Antihydatic and immunomodulatory effects of Algerian propolis ethanolic extract: In vitro and in vivo study
Nahla Deghbar, Dalila Mezioug✉, Touri Kahina, Yacine-Miloud Medjdoub,Chafia Touil- Boukoffa
1Cytokines and NO Synthases-Immunity and Pathogeny team, Laboratory of Cellular and Molecular Biology, Faculty of Biological Sciences, University of Sciences and Technology Houari Boumediene, Algiers, Algeria
2Department of Thoracic Surgery, Mustapha Pacha Hospital, Algiers, Algeria
Keywords:Cystic echinococcosis Algerian propolis Antihydatic effect Immunomodulatory effect
ABSTRACT Objective: To evaluate the in vitro and in vivo effect of the Algerian propolis ethanolic extract(EEP) against Echinococcus granulosus (E. granulosus) infection.Methods: In vitro scolicidal activity of EEP was investigated on the protoscolices of hydatid cyst. This in vitro study was conducted by using an in vivo assay. BALB/c mice were inoculated with E. granulosus and treated with propolis for three months. Hydatid cysts development was assessed. Nitric oxide (NO), tumor necrosis factor alpha (TNF-α) production and inducible NO synthase, NF-κB, and TNF-α spleen expression were estimated by Griess method and immuno fluorescence respectively.Results: Our study revealed that EEP has a high scolicidal activity against E. granulosus. Oral administration of EEP decreased TNF-α, NF-κB and inducible NO synthase expression in the spleen tissues in the CE+EEP group, in comparison with the CE group. Concomitantly, EEP treatment caused an important systemic decrease in NO and TNF-α levels. These findings are associated with the reduction of CE development.Conclusions: This is the first report demonstrating with interest the antihydatic and immunomodulatory effects of the Algerian EEP, suggesting its therapeutic potential for the hydatid disease treatment.
1. Introduction
Cystic echinococcosis (CE) is a major zoonotic infection that affects humans and domestic animals. It is caused by Echinococcus granulosus (E. granulosus) which is a metacestode belonging to the family of Taeniidae[1,2]. Cystic echinococcosis is still endemic and considered as a severe public health concern in many regions of the world, as is the case in Algeria[3].
This parasitic disease manifests frequently as unilocular hydatid cyst(s) which can develop in many organs including liver, lung, and occasionally, kidney, spleen, bone, heart and brain[4]. The clinical evolution of these cysts is slow and in some cases asymptomatic[1].Duration and intensity of infection as well as the variety of immunological responses to parasitic epitopes are the major factors that in fluence the severity and variability of the clinical expression of CE[5-7].
Because of its high efficiency, surgery is still the preferred CE treatment method. However, in many cases, it is dangerous or sometimes impossible. It also increases the risk of scolices dissemination during the surgical operation which contributes to recurrence[1,8]. Chemotherapy using benzimidazole compounds such as albendazole and mebendazole indicated in inoperable cysts and complementarily with cyst puncture, aspiration, injection of chemicals as well as respiration, and surgery treatment were subsequently introduced[2]. However, due to their different side effects, the application of these drugs has become controversial and limited[9,10]. Therefore, enormous efforts and investments have been spent attempting to discover, especially from natural resources,alternative treatment strategies and new anti-hydatid compounds which have low or no adverse effects and high potency for hydatid cyst surgery[11-13].
In this context, we are interested in propolis which is a resinous hive product, composed of exudates of trees mixed by honey bees with enzymes, beeswax and pollen[14]. This substance is used by bees to fill gaps, cover hive walls and mummify the carcasses of external invader insects[15]. Its composition may vary qualitatively and quantitatively according to its harvest[16], the different plant sources and the collection season[17,18]. Basically, resins and balsams are the major constituents of crude propolis (50%). Other substances such as wax (30%), aromatic oils (10%), pollen (5%) and mineral and organic matter (5%) are found[19]. Till date, more than 300 chemical compounds have been identified in propolis, guaranteeing its curative effects. Among them are phenolic and flavonoic compounds[20].Apart from its antibacterial[21], anti-in flammatory[22], antioxidant[23],hepatoprotective[24], anti-proliferative and antifungal[25,26] activities,propolis has also antiparasitic effects[27-30].
In the present work, we investigated the antihydatic and immunomodulatory effects of Algerian propolis ethanolic extract(EEP) in an experimental cystic echinococcosis. In this sense, the in vitro effects of EEP on the protoscoleces of E. granulosus (PSCs)viability and the hydatid cysts development in BALB/c mice were evaluated. In addition, TNF-α, NF-κB and iNOS expression in the spleen was analyzed and NO and TNF-α levels were determined.
