Existing anti-angiogenic therapeutic strategies for patients with metastatic colorectal cancer progressing following first-line bevacizumab-based therapy
Ozkan Kanat, Hulya Ertas
Abstract
Key words: Angiogenesis inhibition; Second-line chemotherapy; Colorectal cancer;Bevacizumab; Aflibercept; Ramucirumab
INTRODUCTION
The medical treatment of metastatic colorectal cancer (mCRC) has become more diversified over the past few decades owing to the successful integration of targeted therapy agents, which block either epidermal growth factor signaling pathway or angiogenesis, into cytotoxic drug combinations[1]. Concordantly, a dramatic improvement in survival has been achieved among patients suffering from mCRC.Moreover, extensive preclinical efforts were able to identify additional targetable molecular alterations in these patients such asBRAFmutation, human epidermal growth factor receptor 2 amplification, and microsatellite instability[2-4]. The clinical application of compounds that can inhibit signaling pathways in cancer cells activated by these genetic events seems to provide additional survival gains in selected patients with mCRC.
Among the molecular targets mentioned above, tumor-driven angiogenesis is still an attractive target in mCRC[5-7]. The United States Food and Drug Administration has approved a total of four drugs that block angiogenesis (bevacizumab, aflibercept,ramucirumab, and regorafenib) in the treatment of mCRC (Table 1). Of these,bevacizumab is the only drug licensed for the treatment of chemotherapy-naïve patients with mCRC.
Bevacizumab is a murine-derived monoclonal antibody (muMAb A4.6.1) that inhibits angiogenesis by targeting the vascular endothelial growth factor (VEGF)-A.Belonging to the VEGF family, (VEGF)-A is a crucial angiogenic cytokine (Figure 1)that is produced by cancer and benign stromal cells, particularly in a hypoxiainducible factor-1-dependent manner. It triggers angiogenic signals via interaction with endothelial cell-surface tyrosine kinase receptors [VEGF receptor-1 (VEGFR-1)and -2 (VEGFR-2)]. The binding of VEGF-A to the extracellular domain of these receptors induces their dimerization and autophosphorylation and the subsequent activation of intracellular pathways that contribute to cell proliferation (e.g.,phospholipase-C-gamma and extracellular signal-regulated kinases 1/2 pathway),migration (e.g., focal adhesion kinase and p38 pathway), and survival (e.g.,phosphatidylinositol 3-kinase/Akt pathway)[8-11]. Other members of the VEGF family,such as VEGF-B, -C, and -D, and placental growth factor (PIGF) play supporting roles in the process of angiogenesis[10,12].
Bevacizumab is conventionally administered in combination with oxaliplatin- or irinotecan-based doublet [i.e., FOLFOX (5-FU, leucovorin, and oxaliplatin) and FOLFIRI (5-FU, leucovorin, and irinotecan)] or triplet [i.e., FOLFOXIRI (5-FU,leucovorin, oxaliplatin, and irinotecan)] chemotherapy regimens. A recent metaanalysis of the first-line chemotherapy for mCRC confirmed that the addition of bevacizumab results in a significant improvement in progression-free survival [PFS;hazard ratio (HR) 0.66,P< 0.0001] and overall survival (OS; HR 0.84,P= 0.0001),compared with chemotherapy alone[13]. In addition, the clinical activity of bevacizumab is not influenced by currently validated predictors of treatment response and/or survival outcomes in mCRC, such as the mutational status (KRASandBRAFgenes) and anatomic location (leftvsright side of the colon) of the primary tumor.
On the other hand, patients undergoing first-line bevacizumab-based therapy eventually develop disease progression (usually within 9 mo) and become candidates for second-line chemotherapy[13]. Available data strongly favor the continuous inhibition of angiogenesis (using maintenance bevacizumab therapy or switching to another antiangiogenic monoclonal antibody) during second-line chemotherapy to achieve a satisfactory clinical outcome[14,15]. In this article, we discuss therapeutic strategies that have been proven to be useful in the treatment of patients with mCRC in whom first-line bevacizumab-based therapy was ineffective.
CONTINUATION OF BEVACIZUMAB BEYOND DISEASE PROGRESSION
Several United States-based non-randomized observational studies, such as theBevacizumab Regimens: Investigation of Treatment Effects and Safety and the Avastin Registry: Investigation of Effectiveness and Safety, initially reported that the continuation of bevacizumab during second-line chemotherapy had a beneficial impact on the survival of patients with mCRC in whom first-line bevacizumab-based therapy was ineffective[16-18]. Further evidence in support of this treatment strategy was provided by the phase III ML18147 trial (Table 2)[19].
