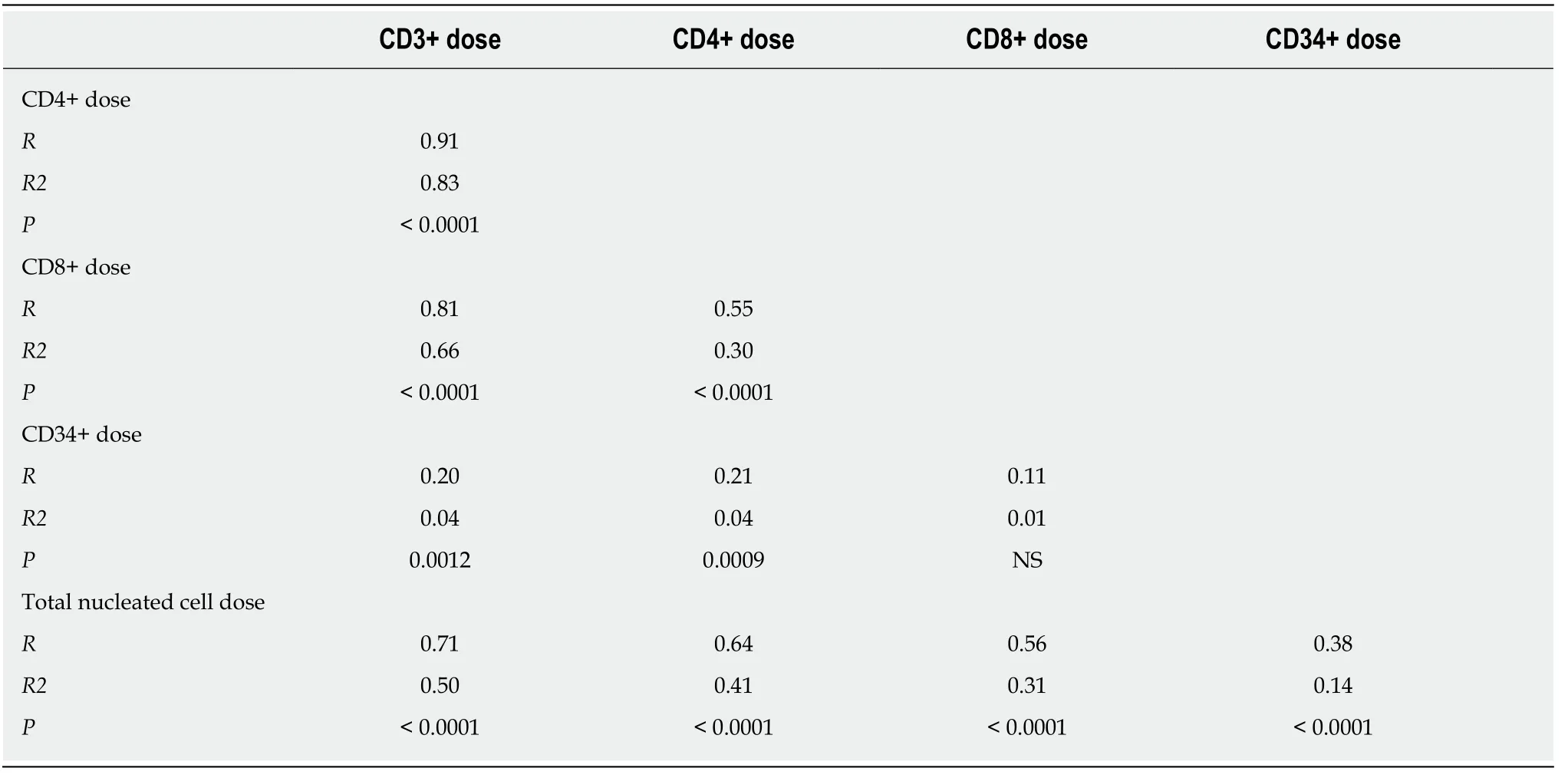
Table 5 Summary of correlations between cell doses demonstrating low correlation between total nucleated cell dose and CD8+ and CD34+ cell doses and moderate correlation with CD3+ and CD4+ cell doses

Figure 2 OS by total nucleated cell dose. A: OS was not significantly different in patients conditioned without TBI; B: OS was significantly better with a higher total nucleated cell dose in patients conditioned with TBI; C: OS was not significantly different in patients conditioned with myeloablative TBI; D: OS was significantly better with a higher total nucleated cell dose in patients conditioned with reduced intensity TBI. OS: Overall survival; TBI: Total body irradiation.