2. Materials and methods
2.1. Preparation of ethanolic propolis extract
Propolis sample was harvested in May in El Bayadh (North-West of Algeria) by scraping it off from the frames of Apis mellifera hives.Propolis was extracted following the protocol described by Baltas with some modifications[31]. Propolis sample was frozen overnight at -20 ℃ and then crushed rapidly to obtain a homogeneous powder. Ethanol at 70% was used for the extraction. A quantity of 20 g of propolis was added to the solvent (1 v: 5 v). Kept in the dark, the solutions were stirred for 12 d at room temperature under agitation. The supernatant was filtered (filter, 2 µm) in order to remove any residual sediment or contaminants and dried at 40 ℃.Freshly isolated propolis was then dissolved in ethanol (70%) and stock solutions with a final concentration of 170 mg/mL have been obtained.
2.2. Phenolic and flavonoid total contents
The phenolic total content in our propolis sample was determined based on the Folin-Ciocalteu assay as previously described by Zongo with slight changes[32]. Brie fly, 0.1 mL of EEP (0.1 mg/mL) was added to 0.5 mL of Folin-Ciocalteu’s reagent. 5 min later, the resulting solution was mixed with 0.4 mL of sodium carbonate (Na2CO3)solution (7.5%) and homogenized immediately. The tubes were kept for 30 min at 30 ℃ and the optical density was measured at 735 nm.The total content of flavonoid was determined by AlCl3colorimetric method[33]. Brie fly, 1 mL of ethanolic solution of the diluted extract(0.1 mg/mL) was added to 1 mL of aluminum chloride (AlCl3) at 1%. After 10 min of incubation in the dark, the mixture absorbance was read at 435 nm.
Phenolic and flavonoic compounds concentrations were calculated using the equation obtained from the standard gallic acid [0-400 µg/mL, y=0.010 8x+0.047 6 (R2=0.997 9)] and quercetin [0-100 µg/mL, y=0.055 1x+0.015 4 (R2=0.999 4)] graph respectively.
2.3. Evaluation of antioxidant capacity of EEP
The antioxidant capacity of our extract was evaluated using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical method, as previously described by Wang, with slight modifications[34]. The ethanolic propolis extract was prepared at several concentrations (0.6 µg/mL to 500.0 µg/mL) in the methanol and mixed with the DPPH methanol solution (0.2 mM). The reaction mixture was gently homogenized with a vortex and incubated in the dark for 15 min. Using methanol as a blank, the optical density was measured at 517 nm. Quercetin and vitamin C were used as standards.
The scavenging rate was calculated according to the following equation:
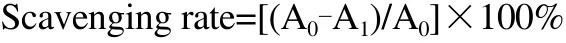
A0=the DPPH absorbance (without the EEP sample).
A1=the DPPH absorbance when the EEP sample is present.
Results were expressed as IC50value that was calculated using the relationship curve between the scavenging activities (%) and the respective sample concentrations.
2.4. Protoscolex collection
The PSCs were obtained, under sterile conditions, from fertile lung hydatid cysts (with no calcification and infection) of patients, after their ponction (Mustapha Pacha Hospital, Department of Surgery,Algiers, Algeria). After the elimination of the supernatant, the solution of sterile PBS (phosphate buffered saline), pH 7.5, was used to wash the PSCs and their viability was examined under a light microscope using eosin solution (0.1%). The viability of the samples was >95% at the time of experiment.
2.5. In vitro scolicidal activity of EEP
In our study, three concentrations of the EEP (5, 10 and 25 mg/mL) were tested for durations of 10, 20, 30 and 60 min. These concentrations were obtained by diluting the EEP solution in a test tube containing PBS (total volume=0.5 mL). Protoscoleces were then added to the tube and kept at room temperature. At each incubation’s end, the supernatant was discarded and 50 µL of eosin solution was mixed with the sediment and incubated for 5 min. The protoscoleces viability was then examined microscopically and the death rate was calculated by counting at least 300 protoscoleces. PBS, ethanol at 10% and 20% hypertonic saline have been used as negative, solvent and positive control groups respectively and the experiments were repeated 3 times.
2.6. Animals
Thirty healthy BALB/c female mice (18-20 g) were purchased from Pasteur Institute (Algiers, Algeria). They were intraperitoneally infected by inoculation of 2 000 protoscoleces suspended in sterile PBS[35]. All the animals were fed ad libitum and kept under normal conditions with a dark/light cycle (12 h/12 h). Our study was approved by the Committee on the Ethics of Animal Experiments of the Thematic Research Agency in Health Sciences ATRSS (ex ANDRS), which supported our project (Code: N°43-ANDRS-2011).