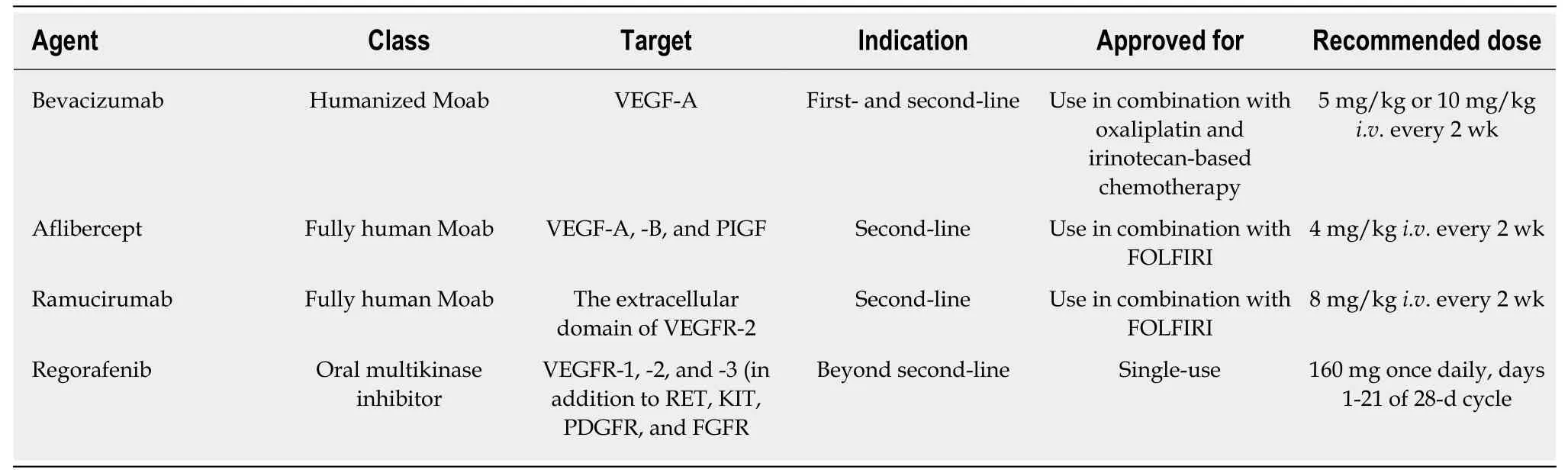
Table 1 Food and Drug Administration-approved antiangiogenic drugs for the treatment of metastatic colorectal cancer
The ML18147 trial was designed by German and Austrian investigators to evaluate the effectiveness of continuing with bevacizumab-based therapy following disease progression in patients with mCRC who had previously received irinotecan- and oxaliplatin-based chemotherapy regimens in combination with bevacizumab[19].However, the study excluded patients who exhibited progression within the first 3 mo of first-line therapy (rapid progressors), those who showed progression 3 mo after the last bevacizumab administration, and those who received bevacizumab for < 3 consecutive months of first-line therapy. Overall, 820 patients were randomized to receive a novel chemotherapy regimen (fluoropyrimidine plus oxaliplatin or irinotecan) plus bevacizumab (equivalent of 2.5 mg/kgi.v. per week) or chemotherapy alone. Therapy was continued until the development of disease progression or intolerable toxicity. Patient stratification was conducted based on the first-line chemotherapy regimen, first-line PFS (≤ 9 movs> 9 mo), time from last bevacizumab administration (≤ 42 dvs> 42 d), and performance status (ECOG 0-1vs2).
In comparison with patients receiving chemotherapy alone, those receiving chemotherapy plus bevacizumab had a significantly longer median PFS (5.7 movs4.0 mo; HR 0.63;P< 0.0001) and median OS [11.2 movs9.8 mo; HR 0.81; 95% confidence interval (CI): 0.69-0.94;P= 0.0062]. Bevacizumab was consistently beneficial across all subgroups, although the response rates were relatively low in both groups (5%vs4%).However, the disease control rate was significantly higher in the chemotherapy plus bevacizumab group (68%vs54%,P< 0.0001). In addition, the chemotherapy plus bevacizumab group was not associated with increased toxicity, with the exception of specific bevacizumab-related (grade 3-5) side effects including bleeding/hemorrhage(2%vs< 1%), gastrointestinal perforation (2%vs< 1%), and venous thromboembolism(5%vs3%). There were four treatment-related deaths in the chemotherapy plus bevacizumab group and three in the chemotherapy alone group.