ARTICLE HIGHLIGHTS
Research background
Peripheral blood hematopoietic cell transplant (PBHCT) is the most commonly used allogeneic hematopoietic cell source due to its quick rate of neutrophil engraftment. A higher total nucleated cell (TNC) dose has been reported to improve survival after PBHCT, but analyses of specific T-cell subsets have been inconsistent. Factors such as T-cell depletion, conditioning regimen intensity, use of total body irradiation (TBI), and donor age may be interacting with graft cell doses to generate different effects on PBHCT outcomes. In addition, flow cytometric enumeration of cell doses are not standardized and may also lead to differences in results between studies.
Research motivation
While the optimal CD34+ cell dose range to minimize time to neutrophil and platelet recovery without increasing risk of acute graft-versus-host disease has been found to be 4-10 × 106/kg,some studies have reported a higher CD34+ cell dose yields improved overall survival while others have found no significant association. Further understanding of the impact of graft composition on post-transplant outcomes, and their potential interactions with conditioning regimens, could allow physicians to better target certain cell doses in order to improve posttransplant survival outcomes.
Research objectives
The objectives of this study were to examine whether the collected and infused CD34+, CD3+,CD4+, CD8+ or TNC dose influenced engraftment, overall survival, progression free survival,and incidence of acute and chronic GvHD, and whether the results were affected by conditioning regimen intensity or use of TBI.
Research methods
Four conditioning regimen groups were defined a priori as (1) myeloablative (MA) without TBI(MA-noTBI), (2) myeloablative with TBI (MA + TBI), (3) Reduced intensity conditioning (RIC)without TBI (RIC-noTBI) and (4) RIC with TBI (RIC + TBI). Correlations between TNC dose and CD3+ dose, CD4+ dose, CD8+ dose, CD34 dose were calculated using the Pearson productmoment correlation coefficient. The cumulative incidence of acute and chronic GvHD was analyzed adjusting for the competing risk of disease relapse. Univariable analysis of OS and progression free survival (PFS) were analyzed as time-to-event; survival curves were generated using the Kaplan-Meier method and were compared using the log-rank test. Multivariable analyses tested each cell dose while adjusting for significant factors in the univariate analysis,first in all patients and then stratified by the four conditioning regimen groups. These analyses allowed us to explore the interaction of conditioning regimen and allogeneic donor apheresis product composition in relation to outcomes after unmanipulated peripheral blood hematopoietic cell transplantation.
Research results
The cohort consisted of 135 sibling and 112 unrelated donor transplant recipients. Overall,patients who received a CD34+ cell dose > 4 × 106/kg experienced faster neutrophil engraftment and platelet engraftment as compared to patients who received a CD34+ cell dose < 4 × 106/kg.Analysis by conditioning regimen demonstrated significantly faster neutrophil engraftment for an infused CD34+ cell dose > 4 × 106/kg in the RIC + TBI group. Overall and progression-free survival was significantly better in patients with a RIC + TBI regimen and TNC dose > 8 ×108/kg. Our results indicated that overall CD34+ cell dose is not associated with OS or PFS in our patient population, similar to other studies. We did find an overall and progression-free survival benefit in patients with a RIC + TBI regimen and TNC dose > 8 × 108/kg, which could indicate there is another graft cell subset that may better predict these outcomes.
Research conclusions
Our single center study is the first to analyze the relationship of conditioning regimen intensity and use of TBI with infused cell doses. Neutrophil engraftment was significantly faster after reduced intensity TBI based conditioning and > 4 × 106CD34+ cells/kg infused. In addition,overall and progression-free survival were significantly better in patients with a RIC + TBI regimen and TNC dose > 8 × 108/kg. Our study suggested that TBI and conditioning intensity may alter the relationship between infused cell doses and outcomes after PBHCT. Our results demonstrated that immune cell subsets may predict improved survival after unmanipulated PBHCT.
Research perspectives
This study suggests that TBI and conditioning intensity may alter the relationship between infused cell doses and outcomes after PBHCT. Further confirmation of our results in a larger,multi-center registry study could be performed. In addition, analysis of other cell populations,such as NK cell dose, could explain our findings. Further understanding of the impact of graft composition on post-transplant outcomes, and their potential interactions with conditioning regimens could allow physicians to better target certain cell doses in order to improve posttransplant survival outcomes.
 World Journal of Clinical Oncology2019年2期
World Journal of Clinical Oncology2019年2期
- World Journal of Clinical Oncology的其它文章
- Challenges in the diagnosis and treatment of gestationaltrophoblastic neoplasia worldwide
- Oligometastases in prostate cancer: Ablative treatment
- Existing anti-angiogenic therapeutic strategies for patients with metastatic colorectal cancer progressing following first-line bevacizumab-based therapy
- Pancreatic cancer screening in patients with presumed branch-duct intraductal papillary mucinous neoplasms
- Retrospective evaluation of FOLFlRl3 alone or in combination with bevacizumab or aflibercept in metastatic colorectal cancer
- Hong Kong female’s breast cancer awareness measure: Crosssectional survey