2.7. Acute toxicity study
Fifty healthy BALB/c female mice were split randomly into 5 groups (10 mice per group). All mice were given access to water while food was withheld for overnight prior to the treatment. They were given the ethanolic propolis extract at 1 000, 2 000, 3 000 and 4 000 mg/kg body weight concentrations while normal control group received hydroalcoholic solution at 20% used as gavage vehicle. The survival of mice as well as side effects were observed for 14 d.
2.8. Therapeutic trials
To estimate the Algerian EEP therapeutic effects during experimental echinococcosis, the animals were assigned randomly into 3 groups (10 mice per group): Group 1 (Ctrl) served as normal control and received tap water for three months and an intraperitoneal injection with PBS at the time point of infection. Group 2 (CE)served as hydatidosis control and received intraperitoneal injection of 2 000 viable hydatid protoscoleces suspended in 200 µL of sterile phosphate buffered saline solution. Group 3 (CE+EEP) was treated by daily intragastric administration of 100 mg/kg of EEP (from the 10th day after protoscoleces inoculation till the 3rd month’s end).During this time period, food and water intakes were daily recorded.A vehicle control (Ethanol at 2%) was confirmed to be not toxic.
2.9. Assessment of cyst development
At the treatment period’s end, the weight of cysts taken from the peritoneal cavity was measured. The following formula was used to determine the inhibition rate and the larval growth:
[(average cysts weight of CE group-average cysts weight of CE+EEP group)/average cysts weight of CE group]×100.
2.10. Serum collection and nitrite measurement
After 3 months of infection, all the mice were anesthetized. The blood samples collected via cardiac puncture and put in tubes without anticoagulant were centrifuged to obtain serum, which was used for measurement of nitrite.
Nitric oxide production in serum was evaluated via the measurement of nitrites using Griess reaction. In brief, a volume of 50 µL of serum was added to 50 µL of Griess reagent (5%sulfanilamide and 0.5% napthylethylenediamine dihydrochloride dissolved in 20% HCl) and incubated for 20 min in obscurity.After incubation, the optical density was read at 543 nm and nitrite concentration was calculated using a standard curve performed with sodium nitrite [NaNO2; (0-200) µmoL/mL].
2.11. Systemic cytokine levels measurement
The TNF-α levels in serum of mice were analyzed by using enzyme-linked immunosorbent assay (ELISA) kit (Sigma-Aldrich)according to the manufacturer’s specifications.
2.12. Immunohistofluorescence
At 3 months post-infection, 2 µm thick sections of spleen tissue of paraffin-embedded samples were dewaxed with xylene, and rehydrated in graded alcohols. They were then saturated by incubation(2 h) in PBS containing 5% skim milk and permeabilised with 0.1%Triton X-100 for 30 min. For the immunohisto fluorescence study, the sections were labeled at 4 ℃ overnight with the appropriate primary:anti TNF-α antibody (diluted 1:100), anti-iNOS antibody (Santa Cruz Biotechnology, diluted 1:100) and anti NF-κB/p50 antibody(Thermo Fisher SCIENTIFIC, diluted 1:100). Finally, the anti IgG-FITC (Life Technologies, diluted 1:500) was applied to tissue sections for 2 h and then rinsed three times with PBS. 90% glycerol-PBS solution was used to cover slides which were examined under fluorescent microscope (Olympus) connected to a digital camera to take photos (×30 magnification).
2.13. Statistical analysis
To express the difference among the groups, our results were analyzed by one-way analysis of variance (ANOVA) or t-test.GraphPad Prism 6.01 (GraphPad Inc.) and ImageJ softwares were used to perform statistical analyses and quantitative analyses of the immunofluorescence staining, respectively. Data are presented as means±SD and P values of less than 0.05 were accepted as statistically significant.
3. Results
3.1. Amount of total phenolic and flavonoid contents
In order to determine the amount of total phenolic and flavonoid contents, AlCl3and Folin-Ciocalteu’s reagents were used respectively. Results showed that EEP contains high polyphenol amount with (210.50±22.45) mg of GAE/g and flavonoid content with (16.4±3.4) mg QE/g.
3.2. DPPH scavenging activity measurement
Phenolic compounds such as flavonoid and phenolic acids, because of their hydroxyl groups, are known for their antioxidant activity[36].Thus, the extracts activity was evaluated using the DPPH free radical scavenging test. Our propolis sample gave an IC50value of(74.65±9.79) µg/mL. This value was close to the standards used in this study (10.35 µg/mL quercetin and 10.27 µg/mL vitamin C). This result indicated a notable antioxidant activity of our natural extract.