The Bevacizumab Beyond Progression (BEBYP) phase III trial was designed by Italian researchers to investigate the clinical effectiveness of continuing bevacizumab or reintroducing it (after a bevacizumab-free interval of > 3 mo) in combination with second-line chemotherapy in patients with mCRC who developed disease progression following first-line bevacizumab-based therapy[20]. However, following the presentation of data from the ML18147 trial, the study was prematurely discontinued after inclusion of only 185 patients. These patients were randomized to receive second-line chemotherapy alone or in combination with bevacizumab and stratified into subgroups according to their performance status, (ECOG 0vs1-2),chemotherapy-free interval (> 3 movs< 3 mo), bevacizumab-free interval (> 3 movs<3 mo), and the second-line chemotherapy regimen administered (FOLFIRIvsFOLFOX). The bevacizumab-free interval was longer than 3 mo in 50% of the patients in the chemotherapy plus bevacizumab group. After a median follow-up of 45.3 mo,when compared with chemotherapy alone, the continuation or reintroduction of bevacizumab with second-line chemotherapy was associated with a significantly higher median PFS (6.8 movs5.0 mo; adjusted HR 0.70; 95%CI: 0.52–0.95; stratified log-rankP= 0.010) and median OS (15.5 movs14.1 mo; adjusted HR 0.77; 95%CI:0.56–1.06; stratified log-rankP= 0.043); this benefit was consistently observed across all patient subgroups. The response rates observed between the groups were not significantly different (17%vs21%;P= 0.573). Subgroup analyses revealed an equivalent survival benefit regardless of whether bevacizumab was continued or reintroduced. The safety profile and frequency of adverse events were also similar in the treatment groups.
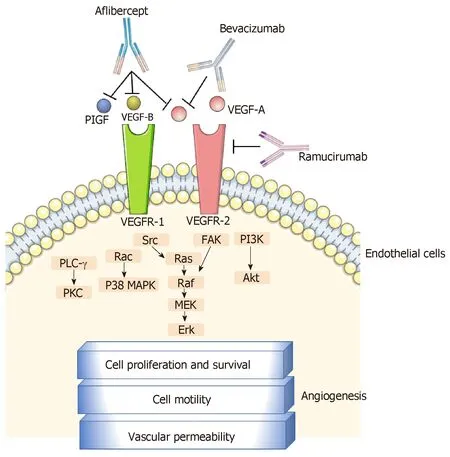
Figure 1 Approved anti-vascular endothelial growth factor monoclonal antibodies in the treatment of metastatic colorectal cancer and their mechanisms of action. VEGF: Vascular endothelial growth factor; PIGF:Placental growth factor; FAK: Focal adhesion kinase; PI3K: Phosphoinositide 3-kinase; PLC-γ: Phospholipase C gamma; PKC: Protein kinase C; MAPK: Mitogen-activated protein kinases; Erk: Extracellular signal-regulated kinase.
SWITCHING TO A DIFFERENT ANTI-VEGF MONOCLONAL ANTIBODY
Aflibercept
Aflibercept is a recombinant protein that is constructed from the second extracellular ligand-binding domain of VEGFR-1 and the third extracellular ligand-binding domain of VEGFR-2, fused to the constant region of a human immunoglobulin G1 molecule[21-25]. In contrast to bevacizumab that only inhibits VEGF-A, aflibercept can bind to other angiogenic cytokines (e.g., VEGF-B and PIGF) that are thought to play a role in resistance to bevacizumab[21-25]. This biological advantage of aflibercept may explain its superior antitumor activity when compared with bevacizumab in patientderived xenograft models of CRC[21]. In addition, studies in tumor xenografts have demonstrated that switching to aflibercept during disease progression following bevacizumab therapy resulted in a higher tumor response than the cases receiving continued bevacizumab-based therapy[26].
The phase III VELOUR trial was designed to evaluate the effectiveness of aflibercept in combination with FOLFIRI regimen during the second-line chemotherapy of patients with mCRC who had developed disease progression either during or after completion of oxaliplatin-based chemotherapy without a biologic agent[27]. Moreover, patients who relapsed within 6 mo of the completion of adjuvant oxaliplatin-based chemotherapy were also included in this study. Patients with prior exposure to irinotecan were not eligible, although those previously treated with bevacizumab were included. Patients were randomized to receive either FOLFIRI plusaflibercept (4 mg/kgi.v. every 2 wk) (n= 612) or FOLFIRI plus placebo (n= 614), and stratified according to ECOG performance status (0vs1vs2), prior bevacizumab exposure (approximately 30.5% of patients in both treatment arms had received firstline bevacizumab-based therapy), age, sex, anatomic location of primary tumor,number of involved organs, hepatic metastasis, prior hypertension, and geographical region. Treatment was continued until the development of disease progression or intolerable toxicity. The primary endpoint was OS.
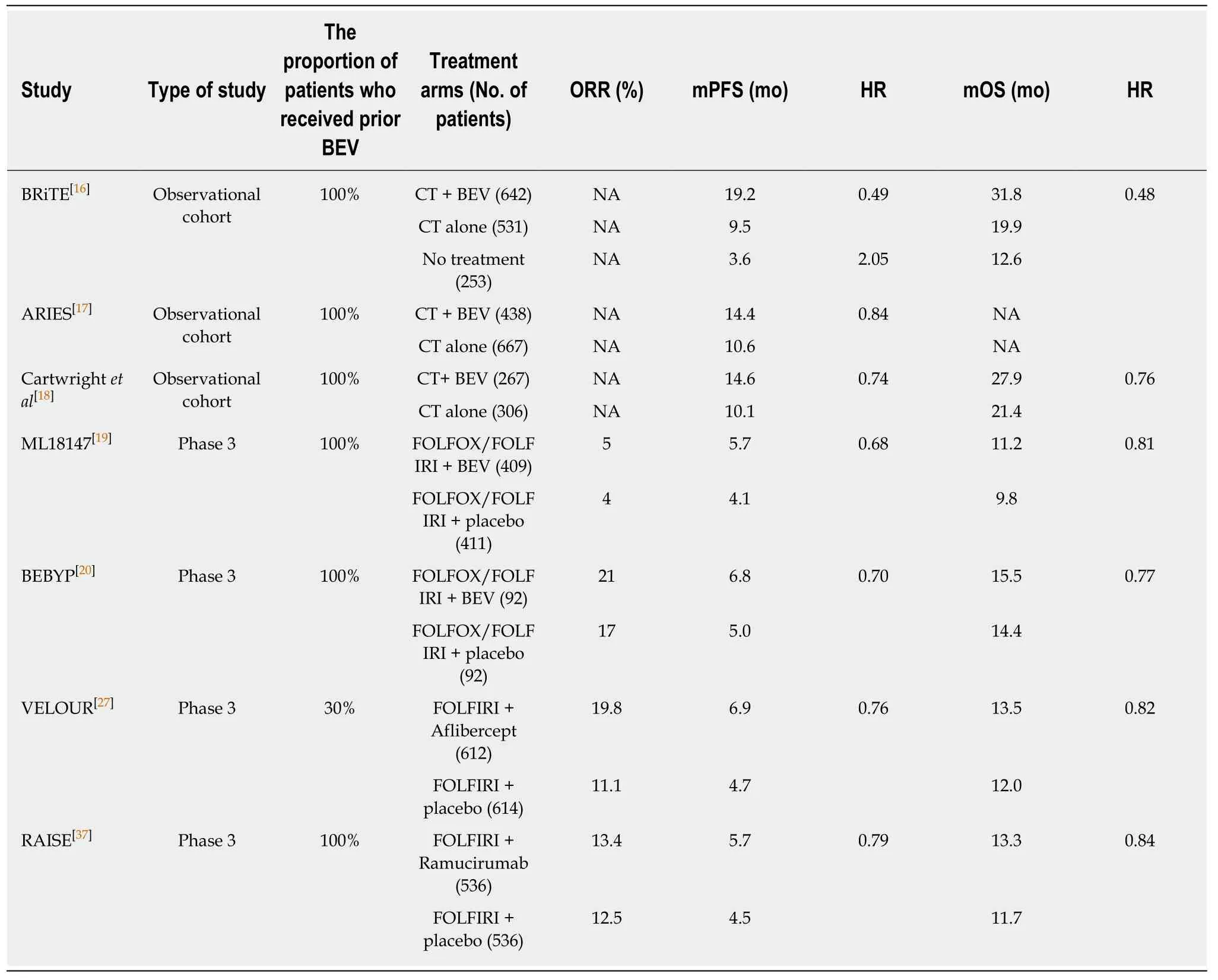
Table 2 Randomized clinical studies comparing the efficacy of second-line chemotherapy plus antiangiogenic agent with chemotherapy alone (or plus placebo) in metastatic colorectal cancer
After a median follow-up of 22.3 mo, patients receiving FOLFIRI plus aflibercept demonstrated a significantly longer PFS (median, 6.90 movs4.67 mo; HR 0.758;95%CI: 0.661-0.869;P< 0.0001) and OS (median, 13.5 movs12 mo; HR 0.817;95.34%CI: 0.713-0.937;P= 0.0032) than those receiving placebo plus FOLFIRI. The aflibercept group had a higher ORR than the placebo group (28%vs18.7%)[28,29].Subsequent subgroup analyses revealed that patients previously exposed to bevacizumab also benefited from a longer OS (albeit less pronounced) through the application of aflibercept; the median OS values were 12.5 and 11.7 mo with the aflibercept and placebo groups, respectively (HR 0.862). However, the most significant benefit from aflibercept treatment was observed among patients with liveronly metastases and among those with no previous exposure to bevacizumab[30,31].