3.3. In vitro scolicidal activity of EEP
Propolis has shown significant scolicidal activity against E.granulosus. Indeed, at all tested concentrations, its ethanolic extract has demonstrated a great effectiveness against protoscoleces. The results of the mortality rate of PSCs after incubation with different concentrations of EEP are summarized in Table 1.
From this table we noticed that the concentration of EEP that has given 100% of mortality rate in shorter time (10 min) was 25 mg/mL. Similarly, the same rate of mortality has been observed after 60 and 30 min of exposure to 5 and 10 mg/mL of propolis concentrations, respectively (Table 1). These results demonstrated that EEP at lower concentrations induced a delayed scolicidal effect.
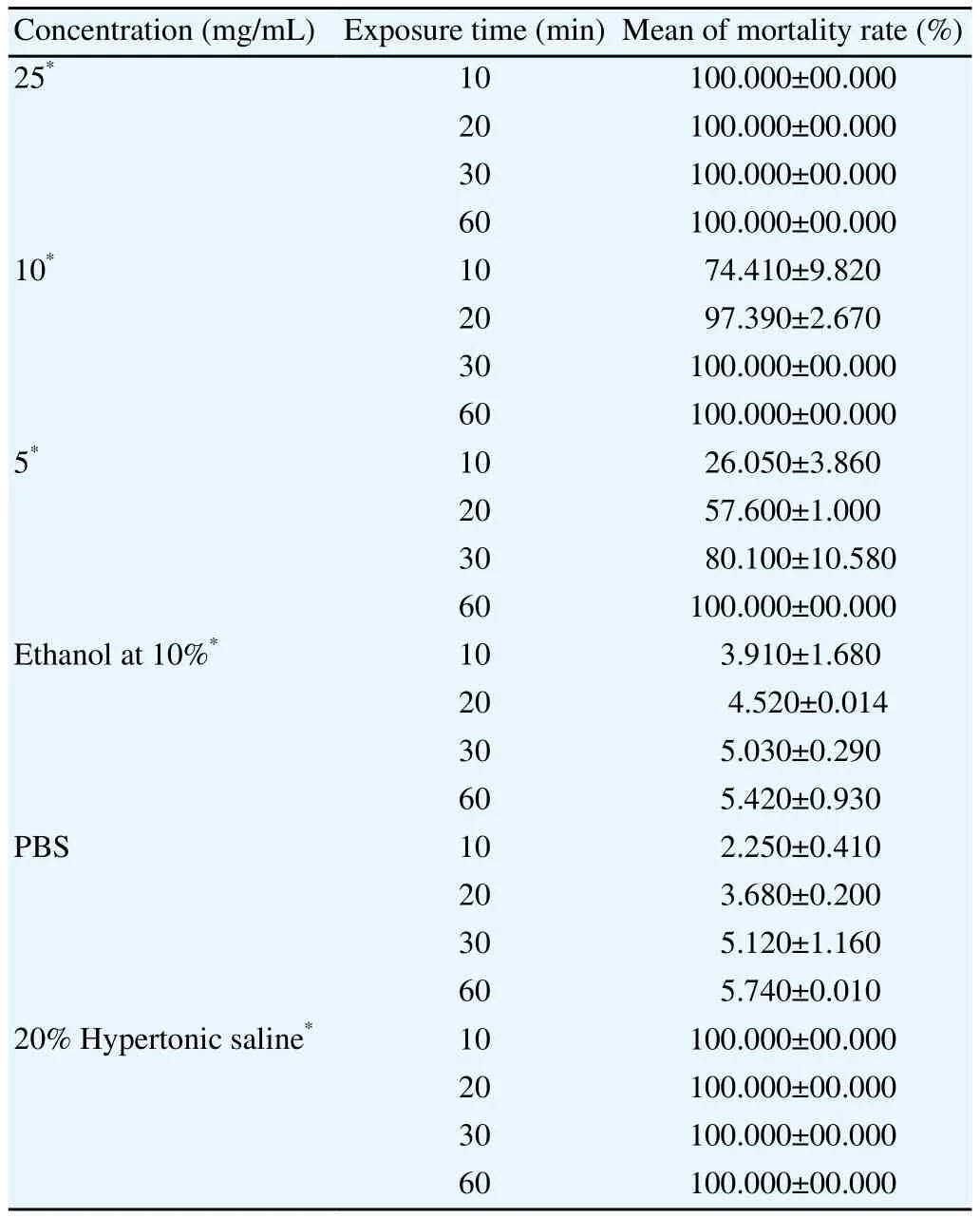
Table 1. Scolicidal effect of EEP at the concentrations of 5, 10, 25 mg/mL after 10, 20, 30 and 60 min of incubation with E. granulosus.