Compared with the placebo group, the aflibercept group were found to experience more grade ≥ 3 anti-VEGF class-specific side effects, which included hypertension(19.5%vs1.5%), hemorrhage (2.9%vs1.7%), arterial thromboembolic events (1.8%vs0.5%), and venous thromboembolic events (7.9%vs6.3%). In addition, aflibercept administration led to an increase in the incidence of chemotherapy-related toxicities such as neutropenia, diarrhea, asthenia, stomatitis, infections, and palmar-plantar erythrodysesthesia. Patients aged ≥ 65 years appeared to be particularly vulnerable to these adverse events[32,33].
Ramucirumab
Ramucirumab is another inhibitor of the VEGF/VEGFR axis. It selectively targets VEGFR-2 and induces conformational changes in the extracellular domain of the receptor, which prevents the binding of all VEGF ligands and receptor activation[34].Several preclinical studies suggest that the inhibition of VEGFR-2 using monoclonal antibodies, such as DC101, inhibits the growth of CRC cells that are resistant to other angiogenesis inhibitors[35,36]. Therefore, the use of potent and selective VEGFR-2 inhibitors, such as ramucirumab, provides a rational therapeutic option for patients with mCRC who developed disease progression despite receiving first-line bevacizumab-based therapy.
The multicenter, randomized, double-blind, phase III RAISE trial compared the effectiveness of ramucirumab versus placebo, both in combination with second-line FOLFIRI regimen[37]. The study included patients with mCRC who developed disease progression within 6 mo after the final dose of first-line oxaliplatin-based chemotherapy plus bevacizumab. Patients who had received bevacizumab (within 28 d) or chemotherapy (within 21 d) before randomization were excluded. Overall, 1072 patients were randomized to receive ramucirumab (8 mg/kg every 2 wk) plus FOLFIRI or placebo plus FOLFIRI (n= 536 in each group). Stratification variables included the geographical location (North AmericavsEuropevsall other regions),KRAS exon 2 status (mutantvswild-type), and time to disease progression after firstline therapy (< 6 movs≥ 6 mo). Of the patients, 83% had received at least 3 mo of first-line bevacizumab-based therapy. Treatment continued until the development of disease progression or intolerable toxicity. The primary endpoint of the study was OS.
After a median follow-up of 21.7 mo, OS was significantly longer in the ramucirumab group than the placebo group (13.3 movs11.7 mo; HR 0.844; 95%CI:0.730–0.976;P= 0.0219). An improved PFS also was detected in patients receiving ramucirumab (5.7 movs4.5 mo; HR 0.793; 95%CI: 0.697-0.903;P= 0.0005). The survival benefit was consistent across all patient subgroups that received ramucirumab plus FOLFIRI. However, the response rates in the ramucirumab and placebo groups were comparable (ORR 13.4%vs12.5%;P= 0.63).