The scolicidal effects of EEP were statistically significant (P<0.05)for the 3 concentrations used in this study, compared to the control group (PBS). In addition, the in-vitro vitality/viability evaluation of protoscoleces after treatment with EEP extract indicated a significant loss in the viability and morphological tegumental destruction leading to protoscoleces disintegration (Figure 1).Control protoscoleces placed in PBS and ethanol at 10% remained viable after 60 min of incubation. Their structure was not affected during the experimental period. They had rapid movements and most of them appeared invaginated and turgid (Figure 2).
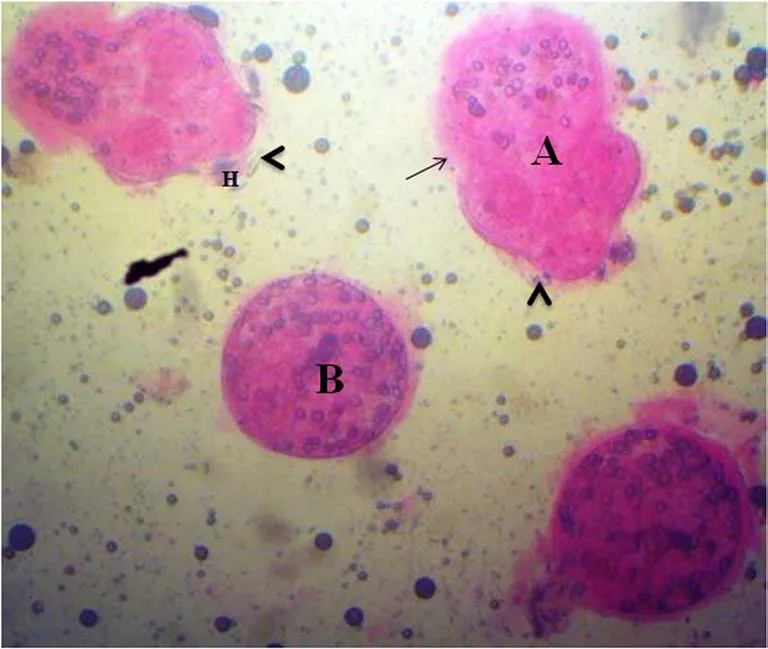
Figure 1. Dead protoscoleces exposed to EEP and 0.1% eosin solutions(×40 magnification).
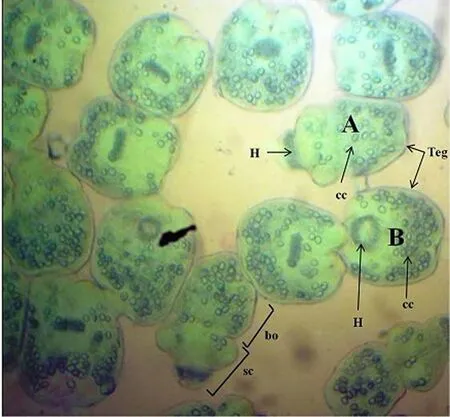
Figure 2. Live protoscoleces after exposure to 0.1% eosin solution (×40 magnification).
3.4. E. granulosus infection model
Three months after the intraperitoneal injection of protoscoleces,hydatid cysts were developed in all the mice with an average of 2.7(1-7) cysts per mouse. These cysts were macroscopically visible and only located in the peritoneal cavity, primarily observed in the pancreas, liver or free in the peritonea. In our study, the used procedure permitted the infection of 100% of the mice, and a reliable and reproducible mouse infection model was therefore established.
3.5. Acute toxicity study
After 14 d of the study, the mice of both the normal control(vehicle) and the treated groups did not show any signs of toxicity and no death cases were observed. Indeed, the mice’s appearance and behavior remained normal throughout the experiment duration.These results indicated that our propolis extract is relatively harmless and its toxic dose could be more than 4 000 mg/kg body weight.
3.6. Effect of EPP on clinical parameters of secondary experimental echinococcosis
The weight of developed cysts was measured to assess the effect of propolis treatment at a dose of 100 mg/mL. Our data revealed that EEP intragastric administration to BALB/c mice for three months has a negative effect on the hydatid cysts development. Indeed,the cysts weight decreased significantly in the propolis treated group in comparison with the untreated control group (P=0.000 2).Propolis treatment inhibited cyst growth by 73.75% in the CE+EEP group; weight (g) of cysts in untreated control water (CE) group and CE+EEP group were (11.280±6.008) g and (2.960±1.814) g respectively (Figure 3).

Figure 3. Inhibition effect of EPP on clinical parameters of secondary experimental echinococcosis.
3.7. Effect of EPP on systemic NO levels
We investigated the effect of propolis on NO production in secondary echinococcosis. Serum NO levels increased significantly in mice inoculated with E. granulosus (CE group) in comparison with its levels in non-infected mice (Ctrl group) [(5.083±0.607) µM vs.(2.610±1.003) µM (P=0.004)] (Figure 4). However, compared to the untreated-CE group, systemic NO levels have significantly decreased in CE group after oral administration of EEP [(2.838±0.289) µM vs.(5.083± 0.607) µM (P=0.004)] (Figure 4).
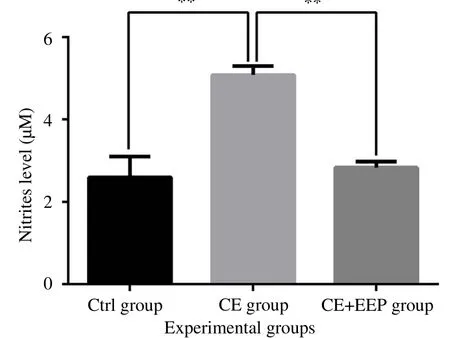
Figure 4. Treatment with EEP reduces the production of NO in serum of infected BALB/c mice.
3.8. Effect of EEP on in vivo cytokine production
To investigate the effect of the EEP on proin flammatory cytokine production in vivo during CE, systemic TNF-α concentrations were determined in the serum of mice (Figure 5). Our results showed that TNF-α levels were increased in CE group compared with the control group[(46.0±13.73) pg/mL vs. (29.50±17.86) pg/mL]. Interestingly,oral administration of EEP to CE group significantly decreased the levels of this in flammatory marker which was close to the Ctrl group[(18.0±13.16)pg/mL vs. (46.0±13.73)pg/mL (P=0.047)] (Figure 5). In addition, no significant difference in TNF-α concentrations between the Ctrl and CE+EEP groups was seen [(29.50±17.86) pg/mL vs.(18.0±13.16)pg/mL].
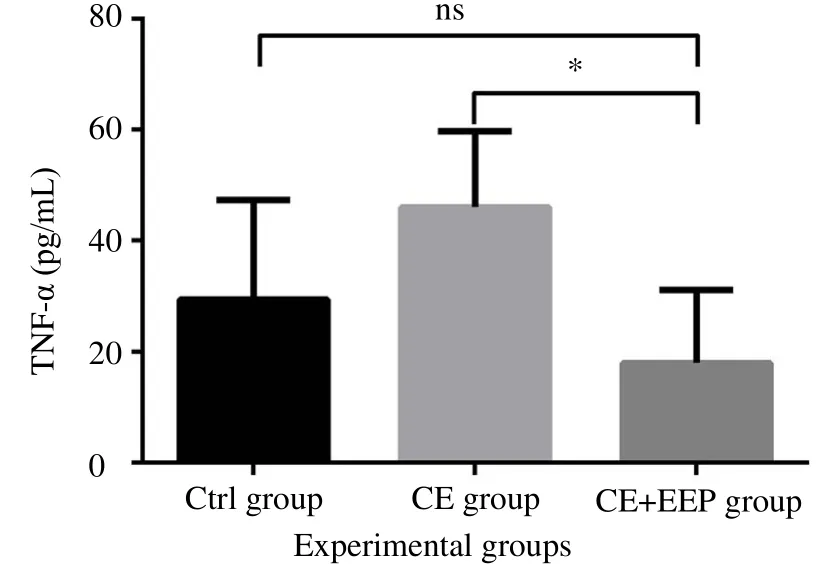
Figure 5. Treatment with EEP reduces the systemic levels of TNF-α in serum of infected BALB/c mice.
3.9. Effect of propolis on the expression of iNOS, TNFα and NF-κB in spleen tissue
The effect of propolis on the expression of spleen iNOS, TNF-α and NF-κB/p50, which is evaluated by immuno fluerecscence, was shown in Figure 6 and Table 2. A significantly increased iNOS,TNF-α and NF-κB/p50 immunoreactivity was observed in the CE group (in comparison with the control water group), particularly in the white pulp (P<0.01 for TNF-α staining, P<0.001 for iNOS and NF-κB/p50 staining). This immunoreactivity decreased significantly in the spleen of treated mice (Figure 6C, 6F and 6I) compared to the untreated ones (P<0.01 for NF-κB/p50 and TNF-α staining,P<0.001 for iNOS staining). None or a low iNOS, TNF-α and NF-κB/p50 expression was detected in the control group (Figure 6A, 6D and 6G; Table 2).