The addition of ramucirumab to chemotherapy was associated with higher rates of neutropenia, hypertension, diarrhea, and fatigue. Despite the transient deterioration in the quality of life of these patients, the adverse events were manageable.
In a prospective biomarker analysis of the RAISE trial, the efficacy of ramucirumab was compared with pretreatment plasma levels of several angiogenic cytokines[38]. In particular, ramucirumab plus FOLFIRI therapy was found to be more beneficial in patients with elevated plasma VEGF-D levels, with an improvement of 2.4 mo in OS(13.9 movs11.5 mo). However, this therapy was associated with reduced OS in patients with low VEGF-D levels, compared with the placebo group (12.6 movs13.1 mo).
Comments and conclusions
The data presented above shows that the maintenance of angiogenesis inhibition using bevacizumab, aflibercept, or ramucirumab beyond the initial development of disease progression is an effective and tolerable strategy with a consistent and significant improvement in OS (approximately 1.4 mo) observed in patients with mCRC. In fact, no notable differences between these three drugs were found in terms of their contribution to survival and safety profile. The estimated HR for OS values were similar in the ML18147 (0.81), BEBYP (0.77), VELOUR (0.82), and RAISE (0.84)studies. Accordingly, the most recent version of the European Society of Medical Oncology consensus guidelines for the management of mCRC recommended either the continuation of bevacizumab or switching to aflibercept or ramucirumab (only in combination with FOLFIRI and in irinotecan-naïve patients) for the second-line chemotherapy of patients in whom first-line bevacizumab-based therapy was ineffective (category 1A)[39].
At present, a head-to-head randomized clinical study comparing the efficacy of these three angiogenesis inhibitors in this setting has not been undertaken. Moreover,useful biomarkers that could be integrated into an ideal treatment protocol are not available. Although the measurement of pretreatment plasma levels of angiogenic cytokines (particularly VEGF-D) is a promising approach in this setting, the process is inconvenient for routine clinical use.
The clinical course of patients during first-line therapy may assist clinicians in their decision-making. In this context, patients who exhibit rapid progression (i.e., within 3 mo) following the initiation of first-line bevacizumab-based therapy are usually good candidates for treatment with aflibercept or ramucirumab. It should be noted that such patients were not included in the ML18147 study, and it is possible that they have an intrinsic resistance to bevacizumab.
Cost-effectiveness is also a factor that influences the clinician’s decision. Goldstein and El-Rayes calculated the costs of these agents for the treatment of mCRC based on average US prices[40]. They estimated that ramucirumab leads to a more than two-fold increase in the cost of treatment compared with bevacizumab and aflibercept.Morlocket al[41]indirectly compared the total cost and clinical outcomes of using bevacizumab plus chemotherapy and aflibercept plus chemotherapy as second-line chemotherapeutic strategies for mCRC using Butcher’s method. Bevacizumab plus chemotherapy was found to be more cost-effective than aflibercept plus chemotherapy ($39104 less per treated patient), with similar effectiveness (OS 13.3 movs12.5 mo; HR 0.94). Therefore, the use of bevacizumab beyond disease progression appears to be the most reasonable therapeutic approach in selected patients.
For patients with RAS wild-type mCRC in whom the first-line bevacizumab-based treatment was ineffective, the optimal second-line chemotherapy remains controversial. The data from two small phase II studies, the SPIRITT and PRODIGE 18, suggests that switching from bevacizumab to an epidermal growth factor inhibitor(panitumumab or cetuximab) in the second-line chemotherapy of patients with KRAS wild-type mCRC does not provide a survival benefit that is superior to the continuation of bevacizumab[42,43]. However, the SPIRITT study demonstrated that a switch from bevacizumab to panitumumab might be associated with increased tumor response (19%vs32%)[42]. Therefore, when a rapid response is desired, the continuation of treatment with an EGFR inhibitor may be more appropriate.
In conclusion, based on current evidence, we propose a simple algorithm for the management of patients with mCRC who developed disease progression following first-line bevacizumab-based therapy (Figure 2). The identification of clinically useful predictive markers reflecting tumor sensitivity to a specific antiangiogenic agent would improve the effectiveness of treatment and reduce costs.