Figure 6. Representative pictures of immuno fluorescence essay results.

Table 2. Percentages of iNOS, NF-κB and TNF-α expression in the sections of the spleen of the Ctrl group, CE group and CE+EEP group (%).
4. Discussion
There is a growing interest to find new agents such as medicinal plant extracts and natural products as alternative options to medicate CE, due to being easily available, inexpensive and safe (low side effects and toxicity)[37]. In this study, we focused on the antihydatic and immunomodulatory effect of EEP during cystic echinococcosis.Our data demonstrated, for the first time, that administration of propolis at 100 mg/kg for 3 months induced a high inhibitory effect on the development of hydatid cyst. Indeed, the results showed a decrease in hydatid cyst weight in CE+EEP group. In addition, lethal effects were not observed after the administration of EEP during 3 months of treatment.
Several studies have reported the propolis anti-parasitic activity against numerous parasitic species both in vitro and in vivo. In their study, Pontin et al have shown that Brazilian green propolis had an in vitro and in vivo anti-leishmanial activity[38]. In addition, Omar et al have reported that Nigerian propolis was active against Trypanosoma brucei[39]. Our findings are also in agreement with a previous study conducted by Mahmoud reporting that propolis exhibited antischistosomal activity in mice[40]. More recently, Afrouzan and Algabbani demonstrated the anti-plasmodial activity of Iranian and Saudi propolis extracts in vitro and in vivo respectively[13,41].
We suggest that the antihydatic effect observed after treatment with Algerian propolis ethanolic extract can be due to its biologically active ingredients such as phenolic compounds. It has been demonstrated that flavonoids and phenolic acid derivatives have antibacterial, antinoceceptive, antifungal, antiseptic and antiparasitic properties[42]. The analysis of our EEP showed high levels of flavonoid and polyphenol. In addition, Soltani et al reported that the major compounds present in Algerian propolis are phenolic compounds, flavonoids, alkaloids, saturated and unsaturated fatty acids[43]. Moreover, Moazeni et al suggested that caffeic acid,quercetin, gallic acid and catechin may be responsible for the antihydatid effect of Zataria multiflora[12].
The advantages of propolis versus other herbal scolicidal agents are its antioxidant, immunostimulatory and hepatoprotective properties[44,45]. Additionally, there is little concern regarding the toxic or intensive adverse effects of propolis. Our study of acute toxicity showed that the ethanolic propolis extract had an LD50higher than 4 000 mg/kg of mice. Indeed, all the animals had a similar behavior and appearance, and they didn’t show any adverse changes throughout the acute toxicity test duration. These results indicated that EEP’s safety margin was high as the mice EEP tolerance exceeded 4 000 mg/kg of extract given orally. Arvouet-Grand et al have shown in their study that propolis has a low toxicity in mice with an LD50ranging from 2 000 to 7 300 mg/kg[46]. In addition, Mohammadzadeh et al also reported that the use of 20 000 mg/kg dose of propolis extract did not cause any death or toxicity in Wistar rats[47].
In our present work, we also focused on the anti-inflammatory effect of EEP treatment during experimental hydatidosis. We reported for the first time that CE increases, in spleen tissue, TNF-α,NF-κB and iNOS expression during E. granulosus infection. This expression was associated with high NO levels. Similar observations found in the liver have previously been reported by Labsi et al. Indeed, TNF-α, NF-κB and iNOS expression increased in Kupffer and hepatocytes cells of hepatic hydatid cysts of mice[48].In addition, the involvement of iNOS pathway was reported in hydatidosis and several protozoan and helminthic diseases[49-52].
Remarkably, oral administration of EEP decreased the immunoreactivity of TNF-α, NF-κB and iNOS in the spleen tissues of treated mice in comparison with untreated ones. Concomitantly,EEP treatment caused an important systemic decrease in NO and TNF-α levels. These results are associated with the reduction of CE development. It is possible that, after treatment, the inhibition of hydatid cysts growth induced the attenuation of immune cells response.
To our knowledge, this work is the first to show that administration of Algerian propolis attenuated inflammation during experimental hydatidosis. The anti-in flammatory effect of propolis in CE appears to be tightly related to the inhibition of the NF-κB pathway. It is well known that iNOS expression and NO production are mostly induced in macrophage via NF-κB by LPS, TNF-α and IL-1β[53,54].