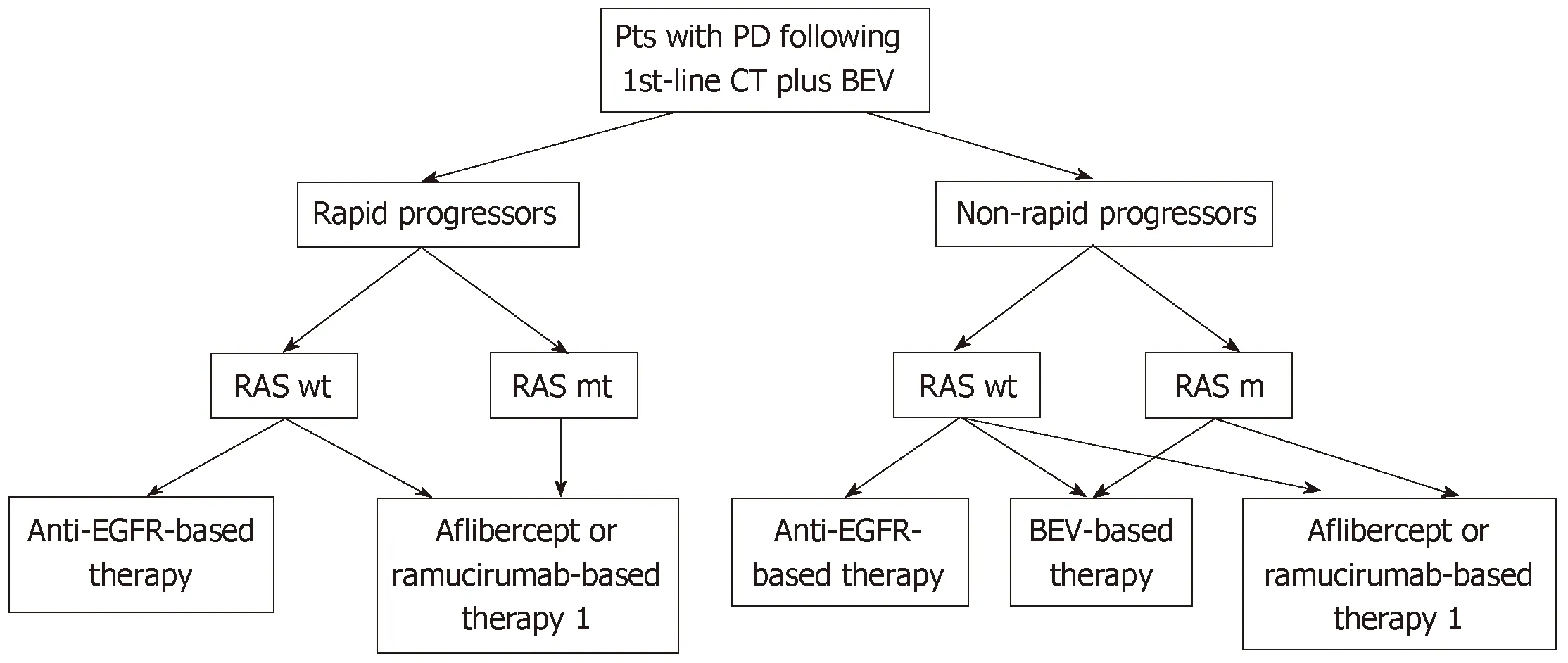
Figure 2 A proposed algorithm for the management of patients with metastatic colorectal cancer after disease progression following bevacizumab-based first-line therapy. Rapid progressors: Patients progressing within 3 mo after starting first-line chemotherapy. 1In patients who did not receive irinotecan-based firstline chemotherapy and only in combination with FOLFIRI. Pts: Patients; PD: Progressive disease; CT: Chemotherapy; BEV: Bevacizumab; wt: Wild-type; mt: Mutant;EGFR: Epidermal growth factor receptor.
 World Journal of Clinical Oncology2019年2期
World Journal of Clinical Oncology2019年2期
- World Journal of Clinical Oncology的其它文章
- Challenges in the diagnosis and treatment of gestationaltrophoblastic neoplasia worldwide
- Oligometastases in prostate cancer: Ablative treatment
- Pancreatic cancer screening in patients with presumed branch-duct intraductal papillary mucinous neoplasms
- Retrospective evaluation of FOLFlRl3 alone or in combination with bevacizumab or aflibercept in metastatic colorectal cancer
- Impact of conditioning regimen on peripheral blood hematopoietic cell transplant
- Hong Kong female’s breast cancer awareness measure: Crosssectional survey