The anti-in flammatory effects of propolis have been investigated in many in vivo and in vitro studies. Ertürküner SP et al showed that propolis treatment caused a significant reduction of ciliary body NF-κB/p65 expression and TNF-α level in LPS-induced uveitis[55].Wang et al have demonstrated that propolis collected from China reduced the LPS-induced NF-κB expression by the inhibition of IkB phosphorylation which led to the TNF-α level decrease[56]. It has been also reported by Al Ghamdi et al that the oral administration of propolis to diabetic mice significantly decreased the plasma TNF-α level[57].It has been shown that propolis has known antioxidant properties and the extracts obtained from this natural product are able to scavenge RL and ROS like hydroxyl radicals and superoxide anions[58]. In addition, the literature reports that the antioxidant effect of propolis could be related to its flavonoids and phenolic content[59].Our data showed that polyphenolics and flavonoids concentration was high in EPP of Algeria. It also showed a high DPPH free radical scavenging activity with an IC50value of 74.29 µg/mL. Several teams have reported a correlation between EEP radical scavenging activity and the concentration of polyphenolic compounds[60,61]. Our results indicated that the propolis obtained from El Bayadh region has higher total polyphenolic content (210.50 mg/g) compared with other propolis extracts from the Algerian north-western regions[62]. In fact,Benhanifia et al have found that total polyphenol contents of EEP collected from 5 regions of north-western Algeria varied between 9.99 mg/g and 46.63 mg/g. These differences may be attributed to the botanical origin, the harvest year and environmental hives[63].In addition, our results corroborate previous findings that reported the polyphenol concentrations in propolis obtained from various countries; Brazil: 232 mg/g[64], China: 43-302 mg/g[65], India: 159-269 mg/g and Iran: 31-187 mg/g[66,67].
Moreover, we explored the effect of ethanolic propolis extract on the hydatid cyst protoscoleces in various concentrations and over different time periods. Our study’s results showed that the highest scolicidal activity was observed at the concentration of 25 mg/mL because 100%of mortality has been obtained in less time (10 min). In addition,all the protoscoleces were killed after 60 and 30 min of exposure to EPP at the concentrations of 5 mg/mL and 10 mg/mL, respectively.Therefore, we notice that the increase in EEP concentrations induced higher protoscolicidal activity.
The protoscolicidal effect of many plants has been reported in several studies. Indeed, Moazeni et al demonstrated that 100% of mortality was obtained when protoscoleces were exposed to 25 mg/mL of garlic methanolic extract for 60 min[68]. Moreover, Sambucus ebulus extract with a concentration of 100 mg/mL killed 98.6% of the protoscolices after 60 min of application[69]. However, in our study, 100% of mortality was observed after only 10 min of EEP application at the concentration of 25 mg/mL. These results suggest that EEP can constitute a source of new compounds that could be used as an effective scolicidal agent. Thus, we can conclude that EEP could be considered as a safe and potent scolicidal agent used in hydatid cyst surgery.
Collectively, our study is the first report demonstrating with interest the antihydatic and immunomodulatory effects of the Algerian EEP, suggesting its possible therapeutic role in hydatid disease treatment. Additional research is needed to fully clarify the exact mechanism by which Algerian propolis exerts its antihydatic and immunomodulatory effects.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Acknowledgement
The authors wish to thank the surgical and technical staff of the Mustapha Pacha Hospital, Department of Thoracic Surgery of Algiers for providing cyst samples. We are grateful to the Thematic Research Agency in Health Sciences ATRSS (ex ANDRS), which supported our project (project manager: Dr. Mezioug Dalila;contract No: N°59/DFPR/ATRSS).
 Asian Pacific Journal of Tropical Medicine2019年3期
Asian Pacific Journal of Tropical Medicine2019年3期
- Asian Pacific Journal of Tropical Medicine的其它文章
- SARS and its treatment strategies
- Phyllanthus acidus (L.) Skeels and Rhinacanthus nasutus (L.) Kurz leaf extracts suppress melanogenesis in normal human epidermal melanocytes and reconstitutive skin culture
- Chemical composition of Mentha suaveolens and Pinus halepensis essential oils and their antibacterial and antioxidant activities
- Isolation and structural elucidation of antifungal compounds from Curcuma amada
- Status of intestinal parasitic infections among rural and urban populations, southwestern Iran
- X-linked Toll-like receptor 7 polymorphism associated with susceptibility to Chikungunya Fever
